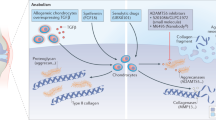
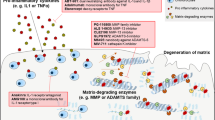
Abstract
Osteoarthritis (OA) is the most common form of arthritis worldwide, affecting ~500 million people, yet there are no effective treatments to halt its progression. Without any structure-modifying agents, management of OA focuses on ameliorating pain and improving function. Treatment approaches typically have modest efficacy, and many patients have contraindications to recommended pharmacological treatments. Drug development for OA is hindered by the gradual and progressive nature of the disease and the targeting of established disease in clinical trials. Additionally, new medications for OA cannot receive regulatory approval without demonstrating improvements in both structure (pathological features of OA) and symptoms (reduced pain and/or improved function). In clinical trials, people with OA show high ‘placebo responses’, which hamper the ability to identify new effective treatments. Placebo responses refer to the individual variability in response to placebos given in the context of clinical trials and other settings. Placebo effects refer specifically to short-lasting improvements in symptoms that occur because of physiological changes. To mitigate the effects of the placebo phenomenon, we must first understand what it is, how it manifests, how to identify placebo responders in OA trials and how these insights can be used to improve clinical trials in OA. Leveraging placebo responses and effects in clinical practice might provide additional avenues to augment symptom management of OA.
Key points
-
An understanding of placebo mechanisms and their role in clinical trials is important to facilitate the development of new treatments for osteoarthritis.
-
Valuable insights on study designs and potential pitfalls for future clinical trials can aid researchers in improving research methodologies across different health conditions.
-
Recognizing the clinical implications and potential benefits of harnessing placebo effects can lead to more effective treatment approaches in the management of diverse medical conditions.
-
Examining opioid reduction in patients undergoing joint-replacement surgery for conditions other than osteoarthritis and its effect on outcomes offers important insights for optimizing postsurgical care in different health contexts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Long, H. et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 74, 1172–1183 (2022).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Sebbag, E. et al. The world-wide burden of musculoskeletal diseases: a systematic analysis of the World Health Organization burden of diseases database. Ann. Rheum. Dis. 78, 844–848 (2019).
Singh, J. A., Yu, S., Chen, L. & Cleveland, J. D. Rates of total joint replacement in the United States: future projections to 2020–2040 using the national inpatient sample. J. Rheumatol. 46, 1134–1140 (2019).
Stokes, A., Berry, K. M., Hempstead, K., Lundberg, D. J. & Neogi, T. Trends in prescription analgesic use among adults with musculoskeletal conditions in the united states, 1999–2016. JAMA Netw. Open 2, e1917228 (2019).
Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2013).
Bingham, C. O. III et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 54, 3494–3507 (2006).
Karsdal, M. A. et al. Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials. Osteoarthritis Cartilage 23, 532–543 (2015).
Conaghan, P. G. et al. Disease-modifying effects of a novel cathepsin K inhibitor in osteoarthritis: a randomized controlled trial. Ann. Intern. Med. 172, 86–95 (2020).
Kraus, V. B. et al. Proposed study designs for approval based on a surrogate endpoint and a post-marketing confirmatory study under FDA’s accelerated approval regulations for disease modifying osteoarthritis drugs. Osteoarthritis Cartilage 27, 571–579 (2019).
Hochberg, M. C. et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. J. Am. Med. Assoc. 322, 1360–1370 (2019).
Bannuru, R. R. et al. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann. Intern. Med. 163, 365–372 (2015).
Zhang, W., Robertson, J., Jones, A. C., Dieppe, P. A. & Doherty, M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 67, 1716–1723 (2008).
Henriksen, M. et al. Exercise and education vs intra-articular saline for knee osteoarthritis: a 1-year follow-up of a randomized trial. Osteoarthritis Cartilage 31, 627–635 (2023).
Englund, M. & Turkiewicz, A. The emperor’s new clothes. Osteoarthritis Cartilage 31, 549–551 (2023).
Colloca, L. The placebo effect in pain therapies. Annu. Rev. Pharmacol. Toxicol. 59, 191–211 (2019).
