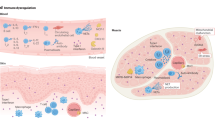
Abstract
The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of systemic autoimmune diseases that affect the skeletal muscles and can also involve the skin, joints, lungs and heart. The epidemiology of IIM is obscured by changing classification criteria and the inherent shortcomings of case identification using healthcare record diagnostic coding. The incidence of IIM is estimated to range from 0.2 to 2 per 100,000 person-years, with prevalence from 2 to 25 per 100,000 people. Although the effects of age and gender on incidence are known, there is only sparse understanding of ethnic differences, particularly in indigenous populations. The incidence of IIM has reportedly increased in the twenty-first century, but whether this is a genuine increase is not yet known. Understanding of the genetic risk factors for different IIM subtypes has advanced considerably. Infections, medications, malignancy and geography are also commonly identified risk factors. Potentially, the COVID-19 pandemic has altered IIM incidence, although evidence of this occurrence is limited to case reports and small case series. Consideration of the current understanding of the epidemiology of IIM can highlight important areas of interest for future research into these rare diseases.
Key points
-
The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of rare, systemic autoimmune conditions with an incidence of 0.2–2 per 100,000 person-years, and a prevalence of 2–25 per 100,000 people.
-
Changing classification criteria, biases in patient identification and heterogeneity within the group of IIMs affect the ascertainment of incidence and prevalence.
-
The incidence and prevalence of IIM varies with age and gender, and between geographical regions, but is still unreported in many areas, particularly in South America, Africa and Asia.
-
Further research is needed in non-white cohorts of IIM to better understand phenotypic, serological and genetic characteristics of ethnically diverse groups.
-
Although mortality from IIM has improved with earlier diagnosis and treatment, substantial morbidity remains in association with malignancy, cardiovascular events, lung disease and infections, resulting in considerable healthcare costs.
-
Understanding is growing of the unique genetic risk factors and gene–environment risk interactions in IIM, and of the influence of infections, medications (including statins and immune-checkpoint inhibitors), malignancy and geography.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Oldroyd, A. G. S. et al. British Society for Rheumatology guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy. Rheumatology 61, 1760–1768 (2022).
Chinoy, H. & Lilleker, J. B. Pitfalls in the diagnosis of myositis. Best. Pract. Res. Clin. Rheumatol. 34, 101486 (2020).
Lundberg, I. E. et al. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Prim. 7, 86 (2021).
Barsotti, S. & Lundberg, I. E. Myositis an evolving spectrum of disease. Immunol. Med. 41, 46–54 (2018).
Namsrai, T. et al. Diagnostic delay of myositis: an integrated systematic review. Orphanet J. Rare Dis. 17, 420 (2022).
Mammen, A. L. Which nonautoimmune myopathies are most frequently misdiagnosed as myositis? Curr. Opin. Rheumatol. 29, 618–622 (2017).
Bernatsky, S., Linehan, T. & Hanly, J. G. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J. Rheumatol. 38, 1612–1616 (2011).
Tanno, L. K. et al. Global implementation of the world health organization’s International Classification of Diseases (ICD)-11: the allergic and hypersensitivity conditions model. Allergy 75, 2206–2218 (2020).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347 (1975).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292, 403–407 (1975).
Carpenter, S., Karpati, G., Heller, I. & Eisen, A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology 28, 8–17 (1978).
Griggs, R. C. et al. Inclusion body myositis and myopathies. Ann. Neurol. 38, 705–713 (1995).
Hoogendijk, J. E. et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 14, 337–345 (2004).
Day, J., Otto, S., Cash, K. & Limaye, V. Clinical and histological features of immune-mediated necrotising myopathy: a multi-centre South Australian cohort study. Neuromuscul. Disord. 30, 186–199 (2020).
Allenbach, Y., Benveniste, O., Stenzel, W. & Boyer, O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat. Rev. Rheumatol. 16, 689–701 (2020).
Allenbach, Y. et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 139, 2131–2135 (2016).
Kassardjian, C. D., Lennon, V. A., Alfugham, N. B., Mahler, M. & Milone, M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol. 72, 996–1003 (2015).
Ma, X., Xu, L., Ji, S., Li, Y. & Bu, B. The clinicopathological distinction between seropositive and seronegative immune-mediated necrotizing myopathy in China. Front. Neurol. 12, 670784 (2021).
Allenbach, Y., Mammen, A. L., Benveniste, O. & Stenzel, W. & Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop:: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. 28, 87–99 (2018).
Bernstein, R. M. et al. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br. Med. J. 289, 151–152 (1984).
Tieu, J., Lundberg, I. E. & Limaye, V. Idiopathic inflammatory myositis. Best. Pract. Res. Clin. Rheumatol. 30, 149–168 (2016).
Opinc, A. H. & Makowska, J. S. Antisynthetase syndrome — much more than just a myopathy. Semin. Arthritis Rheum. 51, 72–83 (2021).
Huang, K. & Aggarwal, R. Antisynthetase syndrome: a distinct disease spectrum. J. Scleroderma Relat. Disord. 5, 178–191 (2020).
Uruha, A. et al. Diagnostic potential of sarcoplasmic myxovirus resistance protein A expression in subsets of dermatomyositis. Neuropathol. Appl. Neurobiol. 45, 513–522 (2019).
Aouizerate, J. et al. Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy. Acta Neuropathol. Commun. 2, 154 (2014).
Dalakas, M. C. Complement in autoimmune inflammatory myopathies, the role of myositis-associated antibodies, COVID-19 associations, and muscle amyloid deposits. Expert. Rev. Clin. Immunol. 18, 413–423 (2022).
