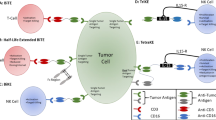
Abstract
Prostate cancer is the most commonly diagnosed cancer but the management of advanced prostate cancer remains a therapeutic challenge, despite the survival benefits imparted by several therapeutic discoveries targeting different molecular pathways. The mechanisms of resistance to androgen deprivation and tumour progression to lethal metastatic variants are often regulated by androgen receptor (AR) bypass mechanisms and/or neuroendocrine differentiation. Moreover, recent data also suggested the involvement of adaptive and innate infiltrated immune cells in prostate tumour progression. Improvements in cancer genome analyses contributed to a better understanding of antitumour immunity and provided solutions for targeting highly cancer-specific neoantigens generated from somatic mutations in individual patients. In this review, we investigated the current knowledge on the interplay between cancer development and the complex mechanisms of immune regulation. Particularly, we focused on the role of tumour immune microenvironment, generally characterised by strong barriers for immunotherapy, and we discuss the rationale for the potential application of single agent and combination immune-targeting strategies that could lead to improved outcomes. Careful selection based on clinical and genomic factors may allow identification of patients who could benefit from this treatment approach in multiple settings (from localised to advanced prostate tumour) and in different histological subtypes (from adenocarcinoma to neuroendocrine prostate cancer).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article, as no data sets were generated or analysed during this study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Davies A, Conteduca V, Zoubeidi A, Beltran H. Biological evolution of castration-resistant prostate cancer. Eur Urol Focus. 2019;5:147–54.
Conteduca V, Oromendia C, Eng KW, Bareja R, Sigouros M, Molina A, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7–18.
de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, et al. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer. 2020;20:455–69.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21.
Kaur HB, Guedes LB, Lu J, Maldonado L, Reitz L, Barber JR, et al. Association of tumour-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod Pathol. 2018;31:1539–52.
Calagua C, Ficial M, Jansen CS, Hirz T, Del Balzo L, Wilkinson S, et al. A subset of localized prostate cancer displays an immunogenic phenotype associated with losses of key tumour suppressor genes. Clin Cancer Res. 2021;27:4836–47.
Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, et al. Tumour infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30.
Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5.
Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–8.
Vicier C, Ravi P, Kwak L, Werner L, Huang Y, Evan C, et al. Association between CD8 and PD-L1 expression and outcomes after radical prostatectomy for localized prostate cancer. Prostate. 2021;81:50–7.
Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22:1969–77.
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129.
Andersen LB, Nørgaard M, Rasmussen M, Fredsøe J, Borre M, Ulhøi BP, et al. Immune cell analyses of the tumour microenvironment in prostate cancer highlight infiltrating regulatory T cells and macrophages as adverse prognostic factors. J Pathol. 2021;255:155–65.
Yamoah K, Awasthi S, Mahal BA, Zhao SG, Grass GD, Berglund A, et al. Novel transcriptomic interactions between immune content and genomic classifier predict lethal outcomes in high-grade prostate cancer. Eur Urol. 2022;81:325–30.
Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–31.
Vidotto T, Melo CM, Castelli E, Koti M, Dos Reis RB, Squire JA. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer. 2020;122:1732–43.
Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–32.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;162:454.
Li L, Ameri AH, Wang S, Jansson KH, Casey OM, Yang Q, et al. 2019 EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene. 2019;38:6241–55.
Zhao D, Cai L, Lu X, Liang X, Li J, Chen P, et al. Chromatin regulator chd1 remodels the immunosuppressive tumour microenvironment in PTEN-deficient prostate cancer. Cancer Discov. 2020;10:1374–87.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumourigenesis. Nature. 2005;436:725–30.
Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N, et al. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur Urol. 2013;64:177–88.
Armstrong CWD, Maxwell PJ, Ong CW, Redmond KM, McCann C, Neisen J, et al. PTEN deficiency promotes macrophage infiltration and hypersensitivity of prostate cancer to IAP antagonist/radiation combination therapy. Oncotarget. 2016;7:7885–98.
Jung SH, Hwang HJ, Kang D, Park HA, Lee HC, Jeong D, et al. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene. 2019;38:1639–50.
Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumours by activating the senescence-associated antitumour immunity. Cell Rep. 2014;9:75–89.
Blattner M, Liu D, Robinson BD, Huang D, Poliakov A, Gao D, et al. SPOP mutation drives prostate tumourigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell. 2017;31:436–51.
Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;55:91–5.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumours with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumours to PD-1 blockade. Science. 2017;357:409–13.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–8.
Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–7.
Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807–13.
Graham LS, Montgomery B, Cheng HH, Yu EY, Nelson PS, Pritchard C, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS ONE. 2020;15:e0233260.
Nava Rodrigues D, Rescigno P, Liu D, Yuan W, Carreira S, Lambros MB, et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J Clin Investig. 2018;128:4441–53.
Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;2:162–74.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53.
Jenzer M, Keß P, Nientiedt C, Endris V, Kippenberger M, Leichsenring J, et al. The BRCA2 mutation status shapes the immune phenotype of prostate cancer. Cancer Immunol Immunother. 2019;68:1621–33.
Kwon JTW, Bryant RJ, Parkes EE. The tumor microenvironment and immune responses in prostate cancer patients. Endocr Relat Cancer. 2021;28:T95–T107.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumour mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9.
Ragu S, Matos-Rodrigues G, Lopez BS. Replication stress, DNA damage, inflammatory cytokines and innate immune response. Genes. 2020;11:409.
Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, et al. cGAS is essential for the antitumour effect of immune checkpoint blockade. Proc Natl Acad Sci USA. 2017;114:1637–42.
Dutta Gupta P, Chaudagar K, Sharma-Saha S, et al. PARP and PI3K inhibitor combination therapy eradicates c-MYC-driven murine prostate cancers via cGAS/STING pathway activation within tumor-associated macrophages [abstract]. Mol Cancer Ther. 2019;18:LB-B03.
Suter MA, Tan NY, Thiam CH, Khatoo M, MacAry PA, Angeli V, et al. cGAS-STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells. Sci Rep. 2021;11:7243.
Härtlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42:332–43.
Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;6:141.
Petrylak DP, Perez-Garcia JL, Lacombe L, Bastos DA, Mahammedi H, Kwanet EM, et al. 579MO: CheckMate 9KD cohort A2 final analysis: Nivolumab (NIVO) + rucaparib for chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2021;32:S629–S630.
Appleman LJ, Kolinsky MP, Berry WR, Retz M, Mourey L, Piulats JM, et al. Keynote-365 cohort B: Pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients with metastatic castration-resistant prostate cancer (mCRPC)- New data after an additional 1 year of follow up. J Clin Oncol. 2021;39:10.
Antonarakis ES. Cyclin-dependent kinase 12, immunity, and prostate cancer. N Engl J Med. 2018;379:1087–9.
Rescigno P, Gurel B, Pereira R, Crespo M, Rekowski J, Rediti M, et al. Characterizing CDK12mutated prostate cancers. Clin Cancer Res. 2021;27:566–74.
Brighi N, Conteduca V, Lolli C, Gurioli G, Schepisi G, Palleschi M, et al. The cyclin-dependent kinases pathway as a target for prostate cancer treatment: rationale and future perspectives. Crit Rev Oncol Hematol. 2021;157:103199.
Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559:363–9.
Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53.
Conteduca V, Caffo O, Scarpi E, Sepe P, Galli L, Fratino L, et al. Immune modulation in prostate cancer patients treated with androgen receptor (AR)-targeted therapy. J Clin Med. 2020;9:1950.
Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17.
Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49.
Long X, Hou H, Wang X, Liu S, Diao T, Lai S, et al. Immune signature driven by ADT-induced immune microenvironment remodeling in prostate cancer is correlated with recurrence-free survival and immune infiltration. Cell Death Dis. 2020;11:779.
Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606:791–6.
Yang C, Jin J, Yang Y, Sun H, Wu L, Shen M, et al. Androgen receptor-mediated CD8+ T cell stemness programs drive sex differences in antitumor immunity. Immunity. 2022;55:1268.e9–83.e9.
Graff JN, Beer TM, Alumkal JJ, Slottke RE, Redmond WL, Thomas GV, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer. 2020;8:e000642.