Colloca, L. & Barsky, A. J. Placebo and nocebo effects. N. Engl. J. Med. 382, 554–561 (2020).
Benedetti, F., Frisaldi, E. & Shaibani, A. Thirty years of neuroscientific investigation of placebo and nocebo: the interesting, the good, and the bad. Annu. Rev. Pharmacol. Toxicol. 62, 323–340 (2022).
Benedetti, F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron 84, 623–637 (2014).
Benedetti, F., Mayberg, H. S., Wager, T. D., Stohler, C. S. & Zubieta, J. K. Neurobiological mechanisms of the placebo effect. J. Neurosci. 25, 10390–10402 (2005).
Wendt, L., Albring, A. & Schedlowski, M. Learned placebo responses in neuroendocrine and immune functions. Handb. Exp. Pharmacol. 225, 159–181 (2014).
Hadamitzky, M., Sondermann, W., Benson, S. & Schedlowski, M. Placebo effects in the immune system. Int. Rev. Neurobiol. 138, 39–59 (2018).
Luckemann, L., Stangl, H., Straub, R. H., Schedlowski, M. & Hadamitzky, M. Learned immunosuppressive placebo response attenuates disease progression in a rodent model of rheumatoid arthritis. Arthritis Rheumatol. 72, 588–597 (2020).
Ader, R. & Cohen, N. Behaviorally conditioned immunosuppression. Psychosom. Med. 37, 333–340 (1975).
Olness, K. & Ader, R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J. Dev. Behav. Pediatr. 13, 124–125 (1992).
Giang, D. W. et al. Conditioning of cyclophosphamide-induced leukopenia in humans. J. Neuropsychiatry Clin. Neurosci. 8, 194–201 (1996).
Goebel, M. U. et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 16, 1869–1873 (2002).
Colloca, L. Placebo, nocebo, and learning mechanisms. Handb. Exp. Pharmacol. 225, 17–35 (2014).
Colloca, L. & Miller, F. G. How placebo responses are formed: a learning perspective. Phil. Trans. R. Soc. B 366, 1859–1869 (2011).
Colloca, L. & Miller, F. G. Role of expectations in health. Curr. Opin. Psychiatry 24, 149–155 (2011).
Langford, D. J. et al. Expectations for improvement: a neglected but potentially important covariate or moderator for chronic pain clinical trials. J. Pain 24, 575–581 (2022).
Gil, M., Menzel, R. & De Marco, R. J. Does an insect’s unconditioned response to sucrose reveal expectations of reward? PLoS ONE 3, e2810 (2008).
Colloca, L. et al. Prior therapeutic experiences, not expectation ratings, predict placebo effects: an experimental study in chronic pain and healthy participants. Psychother. Psychosom. 89, 371–378 (2020).
Colloca, L., Lopiano, L., Lanotte, M. & Benedetti, F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 3, 679–684 (2004).
Suarez-Almazor, M. et al. A randomized controlled trial of acupuncture for osteoarthritis of the knee: effects of provider communication style. Arthritis Rheum. 56, S315 (2007).
Gollub, R. L. et al. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J. Pain 19, 515–527 (2018).
Kong, J. et al. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: a functional neuroimaging study. Neuroimage Clin. 18, 325–334 (2018).
Rosenkjaer, S., Lunde, S. J., Kirsch, I. & Vase, L. Expectations: how and when do they contribute to placebo analgesia. Front. Psychiatry 13, 817179 (2022).
Younger, J., Gandhi, V., Hubbard, E. & Mackey, S. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin. Trials 9, 767–776 (2012).
Ferkin, A. C. et al. A psychometric evaluation of the Stanford Expectations of Treatment Scale (SETS) in the context of a smoking cessation trial. Nicotine Tob. Res. 24, 1914–1920 (2022).
Shedden-Mora, M. C. et al. The treatment expectation questionnaire (TEX-Q): validation of a generic multidimensional scale measuring patients’ treatment expectations. PLoS ONE 18, e0280472 (2023).
Alberts, J. et al. Development of the generic, multidimensional treatment expectation questionnaire (TEX-Q) through systematic literature review, expert surveys and qualitative interviews. BMJ Open 10, e036169 (2020).