Aguila, L. A. et al. Overlap syndromes of idiopathic inflammatory myopathies with systemic lupus erythematosus, systemic sclerosis or rheumatoid arthritis. Lupus 22, 136 (2013).
Keck, A. D., Jaeger, V. K. & Walker, U. A. Myositis in systemic sclerosis, lupus erythematosus and Sjogren’s syndrome. Aktuelle Rheumatol. 42, 310–315 (2017).
Paik, J. J. Myopathy in scleroderma and in other connective tissue diseases. Curr. Opin. Rheumatol. 28, 631–635 (2016).
Leclair, V., Notarnicola, A., Vencovsky, J. & Lundberg, I. E. Polymyositis: does it really exist as a distinct clinical subset? Curr. Opin. Rheumatol. 33, 537–543 (2021).
Betteridge, Z. et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J. Autoimmun. 101, 48–55 (2019).
Hodgkinson, L. M., Wu, T. T. & Fiorentino, D. F. Dermatomyositis autoantibodies: how can we maximize utility? Ann. Transl. Med. 9, 433 (2021).
Chen, Z. et al. Distinct profiles of myositis-specific autoantibodies in Chinese and Japanese patients with polymyositis/dermatomyositis. Clin. Rheumatol. 34, 1627–1631 (2015).
McHugh, N. J. & Tansley, S. L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 14, 290–302 (2018).
Benveniste, O. & Hilton-Jones, D. International workshop on inclusion body myositis held at the institute of myology, Paris, on 29 May 2009. Neuromuscul. Disord. 20, 414–421 (2010).
Dalakas, M. C. & Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 362, 971–982 (2003).
Hilton-Jones, D. & Brady, S. Diagnostic criteria for inclusion body myositis. J. Intern. Med. 280, 52–62 (2016).
Lloyd, T. E. et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology 83, 426–433 (2014).
Mammen, A. L., Allenbach, Y., Stenzel, W., Benveniste, O. & ENMC 239th Workshop Study Group. 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14–16 December 2018. Neuromuscul. Disord. 30, 70–92 (2020).
Rose, M. R. & Group, E. I. W. 188th ENMC international workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul. Disord. 23, 1044–1055 (2013).
Tanimoto, K. et al. Classification criteria for polymyositis and dermatomyositis. J. Rheumatol. 22, 668–674 (1995).
Targoff, I. N., Miller, F. W., Medsger, T. A. Jr. & Oddis, C. V. Classification criteria for the idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 9, 527–535 (1997).
Karasawa, R. & Jarvis, J. N. Using the tools of proteomics to understand the pathogenesis of idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 31, 617–622 (2019).
Lamb, J. A. The genetics of autoimmune myositis. Front. Immunol. 13, 886290 (2022).
Tanboon, J., Uruha, A., Stenzel, W. & Nishino, I. Where are we moving in the classification of idiopathic inflammatory myopathies? Curr. Opin. Neurol. 33, 590–603 (2020).
Lundberg, I. E. et al. European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 69, 2271–2282 (2017).
Hervier, B. & Uzunhan, Y. Inflammatory myopathy-related interstitial lung disease: from pathophysiology to treatment. Front. Med. 6, 326 (2019).
Luu, Q., Day, J., Hall, A., Limaye, V. & Major, G. External validation and evaluation of adding MRI or extended myositis antibody panel to the 2017 EULAR/ACR myositis classification criteria. ACR Open Rheumatol. 1, 462–468 (2019).
Lundberg, I. E. & Svensson, J. Registries in idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 25, 729–734 (2013).
Meyer, A. et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology 54, 50–63 (2014).
Anagnostopoulos, I. et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet. Disord. 11, 98 (2010).
Julian-Santiago, F., Garcia-Garcia, C., Garcia-Olivera, I., Goycochea-Robles, M. V. & Pelaez-Ballestas, I. Epidemiology of rheumatic diseases in Mixtec and Chontal indigenous communities in Mexico: a cross-sectional community-based study. Clin. Rheumatol. 35, 35–42 (2016).
Tolentino, D. S., de Oliveira, C. M. & de Assis, E. M. Population-based study of 24 autoimmune diseases carried out in a Brazilian microregion. J. Epidemiol. Glob. Health 9, 243–251 (2019).
Eaton, W. W., Rose, N. R., Kalaydjian, A., Pedersen, M. G. & Mortensen, P. B. Epidemiology of autoimmune diseases in Denmark. J. Autoimmun. 29, 1–9 (2007).
Pawlitzki, M. et al. Myositis in Germany: epidemiological insights over 15 years from 2005 to 2019. Neurol. Res. Pract. 4, 62 (2022).
Cho, S. K. et al. Incidence and prevalence of idiopathic inflammatory myopathies in Korea: a nationwide population-based study. J. Korean Med. Sci. 34, e55 (2019).
Furst, D. E., Amato, A. A., Iorga, S. R., Gajria, K. & Fernandes, A. W. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve 45, 676–683 (2012).
Smoyer-Tomic, K. E., Amato, A. A. & Fernandes, A. W. Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, Medicare supplemental insured, and Medicaid enrolled populations: an administrative claims analysis. BMC Musculoskelet. Disord. 13, 103 (2012).
Bolender, C. M. et al. Incidence of dermatomyositis in a nationwide cohort study of US veterans. JAMA Dermatol. 158, 1321–1323 (2022).
Essouma, M., Noubiap, J. J., Singwe-Ngandeu, M. & Hachulla, E. Epidemiology of idiopathic inflammatory myopathies in Africa: a contemporary systematic review. J. Clin. Rheumatol. 28, E552–E562 (2022).