Powles T, Yuen KC, Gillessen S, Kadel EE 3rd, Rathkopf D, Matsubara N, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med. 2022;28:144–53.
Alizadeh-Ghodsi M, Owen KL, Townley SL, Zanker D, Rollin SPG, Hanson AR, et al. Potent stimulation of the androgen receptor instigates a viral mimicry response in prostate. Cancer Cancer Res Commun. 2022;2:706–24.
Kumar R, Mendonca J, Owoyemi O, Boyapati K, Thomas N, Kanacharoen S, et al. Supraphysiologic testosterone induces ferroptosis and activates immune pathways through nucleophagy in prostate cancer. Cancer Res. 2021;81:5948–62.
Pu Y, Xu M, Liang Y, Yang K, Guo Y, Yang X, et al. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Sci Transl Med. 2016;8:333ra47.
Ollivier L, Labbé M, Fradin D, Potiron V, Supiot S. Interaction between modern radiotherapy and immunotherapy for metastatic prostate cancer. Front Oncol. 2021;11:744679.
Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126.
Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014;29:153–61.
Chiang CS, Fu SY, Wang SC, Yu CF, Chen FH, Lin CM, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89.
Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, et al. Tumour-reprogrammed resident T cells resist radiation to control tumours. Nat Commun. 2019;10:3959.
Eke I, Aryankalayil MJ, Bylicky MA, Sandfort V, Vanpouille-Box C, Nandagopal S, et al. Long-term expression changes of immune-related genes in prostate cancer after radiotherapy. Cancer Immunol Immunother. 2022;71:839–50.
Yamada Y, Beltran H. Clinical and biological features of neuroendocrine prostate cancer. Curr Oncol Rep. 2021;23:15.
Bhinder B, Ferguson A, Sigouros M, Uppal M, Elsaeed AG, Bareja R, et al. The immunogenomic landscape of neuroendocrine prostate cancer. Clin Cancer Res. 2023:CCR-22-3743. https://doi.org/10.1158/1078-0432.CCR-22-3743.
Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305.
DeLucia DC, Cardillo TM, Ang L, Labrecque MP, Zhang A, Hopkins JE, et al. Regulation of CEACAM5 and therapeutic efficacy of an anti-CEACAM5-SN38 antibody-drug conjugate in neuroendocrine prostate cancer. Clin Cancer Res. 2021;27:759–74.
Puca L, Gavyert K, Sailer V, Conteduca V, Dardenne E, Sigouros M, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med. 2019;11:eaav0891.
Brady L, Kriner M, Coleman I, Morrissey C, Roudier M, True LD, et al. Inter- and intra-tumour heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat Commun. 2021;12:1426.
Brown LC, Halabi S, Somarelli JA, Humeniuk M, Wu Y, Oyekunle T, et al. A phase 2 trial of avelumab in men with aggressive-variant or neuroendocrine prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:762–9.
Lopez-Bujanda ZA, Haffner MC, Chaimowitz MG, Chowdhury N, Venturini NJ, Patel RA, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat Cancer. 2021;2:803–18.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78:822–30.
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405.
Petrylak DP, Loriot Y, Shaffer DR, Braiteh F, Powderly J, Harshman LC, et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin Cancer Res. 2021;27:3360–9.
Antonarakis ES, Isaacsson Velho P, Fu W, Wang H, Agarwal N, Sacristan Santos V, et al. CDK12-altered prostate cancer: clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-ribose) polymerase inhibitors, and PD-1 inhibitors. JCO Precis Oncol. 2020;4:370–81.
Subudhi SK, Vence L, Zhao H, Blando J, Yadav SS, Xiong Q, et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci Transl Med. 2020;12:eaaz3577.
Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 Trial. Cancer Cell. 2020;38:489.e3–99.e3.
Fizazi K, Gonzalez Mella P, Castellano D, Minatta JN, Rezazadeh Kalebasty A, Shaffer D, et al. Nivolumab plus Docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: results from the phase II CheckMate9KD trial. Eur J Cancer. 2022;160:61–71.
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714.