Spisak, T., Bingel, U. & Wager, T. D. Multivariate BWAS can be replicable with moderate sample sizes. Nature 615, E4–E7 (2023).
Tetreault, P. et al. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol. 14, e1002570 (2016).
Vachon-Presseau, E. et al. Validating a biosignature-predicting placebo pill response in chronic pain in the settings of a randomized controlled trial. Pain 163, 910–922 (2022).
Davis, K. D. et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat. Rev. Neurol. 13, 624–638 (2017).
Colagiuri, B., Schenk, L. A., Kessler, M. D., Dorsey, S. G. & Colloca, L. The placebo effect: from concepts to genes. Neuroscience 307, 171–190 (2015).
Hall, K. T., Loscalzo, J. & Kaptchuk, T. J. Genetics and the placebo effect: the placebome. Trends Mol. Med. 21, 285–294 (2015).
Eippert, F., Finsterbusch, J., Bingel, U. & Buchel, C. Direct evidence for spinal cord involvement in placebo analgesia. Science 326, 404 (2009).
Wang, Y. et al. Modeling learning patterns to predict placebo analgesic effects in healthy and chronic orofacial pain participants. Front. Psychiatry 11, 39 (2020).
Colloca, L., Thomas, S., Yin, M., Haycock, N. R. & Wang, Y. Pain experience and mood disorders during the lockdown of the COVID-19 pandemic in the United States: an opportunistic study. Pain Rep. 6, e958 (2021).
Shafir, R., Olson, E. & Colloca, L. The neglect of sex: a call to action for including sex as a biological variable in placebo and nocebo research. Contemp. Clin. Trials 116, 106734 (2022).
Olson, E. M. et al. Effects of sex on placebo effects in chronic pain participants: a cross-sectional study. Pain 162, 531–542 (2021).
Wang, Y., Chan, E., Dorsey, S. G., Campbell, C. M. & Colloca, L. Who are the placebo responders? A cross-sectional cohort study for psychological determinants. Pain 163, 1078–1090 (2022).
Sanislow, C. A. et al. Developing constructs for psychopathology research: research domain criteria. J. Abnorm. Psychol. 119, 631–639 (2010).
Insel, T. et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010).
Dworkin, R. H. et al. Meta-analysis of assay sensitivity and study features in clinical trials of pharmacologic treatments for osteoarthritis pain. Arthritis Rheumatol. 66, 3327–3336 (2014).
Finnerup, N. B. et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 159, 2339–2346 (2018).
Farrar, J. T. et al. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: an ACTTION study. Pain 155, 1622–1631 (2014).
Treister, R., Honigman, L., Lawal, O. D., Lanier, R. K. & Katz, N. P. A deeper look at pain variability and its relationship with the placebo response: results from a randomized, double-blind, placebo-controlled clinical trial of naproxen in osteoarthritis of the knee. Pain 160, 1522–1528 (2019).
Okusogu, C. et al. Placebo hypoalgesia: racial differences. Pain 161, 1872–1883 (2020).
Olson, E. M. et al. Effects of sex on placebo effects in chronic pain participants: a cross-sectional study. Pain 162, 531–542 (2020).
Dworkin, R. H. & Edwards, R. R. Phenotypes and treatment response: it’s difficult to make predictions, especially about the future. Pain 158, 187–189 (2017).
Hohenschurz-Schmidt, D., Draper-Rodi, J. & Vase, L. Dissimilar control interventions in clinical trials undermine interpretability. JAMA Psychiatry 79, 271–272 (2022).
Hohenschurz-Schmidt, D. et al. Blinding and sham control methods in trials of physical, psychological, and self-management interventions for pain (article II): a meta-analysis relating methods to trial results. Pain 164, 509–533 (2022).
Colloca, L., Benedetti, F. & Porro, C. A. Experimental designs and brain mapping approaches for studying the placebo analgesic effect. Eur. J. Appl. Physiol. 102, 371–380 (2008).
Parsons, H. M. What happened at Hawthorne?: new evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 183, 922–932 (1974).