Limaye, V. et al. The epidemiology of dermatomyositis in South Australia. APLAR J. Rheumatol. 10, 94–100 (2007).
Ostrovršnik, J. et al. The incidence of idiopathic inflammatory myopathies in the adult Slovenian population. Clin. Rheumatol. 38, 279–283 (2019).
Parker, M. J. S. et al. Increasing incidence of adult idiopathic inflammatory myopathies in the City of Salford, UK: a 10-year epidemiological study. Rheumatol. Adv. Pract. 2, rky035 (2018).
Patrick, M., Buchbinder, R., Jolley, D., Dennett, X. & Buchanan, R. Incidence of inflammatory myopathies in Victoria, Australia, and evidence of spatial clustering. J. Rheumatol. 26, 1094–1100 (1999).
See, L. C., Kuo, C. F., Chou, I. J., Chiou, M. J. & Yu, K. H. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin. Arthritis Rheumatism 43, 381–386 (2013).
Yu, K. H., See, L. C., Kuo, C. F., Chou, I. J. & Chou, M. J. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res. 65, 244–250 (2013).
Tan, J. A. et al. Incidence and prevalence of idiopathic inflammatory myopathies in South Australia: a 30-year epidemiologic study of histology-proven cases. Int. J. Rheum. Dis. 16, 331–338 (2013).
Medsger, T. A. Jr, Dawson, W. N. Jr & Masi, A. T. The epidemiology of polymyositis. Am. J. Med. 48, 715–723 (1970).
Benbassat, J., Geffel, D. & Zlotnick, A. Epidemiology of polymyositis-dermatomyositis in Israel, 1960–76. Isr. J. Med. Sci. 16, 197–200 (1980).
Svensson, J., Arkema, E. V., Lundberg, I. E. & Holmqvist, M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology 56, 802–810 (2017).
Kuo, C. F. et al. Incidence, cancer risk and mortality of dermatomyositis and polymyositis in Taiwan: a nationwide population study. Br. J. Dermatol. 165, 1273–1279 (2011).
Kronzer, V. L. et al. Incidence, prevalence, and mortality of dermatomyositis: a population-based cohort study. Arthritis Care Res. 75, 348–355 (2023).
Carey, I. M. et al. Prevalence and incidence of neuromuscular conditions in the UK between 2000 and 2019: a retrospective study using primary care data. PLoS One 16, e0261983 (2021).
Badrising, U. A. et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology 55, 1385–1387 (2000).
Dobloug, G. C. et al. High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur. J. Neurol. 22, 672–e641 (2015).
Felice, K. J. & North, W. A. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine 80, 320–327 (2001).
Lefter, S., Hardiman, O. & Ryan, A. M. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology 88, 304–313 (2017).
Lindgren, U., Pullerits, R., Lindberg, C. & Oldfors, A. Epidemiology, survival, and clinical characteristics of inclusion body myositis. Ann. Neurol. 92, 201–212 (2022).
Phillips, B. A., Zilko, P. J. & Mastaglia, F. L. Prevalence of sporadic inclusion body myositis in Western Australia. Muscle Nerve 23, 970–972 (2000).
Serdaroglu Oflazer, P., Deymeer, F. & Parman, Y. Sporadic-inclusion body myositis (s-IBM) is not so prevalent in Istanbul/Turkey: a muscle biopsy based survey. Acta Myol. 30, 34–36 (2011).
Shelly, S. et al. Epidemiology and natural history of inclusion body myositis: a 40-year population-based study. Neurology 96, e2653–e2661 (2021).
Balakrishnan, A., Aggarwal, R., Agarwal, V. & Gupta, L. Inclusion body myositis in the rheumatology clinic. Int. J. Rheum. Dis. 23, 1126–1135 (2020).
Wilson, F. C., Ytterberg, S. R., St Sauver, J. L. & Reed, A. M. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J. Rheumatol. 35, 445–447 (2008).
Arkachaisri, T. et al. Paediatric rheumatology clinic population in Southeast Asia: are we different? Rheumatology 56, 390–398 (2017).
Barber, M. R. W. et al. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 17, 515–532 (2021).
Al-Sheikh, H., Ahmad, Z. & Johnson, S. R. Ethnic variations in systemic sclerosis disease manifestations, internal organ involvement, and mortality. J. Rheumatol. 46, 1103–1108 (2019).
Mendez, E. P. et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Care Res. 49, 300–305 (2003).
Concannon, A. & Han, D. Y. Incidence, severity and clinical manifestations of juvenile dermatomyositis among Maori and Pacific Island compared to European children. J. Paediatrics Child. Health 57, 1881–1885 (2021).
Day, J. & Limaye, V. Over-representation of statin-associated necrotising myopathy in patients of Aboriginal and Torres Strait Islander heritage. Intern. Med. J. 48, 749–751 (2018).
Woolley, M., Stebbings, S. & Highton, J. Statin-associated immune-mediated necrotising myopathy: a New Zealand case series showing possible overrepresentation in Pacific Islanders. Intern. Med. J. 48, 32–36 (2018).
Close, R. M. et al. Potential implications of six American Indian patients with myopathy, statin exposure and anti-HMGCR antibodies. Rheumatology 60, 692–698 (2021).
Mammen, A. L. Statin-associated autoimmune myopathy. N. Engl. J. Med. 374, 664–669 (2016).
Woolley, M. C. & Stebbings, S. Author reply. Intern. Med. J. 48, 751–752 (2018).
Izmirly, P. M. et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus registries. Arthritis Rheumatol. 73, 991–996 (2021).