Agarwal NMB, Maughan BL, Dorff T, Kelly W, Fang B, McKay R, et al. Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): results of expanded cohort 6 of the COSMIC-021 study. Ann Oncol. 2021;32:S1283–46.
Sandhu S, Joshua AM, Emmett L, Spain LA, Horvath L, Crumbaker M, et al. PRINCE: phase I trial of 177Lu-PSMA-617 in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40:5017.
Aggarwal R, Trihy L, Hernandez Romero E, Luch Sam S, Rastogi M, De Kouchkovsky I, et al. A phase Ib study of a single priming dose of 177Lu-PSMA-617 coupled with pembrolizumab in metastatic castration resistant prostate cancer (mCRPC). Ann Oncol. 2022;33:S616–52.
Schepisi G, Farolfi A, Conteduca V, Martignano F, De Lisi D, Ravaglia G, et al. Immunotherapy for prostate cancer: where we are headed. Int J Mol Sci. 2017;18:2627.
Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, et al. Efficacy and safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. 2022;8:546–52.
Schuhmacher J, Heidu S, Balchen T, Richardson JR, Schmeltz C, Sonne J, et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: results from a phase I/II clinical trial. J Immunother Cancer. 2020;8:e001157.
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105.
Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18:727–43.
Stein MN, Fong L, Mega AE, Lam ET, Heyburn JW, Gutierrez AA, et al. KEYNOTE-046 (Part B): effects of ADXS-PSA in combination with pembrolizumab on survival in metastatic, castration-resistant prostate cancer patients with or without prior exposure to docetaxel. J Clin Oncol. 2020;38:126.
McNeel DG, Eickhoff JC, Johnson LE, Roth AR, Perk TG, Fong L. et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP[MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. 2019;37:3507–17.
Kyriakopoulos CE, Eickhoff JC, Ferrari AC, Schweizer MT, Wargowski E, Olson BM, et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR,MVI-118) in patients with metastatic prostate cancer. Clin Cancer Res. 2020;26:5162–71.
Deegen P, Thomas O, Nolan-Stevaux O, Li S, Wahl J, Bogner P, et al. The PSMA-targeting half-life extended BiTE therapy AMG 160 has potent antitumour activity in preclinical models of metastatic castration-resistant prostate cancer. Clin Cancer Res. 2021;27:2928–37.
Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS, et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2 - T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–70.
Zhang Q, Helfand BT, Carneiro BA, Qin W, Yang XJ, Lee C, et al. Efficacy against human prostate cancer by prostate-specific membrane antigen-specific, transforming growth factor-β insensitive genetically targeted CD8+ T-cells derived from patients with metastatic castrate-resistant disease. Eur Urol. 2018;73:648–52.
Di Mitri D, Mirenda M, Vasilevska J, Calcinotto A, Delaleu N, Revandkar A, et al. Re-education of tumor associated macrophages by CXCR2 blockade drives senescence and tumor inhibition in advanced prostate cancer. Cell Rep. 2019;28:2156–68.
Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570.
Guo C, Crespo M, Gurel B, Dolling D, Rekowski J, Sharp A, et al. CD38 in advanced prostate cancers. Eur Urol 2021;79:736–46.
Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang J, et al. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol 2019;75:752–63.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the review. VC, NB and GS drafted the manuscript. NB prepared the figures of the manuscript. UDG revised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
VC has served as a consultant/advisory board member for Janssen, Astellas, Merck, AstraZeneca, Amgen, EISAI, Recordati and Bayer and has received speaker honoraria or travel support from Astellas, Janssen, Ipsen, Bayer, Gilead and BristolMyers Squibb. UDG reports research support from AstraZeneca, Roche and Sanofi; and consultancy fees from Astellas, Bayer, BristolMyers Squibb, Ipsen, Janssen, Merck, Pfizer and Sanofi. No potential conflicts of interest were disclosed by the other authors.
Ethics approval and consent to participate
This work does not require any ethics approval or participating consent.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Conteduca, V., Brighi, N., Schepisi, G. et al. Immunogenomic profiles associated with response to life-prolonging agents in prostate cancer. Br J Cancer 129, 1050–1060 (2023). https://doi.org/10.1038/s41416-023-02354-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02354-3