Ulmer, F. C. The Hawthorne effect. Educ. Dir. Dent. Aux. 1, 28 (1976).
Benedetti, F., Carlino, E. & Piedimonte, A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 15, 736–747 (2016).
Berthelot, J. M., Le Goff, B. & Maugars, Y. The Hawthorne effect: stronger than the placebo effect. Jt. Bone Spine 78, 335–336 (2011).
Colloca, L. & Benedetti, F. Placebo analgesia induced by social observational learning. Pain 144, 28–34 (2009).
Wolfe, F. & Michaud, K. The Hawthorne effect, sponsored trials, and the overestimation of treatment effectiveness. J. Rheumatol. 37, 2216–2220 (2010).
Ellingsen, D. M. et al. Brain-to-brain mechanisms underlying pain empathy and social modulation of pain in the patient-clinician interaction. Proc. Natl Acad. Sci. USA 120, e2212910120 (2023).
Ernst, E. & Resch, K. L. Concept of true and perceived placebo effects. Br. Med. J. 311, 551–553 (1995).
Colloca, L. & Benedetti, F. Placebos and painkillers: is mind as real as matter? Nat. Rev. Neurosci. 6, 545–552 (2005).
Ross, S., Krugman, A. D., Lyerly, S. B., Clyde & J, D. Drugs and placebos: a model design. Psychol. Rep. 10, 383–392 (1962).
Kirsch, I. & Weixel, L. J. Double-blind versus deceptive administration of a placebo. Behav. Neurosci. 102, 319–323 (1988).
Benedetti, F. et al. Open versus hidden medical treatments: the patient’s knowledge about a therapy affects the therapy outcome. Prev. Treat. 6, 1a (2003).
Benedetti, F. et al. Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Res. Bull. 63, 203–211 (2004).
Park, L. C. & Covi, L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch. Gen. Psychiatry 12, 36–45 (1965).
Kaptchuk, T. J. et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE 5, e15591 (2010).
Colloca, L., Enck, P. & DeGrazia, D. Relieving pain using dose-extending placebos: a scoping review. Pain 157, 1590–1598 (2016).
Enck, P., Grundy, D. & Klosterhalfen, S. A novel placebo-controlled clinical study design without ethical concerns — the free choice paradigm. Med. Hypotheses 79, 880–882 (2012).
Fava, M., Evins, A. E., Dorer, D. J. & Schoenfeld, D. A. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72, 115–127 (2003).
Staud, R. & Price, D. D. Role of placebo factors in clinical trials with special focus on enrichment designs. Pain 139, 479–480 (2008).
Wendler, D. & Miller, F. G. Deception in the pursuit of science. Arch. Intern. Med. 164, 597–600 (2004).
Pollo, A. et al. Response expectancies in placebo analgesia and their clinical relevance. Pain 93, 77–84 (2001).
Colloca, L. et al. Veteran engagement in opioid tapering research: a mission to optimize pain management. Pain Rep. 6, e932 (2021).
Park, L. & TRIAL, C. L. N. P. An exploration of neurotic patients’responses to placebo when its inert content is disclosed. Arch. Gen. Psychiatry 12, 36–45 (1965).
Young, N. S., Ioannidis, J. P. & Al-Ubaydli, O. Why current publication practices may distort science. PLoS Med. 5, e201 (2008).
Fanelli, D., Costas, R. & Ioannidis, J. P. Meta-assessment of bias in science. Proc. Natl Acad. Sci. USA 114, 3714–3719 (2017).
Schooler, J. Unpublished results hide the decline effect. Nature 470, 437–437 (2011).
Harrison, R. K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 15, 817–818 (2016).
Scott, A. J., Sharpe, L., Quinn, V. & Colagiuri, B. Association of single-blind placebo run-in periods with the placebo response in randomized clinical trials of antidepressants: a systematic review and meta-analysis. JAMA Psychiatry 79, 42–49 (2022).
Coleshill, M. J., Sharpe, L., Colloca, L., Zachariae, R. & Colagiuri, B. Placebo and active treatment additivity in placebo analgesia: research to date and future directions. Int. Rev. Neurobiol. 139, 407–441 (2018).