Ly, E., Thein, H. & Lam Po Tang, M. Retrospective review of lupus nephritis in a New Zealand multi-ethnic cohort. Lupus 26, 893–897 (2017).
Nigam, A. et al. Lupus nephritis in indigenous Australians: a single-centre study. Intern. Med. J. 50, 830–837 (2020).
Gracey, M. Why closing the Aboriginal health gap is so elusive. Intern. Med. J. 44, 1141–1143 (2014).
Stoneman, J. & Taylor, S. J. Improving access to medicines in urban, regional and rural Aboriginal communities — is expansion of Section 100 the answer? Rural. Remote. Health 7, 738 (2007).
Thurber, K. A. & Bell, K. J. Socio-economic disadvantage and cardiovascular risk factors in young Aboriginal and Torres Strait Islander Australians. Med. J. Aust. 211, 259–260 (2019).
Ohta, A., Nagai, M., Nishina, M., Tomimitsu, H. & Kohsaka, H. Prevalence and incidence of polymyositis and dermatomyositis in Japan. Mod. Rheumatol. 24, 477–480 (2014).
Gerami, P., Walling, H. W., Lewis, J., Doughty, L. & Sontheimer, R. D. A systematic review of juvenile-onset clinically amyopathic dermatomyositis. Br. J. Dermatol. 157, 637–644 (2007).
Bendewald, M. J., Wetter, D. A., Li, X. & Davis, M. D. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch. Dermatol. 146, 26–30 (2010).
Greenfield, J. et al. A comparison of health-related quality of life (HRQoL) across four systemic autoimmune rheumatic diseases (SARDs). PLoS One 12, e0189840 (2017).
van de Vlekkert, J., Hoogendijk, J. E. & de Visser, M. Long-term follow-up of 62 patients with myositis. J. Neurol. 261, 992–998 (2014).
Namsrai, T. et al. Diagnostic delay of myositis: protocol for an integrated systematic review. BMJ Open 12, e060312 (2022).
Gong, J. et al. Nowcasting and forecasting the care needs of the older population in China: analysis of data from the China Health and Retirement Longitudinal Study (CHARLS). Lancet Public Health 7, e1005–e1013 (2022).
van Onna, M. & Boonen, A. Challenges in the management of older patients with inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 18, 326–334 (2022).
Bernatsky, S. et al. Healthcare costs of inflammatory myopathies. J. Rheumatol. 38, 885–888 (2011).
Furst, D. E., Amato, A. A., Iorga, S. R., Bancroft, T. & Fernandes, A. W. Medical costs and health-care resource use in patients with inflammatory myopathies in an insured population. Muscle Nerve 46, 496–505 (2012).
Leclair, V. et al. Distribution and trajectory of direct and indirect costs of idiopathic inflammatory myopathies. Semin. Arthritis Rheum. 51, 983–988 (2021).
Regardt, M., Welin Henriksson, E., Sandqvist, J., Lundberg, I. E. & Schult, M. L. Work ability in patients with polymyositis and dermatomyositis: an explorative and descriptive study. Work 53, 265–277 (2015).
Xu, A., Sun, C., Metcalf, R. & Limaye, V. Health-related quality of life and work impairment in idiopathic inflammatory myopathies in South Australia. Int. J. Rheum. Dis. 24, 809–814 (2021).
Christopher-Stine, L. et al. Patient-reported dermatomyositis and polymyositis flare symptoms are associated with disability, productivity loss, and health care resource use. J. Manag. Care Spec. Pharm. 26, 1424–1433 (2020).
Riddoch, D. & Morgan-Hughes, J. A. Prognosis in adult polymyositis. J. Neurol. Sci. 26, 71–80 (1975).
Bronner, I. M. et al. Long-term outcome in polymyositis and dermatomyositis. Ann. Rheum. Dis. 65, 1456–1461 (2006).
Dobloug, G. C., Svensson, J., Lundberg, I. E. & Holmqvist, M. Mortality in idiopathic inflammatory myopathy: results from a Swedish nationwide population-based cohort study. Ann. Rheum. Dis. 77, 40–47 (2018).
Sultan, S. M., Ioannou, Y., Moss, K. & Isenberg, D. A. Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology 41, 22–26 (2002).
Marie, I. et al. Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J. Rheumatol. 28, 2230–2237 (2001).
Amaral Silva, M., Cogollo, E. & Isenberg, D. A. Why do patients with myositis die? A retrospective analysis of a single-centre cohort. Clin. Exp. Rheumatol. 34, 820–826 (2016).
Hocevar, A. et al. Survival of patients with idiopathic inflammatory myopathies in Slovenia. Front. Med. 8, 801078 (2021).
Limaye, V., Hakendorf, P., Woodman, R. J., Blumbergs, P. & Roberts-Thomson, P. Mortality and its predominant causes in a large cohort of patients with biopsy-determined inflammatory myositis. Intern. Med. J. 42, 191–198 (2012).
Nuno-Nuno, L. et al. Mortality and prognostic factors in idiopathic inflammatory myositis: a retrospective analysis of a large multicenter cohort of Spain. Rheumatol. Int. 37, 1853–1861 (2017).
Danieli, M. G. et al. Impact of treatment on survival in polymyositis and dermatomyositis. A single-centre long-term follow-up study. Autoimmun. Rev. 13, 1048–1054 (2014).
DeVere, R. & Bradley, W. G. Polymyositis: its presentation, morbidity and mortality. Brain 98, 637–666 (1975).
Medsger, T. A. Jr., Robinson, H. & Masi, A. T. Factors affecting survivorship in polymyositis. A life-table study of 124 patients. Arthritis Rheum. 14, 249–258 (1971).
Cobo-Ibanez, T. et al. Long-term pulmonary outcomes and mortality in idiopathic inflammatory myopathies associated with interstitial lung disease. Clin. Rheumatol. 38, 803–815 (2019).
Schiopu, E., Phillips, K., MacDonald, P. M., Crofford, L. J. & Somers, E. C. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: effect of corticosteroids, methotrexate and azathioprine. Arthritis Res. Ther. 14, R22 (2012).
Torres, C. et al. Survival, mortality and causes of death in inflammatory myopathies. Autoimmunity 39, 205–215 (2006).
Danko, K., Ponyi, A., Constantin, T., Borgulya, G. & Szegedi, G. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine 83, 35–42 (2004).
Johnson, C. et al. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung 194, 733–737 (2016).
Aggarwal, R. et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann. Rheum. Dis. 73, 227–232 (2014).
Rojas-Serrano, J. et al. Prognostic factors in a cohort of antisynthetase syndrome (ASS): serologic profile is associated with mortality in patients with interstitial lung disease (ILD). Clin. Rheumatol. 34, 1563–1569 (2015).
Gasparotto, M. et al. Pulmonary involvement in antisynthetase syndrome. Curr. Opin. Rheumatol. 31, 603–610 (2019).
Marie, I. et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun. Rev. 11, 739–745 (2012).
Nombel, A., Fabien, N. & Coutant, F. Dermatomyositis with anti-MDA5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front. Immunol. 12, 773352 (2021).
So, J. et al. Predictors of rapidly progressive interstitial lung disease and mortality in patients with autoantibodies against melanoma differentiation-associated protein 5 dermatomyositis. Rheumatology 61, 4437–4444 (2022).
Niu, Q. et al. A new predictive model for the prognosis of MDA5+ DM-ILD. Front. Med. 9, 908365 (2022).
Yang, Q. et al. Initial predictors for short-term prognosis in anti-melanoma differentiation-associated protein-5 positive patients. Orphanet J. Rare Dis. 16, 58 (2021).
Zhou, J. et al. Evaluation of prognostic factors in anti-MDA5 antibody-positive patients in Chongqing, China: a retrospective study. Int. J. Gen. Med. 14, 4775–4781 (2021).
Tsuji, H. et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 72, 488–498 (2020).
Che, W. I. et al. Familial aggregation and heritability: a nationwide family-based study of idiopathic inflammatory myopathies. Ann. Rheum. Dis. 80, 1461–1466 (2021).
Che, W. I. et al. Familial autoimmunity in patients with idiopathic inflammatory myopathies. J. Intern. Med. 293, 200–211 (2023).
Rothwell, S. et al. Genome-wide imputation identifies novel associations and localises signals in idiopathic inflammatory myopathies. Arthritis Rheumatol. https://doi.org/10.1002/art.42434 (2022).
Rothwell, S., Chinoy, H. & Lamb, J. A. Genetics of idiopathic inflammatory myopathies: insights into disease pathogenesis. Curr. Opin. Rheumatol. 31, 611–616 (2019).
Rothwell, S. et al. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann. Rheum. Dis. 75, 1558–1566 (2016).
Gambino, C. M., Aiello, A., Accardi, G., Caruso, C. & Candore, G. Autoimmune diseases and 8.1 ancestral haplotype: an update. HLA 92, 137–143 (2018).
Rothwell, S. et al. Immune-array analysis in sporadic inclusion body myositis reveals HLA-DRB1 amino acid heterogeneity across the myositis spectrum. Arthritis Rheumatol. 69, 1090–1099 (2017).
Rothwell, S. et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 78, 996–1002 (2019).
Oldroyd, A. G. S. et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology 60, 2615–2628 (2021).
Kwiatkowska, D. & Reich, A. The significance of autoantibodies in juvenile dermatomyositis. Biomed. Res. Int. 2021, 5513544 (2021).
Basharat, P. et al. Statin-induced anti-HMGCR-associated myopathy. J. Am. Coll. Cardiol. 68, 234–235 (2016).
Gonzalez-Galarza, F. F. et al. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 48, D783–D788 (2020).
Limaye, V. et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 52, 196–203 (2015).
Rothwell, S. et al. Identification of novel associations and localization of signals in idiopathic inflammatory myopathies using genome-wide imputation. Arthritis Rheumatol. 75, 1021–1027 (2022).
SEARCH Collaborative Group et al. SLCO1B1 variants and statin-induced myopathy — a genomewide study. N. Engl. J. Med. 359, 789–799 (2008).
Carr, D. F. et al. Genomewide association study of statin-induced myopathy in patients recruited using the UK clinical practice research datalink. Clin. Pharmacol. Ther. 106, 1353–1361 (2019).
Chinoy, H. et al. Interaction of HLA-DRB1*03 and smoking for the development of anti-Jo-1 antibodies in adult idiopathic inflammatory myopathies: a European-wide case study. Ann. Rheum. Dis. 71, 961–965 (2012).
Schiffenbauer, A. et al. The effect of cigarette smoking on the clinical and serological phenotypes of polymyositis and dermatomyositis. Semin. Arthritis Rheum. 48, 504–512 (2018).
Linn-Rasker, S. P. et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann. Rheum. Dis. 65, 366–371 (2006).
Mammen, A. L. et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 64, 1233–1237 (2012).
O’Hanlon, T. P. et al. HLA polymorphisms in African Americans with idiopathic inflammatory myopathy: allelic profiles distinguish patients with different clinical phenotypes and myositis autoantibodies. Arthritis Rheum. 54, 3670–3681 (2006).
Furuya, T. et al. Immunogenetic features in 120 Japanese patients with idiopathic inflammatory myopathy. J. Rheumatol. 31, 1768–1774 (2004).