Colagiuri, B. Participant expectancies in double-blind randomized placebo-controlled trials: potential limitations to trial validity. Clin. Trials 7, 246–255 (2010).
Vollert, J. et al. Assessment of placebo response in objective and subjective outcome measures in rheumatoid arthritis clinical trials. JAMA Netw. Open 3, e2013196 (2020).
Wechsler, M. E. et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365, 119–126 (2011).
Kent, D. M. et al. The predictive approaches to treatment effect heterogeneity (PATH) statement. Ann. Intern. Med. 172, 35–45 (2020).
Schnitzer, T. J. et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. J. Am. Med. Assoc. 322, 37–48 (2019).
Berenbaum, F. et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 79, 800–810 (2020).
Vase, L., Amanzio, M. & Price, D. D. Nocebo vs. placebo: the challenges of trial design in analgesia research. Clin. Pharmacol. Ther. 97, 143–150 (2015).
Morton, D. L., Watson, A., El-Deredy, W. & Jones, A. K. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain 146, 194–198 (2009).
Vase, L. et al. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain 156, 1795–1802 (2015).
Lang, T. A. & Stroup, D. F. Who knew? The misleading specificity of “double-blind” and what to do about it. Trials 21, 697 (2020).
Wood, L. et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. Br. Med. J. 336, 601–605 (2008).
Moerman, D. E. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med. Anthropol. Q. 14, 51–72 (2000).
Blease, C. R. et al. Sharing clinical notes, and placebo and nocebo effects: can documentation affect patient health? J. Health Psychol. 27, 135–146 (2022).
Garcia, M. K. et al. Effect of true and sham acupuncture on radiation-induced xerostomia among patients with head and neck cancer: a randomized clinical trial. JAMA Netw. Open 2, e1916910 (2019).
Karst, M. & Li, C. Acupuncture—a question of culture. JAMA Netw. Open 2, e1916929 (2019).
Waber, R. L., Shiv, B., Carmon, Z. & Ariely, D. Commercial features of placebo and therapeutic efficacy. J. Am. Med. Assoc. 299, 1016–1017 (2008).
Kam-Hansen, S. et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl Med. 6, 218ra215 (2014).
Faasse, K., Martin, L. R., Grey, A., Gamble, G. & Petrie, K. J. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 35, 187–190 (2016).
Meissner, K. et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern. Med. 173, 1941–1951 (2013).
Blease, C., Colloca, L. & Kaptchuk, T. J. Are open-label placebos ethical? Informed consent and ethical equivocations. Bioethics 30, 407–414 (2016).
Lembo, A. et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain 162, 2428–2435 (2021).
Nurko, S. et al. Effect of open-label placebo on children and adolescents with functional abdominal pain or irritable bowel syndrome: a randomized clinical trial. JAMA Pediatrics 176, 349–356 (2022).
Carvalho, C. et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain 157, 2766–2772 (2016).
Carvalho, C. et al. Open-label placebo for chronic low back pain: a 5-year follow-up. Pain 162, 1521–1527 (2021).
Kelley, J. M., Kaptchuk, T. J., Cusin, C., Lipkin, S. & Fava, M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother. Psychosom. 81, 312–314 (2012).
Schaefer, M., Harke, R. & Denke, C. Open-label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother. Psychosom. 85, 373–374 (2016).
Hoenemeyer, T. W., Kaptchuk, T. J., Mehta, T. S. & Fontaine, K. R. Open-label placebo treatment for cancer-related fatigue: a randomized-controlled clinical trial. Sci. Rep. 8, 2784 (2018).
Pan, Y. et al. Open-label placebos for menopausal hot flushes: a randomized controlled trial. Sci. Rep. 10, 20090 (2020).
Olliges, E. et al. Open-label placebo administration decreases pain in elderly patients with symptomatic knee osteoarthritis — a randomized controlled trial. Front. Psychiatry 13, 853497 (2022).
Ader, R. et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom. Med. 72, 192–197 (2010).
Morales-Quezada, L. et al. Conditioning open-label placebo: a pilot pharmacobehavioral approach for opioid dose reduction and pain control. Pain Rep. 5, e828 (2020).