Kang, E. H. et al. Novel susceptibility alleles in HLA region for myositis and myositis specific autoantibodies in Korean patients. Semin. Arthritis Rheum. 49, 283–287 (2019).
Guo, L. et al. WDFY4 polymorphisms in Chinese patients with anti-MDA5 dermatomyositis is associated with rapid progressive interstitial lung disease. Rheumatology 62, 2320–2324 (2023).
Kochi, Y. et al. Splicing variant of WDFY4 augments MDA5 signalling and the risk of clinically amyopathic dermatomyositis. Ann. Rheum. Dis. 77, 602–611 (2018).
Chan, S. H. et al. Analysis of clinically relevant variants from ancestrally diverse Asian genomes. Nat. Commun. 13, 6694 (2022).
Lee, M. H. et al. A meta-analysis of clinical manifestations in Asian systemic lupus erythematous: the effects of ancestry, ethnicity and gender. Semin. Arthritis Rheum. 52, 151932 (2022).
Ng, S. A. & Low, A. H. L. Systemic sclerosis in Asians: are there racial differences? J. Scleroderma Relat. Disord. 7, 98–109 (2022).
Crum-Cianflone, N. F. Bacterial, fungal, parasitic, and viral myositis. Clin. Microbiol. Rev. 21, 473–494 (2008).
Movahedi, N. & Ziaee, V. COVID-19 and myositis; true dermatomyositis or prolonged post viral myositis? Pediatr. Rheumatol. Online J. 19, 86 (2021).
Saud, A., Naveen, R., Aggarwal, R. & Gupta, L. COVID-19 and myositis: what we know so far. Curr. Rheumatol. Rep. 23, 63 (2021).
Kharouf, F. et al. Increased rates of idiopathic inflammatory myopathies during the COVID-19 pandemic: a single-centre experience. Clin. Exp. Rheumatol. 41, 316–321 (2023).
Allenbach, Y. et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology 95, e70–e78 (2020).
Mehta, P., Machado, P. M. & Gupta, L. Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol. Int. 41, 1021–1036 (2021).
Wang, G. et al. Presence of anti-MDA5 antibody and its value for the clinical assessment in patients with COVID-19: a retrospective cohort study. Front. Immunol. 12, 791348 (2021).
Ding, Y. & Ge, Y. Inflammatory myopathy following coronavirus disease 2019 vaccination: a systematic review. Front. Public. Health 10, 1007637 (2022).
Roszkiewicz, J. & Smolewska, E. Kaleidoscope of autoimmune diseases in HIV infection. Rheumatol. Int. 36, 1481–1491 (2016).
Lloyd, T. E. et al. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology 88, 1454–1460 (2017).
Landon-Cardinal, O. et al. Expanding the spectrum of HIV-associated myopathy. J. Neurol. Neurosurg. Psychiatry 90, 1296–1298 (2019).
Nojima, T. et al. A case of polymyositis associated with hepatitis B infection. Clin. Exp. Rheumatol. 18, 86–88 (2000).
Milisenda, J. C. et al. Polymyositis-associated to chronic hepatitis C virus infection. J. Neuromuscul. Dis. 1, S261–S262 (2014).
Uruha, A. et al. Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology 86, 211–217 (2016).
Peravali, R., Acharya, S., Raza, S. H., Pattanaik, D. & Randall, M. B. Dermatomyositis developed after exposure to Epstein-Barr virus infection and antibiotics use. Am. J. Med. Sci. 360, 402–405 (2020).
Sakthivadivel, V. et al. Concurrent acute myositis and Guillain-Barre syndrome in Cytomegalovirus infection — a rare case report. BMC Infect. Dis. 20, 768 (2020).
Oliver, N. D., Millar, A. & Pendleton, A. A case report on parvovirus B19 associated myositis. Case Rep. Rheumatol. 2012, 250537 (2012).
Megremis, S. et al. Analysis of human total antibody repertoires in TIF1γ autoantibody positive dermatomyositis. Commun. Biol. 4, 419 (2021).
Top 10 drugs 2019–20. Aust. Prescr. 43, 209 (2020).
Audi, S. et al. The ‘top 100’ drugs and classes in England: an updated ‘starter formulary’ for trainee prescribers. Br. J. Clin. Pharmacol. 84, 2562–2571 (2018).
Fuentes, A. V., Pineda, M. D. & Venkata, K. C. N. Comprehension of top 200 prescribed drugs in the us as a resource for pharmacy teaching, training and practice. Pharmacy 6, 43 (2018).
Adhyaru, B. B. & Jacobson, T. A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 15, 757–769 (2018).
Alfirevic, A. et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 96, 470–476 (2014).
Ladislau, L., Arouche-Delaperche, L., Allenbach, Y. & Benveniste, O. Potential pathogenic role of anti-signal recognition protein and anti-3-hydroxy-3-methylglutaryl-CoA reductase antibodies in immune-mediated necrotizing myopathies. Curr. Rheumatol. Rep. 20, 56 (2018).
Abed, W., Abujbara, M., Batieha, A. & Ajlouni, K. Statin induced myopathy among patients attending the National Center for Diabetes, Endocrinology, & Genetics. Ann. Med. Surg. 74, 103304 (2022).
Pasternak, R. C. et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation 106, 1024–1028 (2002).
Banach, M. et al. Statin intolerance — an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 11, 1–23 (2015).
Mueller, A. M. et al. The risk of muscular events among new users of hydrophilic and lipophilic statins: an observational cohort study. J. Gen. Intern. Med. 36, 2639–2647 (2021).
Cholesterol Treatment Trialists’ Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet 400, 832–845 (2022).
McClure, D. L., Valuck, R. J., Glanz, M., Murphy, J. R. & Hokanson, J. E. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. J. Clin. Epidemiol. 60, 812–818 (2007).
Mancini, G. B. et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian consensus working group update (2016). Can. J. Cardiol. 32, S35–65 (2016).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38 (2020).
Chennamadhavuni, A., Abushahin, L., Jin, N., Presley, C. J. & Manne, A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front. Immunol. 13, 779691 (2022).
Calabrese, L. H., Calabrese, C. & Cappelli, L. C. Rheumatic immune-related adverse events from cancer immunotherapy. Nat. Rev. Rheumatol. 14, 569–579 (2018).
Hamada, N. et al. Incidence and distinct features of immune checkpoint inhibitor-related myositis from idiopathic inflammatory myositis: a single-center experience with systematic literature review and meta-analysis. Front. Immunol. 12, 803410 (2021).
Saygin, D., Ghosh, N. & Reid, P. Immune checkpoint inhibitor-associated myositis: a distinct form of inflammatory myopathy. J. Clin. Rheumatol. 28, 367–373 (2022).
Shelly, S. et al. Immune checkpoint inhibitor-associated myopathy: a clinicoseropathologically distinct myopathy. Brain Commun. 2, fcaa181 (2020).
Longinow, J. et al. Immune checkpoint inhibitor induced myocarditis, myasthenia gravis, and myositis: a single-center case series. Cancer Med. 12, 2281–2289 (2023).
Hill, C. L. et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357, 96–100 (2001).
Lilleker, J. B. et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann. Rheum. Dis. 77, 30–39 (2018).
Sung, Y. K. et al. Temporal relationship between idiopathic inflammatory myopathies and malignancies and its mortality: a nationwide population-based study. Clin. Rheumatol. 39, 3409–3416 (2020).
Dani, L., Ian Che, W., Lundberg, I. E., Hellgren, K. & Holmqvist, M. Overall and site-specific cancer before and after diagnosis of idiopathic inflammatory myopathies: a nationwide study 2002–2016. Semin. Arthritis Rheum. 51, 331–337 (2021).
Stubgen, J. P. Juvenile dermatomyositis/polymyositis and lymphoma. J. Neurol. Sci. 377, 19–24 (2017).
Hida, A. et al. Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology 87, 299–308 (2016).
Oldroyd, A. et al. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatology 58, 650–655 (2019).
De Vooght, J. et al. Anti-TIF1-γ autoantibodies: warning lights of a tumour autoantigen. Rheumatology 59, 469–477 (2020).
Fiorentino, D. et al. Anti-CCAR1 autoantibodies are specific for anti-TIF1γ-positive dermatomyositis and decrease cancer risk relative to the general population. Arthritis Rheumatol. 75, 1238–1245 (2023).
Hosono, Y. et al. Coexisting autoantibodies against transcription factor Sp4 are associated with decreased cancer risk in patients with dermatomyositis with anti-TIF1γ autoantibodies. Ann. Rheum. Dis. 82, 246–252 (2023).
Sherman, M. A. et al. Anti-Sp4 autoantibodies co-occur with anti-TIF1 and are associated with distinct clinical features and immunogenetic risk factors in juvenile myositis. Arthritis Rheumatol. 75, 1668–1677(2023).
Limaye, V. et al. The incidence and associations of malignancy in a large cohort of patients with biopsy-determined idiopathic inflammatory myositis. Rheumatol. Int. 33, 965–971 (2013).
Lee, S. J. et al. Cancer risks in Korean patients with myositis: comparison between cancers related and unrelated to myositis activity. Ann. Rheum. Dis. 74, 832 (2015).
Liu, Y. et al. Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol. Lett. 16, 5960–5968 (2018).
Okada, S. et al. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 48, 2285–2293 (2003).
Hossain, M. M. et al. The geospatial distribution of myositis and its phenotypes in the united states and associations with roadways: findings from a national myositis patient registry. Front. Med. 9, 842586 (2022).
Nishina, N., Sato, S., Masui, K., Gono, T. & Kuwana, M. Seasonal and residential clustering at disease onset of anti-MDA5-associated interstitial lung disease. RMD Open. 6, e001202 (2020).
So, H. et al. Seasonal variation in idiopathic inflammatory myopathies incidence and presentation: a retrospective study in Beijing and Hong Kong. Ann. Rheum. Dis. 79, 1601–1602 (2020).
Toquet, S. et al. The seasonality of dermatomyositis associated with anti-MDA5 antibody: an argument for a respiratory viral trigger. Autoimmun. Rev. 20, 102788 (2021).
Albayda, J. et al. Antinuclear matrix protein 2 autoantibodies and edema, muscle disease, and malignancy risk in dermatomyositis patients. Arthritis Care Res. 69, 1771–1776 (2017).
Greenberg, S. A. Inclusion body myositis: clinical features and pathogenesis. Nat. Rev. Rheumatol. 15, 257–272 (2019).
Gunawardena, H. The clinical features of myositis-associated autoantibodies: a review. Clin. Rev. Allergy Immunol. 52, 45–57 (2017).
Lundberg, I. E., de Visser, M. & Werth, V. P. Classification of myositis. Nat. Rev. Rheumatol. 14, 269–278 (2018).
McHugh, N. J. Ro52, myositis, and interstitial lung disease. J. Rheumatol. 50, 161–163 (2023).
Satoh, M., Tanaka, S., Ceribelli, A., Calise, S. J. & Chan, E. K. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin. Rev. Allergy Immunol. 52, 1–19 (2017).
Monaghan, M. et al. Inflammatory myositis secondary to anti-retroviral therapy in a child; case report and review of the literature. J. Neuromuscul. Dis. 8, 1089–1095 (2021).
Nofal, A. & El-Din, E. S. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int. J. Dermatol. 51, 535–541 (2012).
Hahn, M., Sriharan, K. & McFarland, M. S. Gemfibrozil-induced myositis in a patient with normal renal function. Ann. Pharmacother. 44, 211–214 (2010).
Lim, A. Y., Kek, P. C. & Soh, A. W. Carbimazole-induced myositis in the treatment of Graves’ disease: a complication in genetically susceptible individuals? Singap. Med. J. 54, e133–136 (2013).
Syrmou, V. et al. COVID-19 vaccine-associated myositis: a comprehensive review of the literature driven by a case report. Immunol. Res. 71, 537–546 (2023).
Ferri, C. et al. Polymyositis following pandemic influenza A (H1N1) and 2009–10 seasonal trivalent vaccines. Case Rep. Rheumatol. 2012, 836930 (2012).
Altman, A., Szyper-Kravitz, M. & Shoenfeld, Y. HBV vaccine and dermatomyositis: is there an association? Rheumatol. Int. 28, 609–612 (2008).
Chanbour, H., Jiblawi, A., Aboudalle, A., Alalman, O. & Chahine Elsett, Z. Association of silicosis and dermatomyositis: case report and literature review. Cureus 13, e19875 (2021).
O’Hanlon, T. et al. Immunogenetic differences between Caucasian women with and those without silicone implants in whom myositis develops. Arthritis Rheum. 50, 3646–3650 (2004).
Selva-O’Callaghan, A. et al. Silicone gel filled breast implants and dermatomyositis. Clin. Exp. Rheumatol. 22, 376 (2004).
Limaye, S. & Limaye, V. Clinical characteristics of myositis associated with graft-versus-host disease. Curr. Rheumatol. Rep. 23, 30 (2021).
Reed, A. M., McNallan, K., Wettstein, P., Vehe, R. & Ober, C. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositis? J. Immunol. 172, 5041–5046 (2004).
Allanore, Y. et al. Systemic sclerosis. Nat. Rev. Dis. Prim. 1, 15002 (2015).
Brito-Zeron, P. et al. Sjogren syndrome. Nat. Rev. Dis. Prim. 2, 16047 (2016).
Crossfield, S. S. R., Marzo-Ortega, H., Kingsbury, S. R., Pujades-Rodriguez, M. & Conaghan, P. G. Changes in ankylosing spondylitis incidence, prevalence and time to diagnosis over two decades. RMD Open. 7, e001888 (2021).
Gladman, D. D., Antoni, C., Mease, P., Clegg, D. O. & Nash, P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 64, ii14–17 (2005).
Watts, R. A., Hatemi, G., Burns, J. C. & Mohammad, A. J. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 18, 22–34 (2022).
Safiri, S. et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 78, 1463–1471 (2019).
Connors, G. R., Christopher-Stine, L., Oddis, C. V. & Danoff, S. K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138, 1464–1474 (2010).
Solomon, J., Swigris, J. J. & Brown, K. K. Myositis-related interstitial lung disease and antisynthetase syndrome. J. Bras. Pneumol. 37, 100–109 (2011).
Prieto-Pena, D. et al. Epidemiological and genetic features of anti-3-hydroxy-3-methylglutaryl-CoA reductase necrotizing myopathy: single-center experience and literature review. Eur. J. Intern. Med. 101, 86–92 (2022).
Shelly, S. et al. Incidence and prevalence of immune-mediated necrotizing myopathy in adults in Olmsted County, Minnesota. Muscle Nerve 65, 541–546 (2022).
Mohassel, P. & Mammen, A. L. Anti-HMGCR myopathy. J. Neuromuscul. Dis. 5, 11–20 (2018).
Adler, B., Christopher-Stine, L. & Tiniakou, E. Mushroom supplements triggering a flare of HMGCR immune mediated necrotising myopathy. BMJ Case Rep. 15, e248880 (2022).
Mueller, P. S. Symptomatic myopathy due to red yeast rice. Ann. Intern. Med. 145, 474–475 (2006).
Jeng, K. C., Chen, C. S., Fang, Y. P., Hou, R. C. & Chen, Y. S. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J. Agric. Food Chem. 55, 8787–8792 (2007).
Yaworski, A. M., Blyumin, M., Chang, T., Mammen, A. L. & Greene, M. Necrotizing myopathy with elevated anti-HMGCR antibodies following exposure to the supplement Bacopa. Muscle Nerve 67, E1–E3 (2023).
Acknowledgements
This research was supported by the NIHR Manchester Biomedical Research Centre (NIHR203308). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. T.K. is supported by the Ken Muirden ARA Fellowship as part of the 2023 Arthritis Australia National Research Program, and an Australian Government Postgraduate Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
All authors researched data and contributed substantially to discussion of the content. T.K. wrote the article. All authors contributed to reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Teerin Liewluck, Julie Paik and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoo, T., Lilleker, J.B., Thong, B.YH. et al. Epidemiology of the idiopathic inflammatory myopathies. Nat Rev Rheumatol 19, 695–712 (2023). https://doi.org/10.1038/s41584-023-01033-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01033-0
This article is cited by
-
Current classification criteria underestimate the incidence of idiopathic inflammatory myopathies by ignoring subgroups
Nature Reviews Rheumatology (2024)
-
Reply to: Current classification criteria underestimate the incidence of idiopathic inflammatory myopathies by ignoring subgroups
Nature Reviews Rheumatology (2024)