Sandler, A. D. & Bodfish, J. W. Open-label use of placebos in the treatment of ADHD: a pilot study. Child Care Health Dev. 34, 104–110 (2008).
Perlis, M. et al. Durability of treatment response to zolpidem with three different maintenance regimens: a preliminary study. Sleep Med. 16, 1160–1168 (2015).
Goebel, M. U., Meykadeh, N., Kou, W., Schedlowski, M. & Hengge, U. R. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother. Psychosom. 77, 227–234 (2008).
Kirchhof, J. et al. Learned immunosuppressive placebo responses in renal transplant patients. Proc. Natl Acad. Sci. USA 115, 4223–4227 (2018).
Colloca, L. & Miller, F. G. Harnessing the placebo effect: the need for translational research. Phil. Trans. R. Soc. B 366, 1922–1930 (2011).
Doering, B. K. & Rief, W. Utilizing placebo mechanisms for dose reduction in pharmacotherapy. Trends Pharmacol. Sci. 33, 165–172 (2012).
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12, 191–204 (2013).
Benedetti, F., Pollo, A. & Colloca, L. Opioid-mediated placebo responses boost pain endurance and physical performance: is it doping in sport competitions. J. Neurosci. 27, 11934–11939 (2007).
Amanzio, M. & Benedetti, F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 19, 484–494 (1999).
Guo, J. Y., Wang, J. Y. & Luo, F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J. Psychopharmacol. 24, 1561–1567 (2010).
Colloca, L. & Benedetti, F. How prior experience shapes placebo analgesia. Pain 124, 126–133 (2006).
Fiorio, M. et al. Enhancing non-noxious perception: behavioural and neurophysiological correlates of a placebo-like manipulation. Neuroscience 217, 96–104 (2012).
Fiorio, M., Recchia, S., Corra, F. & Tinazzi, M. Behavioural and neurophysiological investigation of the influence of verbal suggestion on tactile perception. Neuroscience 258, 332–339 (2014).
Klinger, R., Soost, S., Flor, H. & Worm, M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain 128, 31–39 (2007).
Adie, S., Harris, I., Chuan, A., Lewis, P. & Naylor, J. M. Selecting and optimising patients for total knee arthroplasty. Med. J. Aust. 210, 135–141 (2019).
Quinlan, J., Levy, N., Lobo, D. N. & Macintyre, P. E. Preoperative opioid use: a modifiable risk factor for poor postoperative outcomes. Br. J. Anaesth. 127, 327–331 (2021).
Ravi, B. et al. Patterns of pre-operative opioid use affect the risk for complications after total joint replacement. Sci. Rep. 11, 22124 (2021).
Shadbolt, C. et al. Opioid use and total joint replacement. Curr. Rheumatol. Rep. 22, 58 (2020).
Colloca, L. Informed consent: hints from placebo and nocebo research. Am. J. Bioeth. 15, 17–19 (2015).
Miller, F. G. & Colloca, L. The placebo phenomenon and medical ethics: rethinking the relationship between informed consent and risk-benefit assessment. Theor. Med. Bioeth. 32, 229–243 (2011).
Brody, H., Colloca, L. & Miller, F. G. The placebo phenomenon: implications for the ethics of shared decision-making. J. Gen. Intern. Med. 27, 739–742 (2012).
Barnes, K. et al. Can positive framing reduce nocebo side effects? Current evidence and recommendation for future research. Front. Pharmacol. 10, 167 (2019).
Brody, H. & Colloca, L. Patient autonomy and provider beneficence are compatible. Hastings Cent. Rep. 43, 6 (2013).
Acknowledgements
The authors thank Y. Shaham for his critical review of the manuscript. Part of this research is supported by the National Institutes of Health (L.C. R01AT01033, R01AT011347, 1R01DE025946 and R21DE032532; T.N. K24AR070892, P30AR072571, R01AG066010, R01AG066914, R01NS121419).
Author information
Authors and Affiliations
Contributions
L.C. researched data and wrote the article. Both authors contributed substantially to the discussion of content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Jean-Marie Berthelot, Damien Finniss and Serge Marchand for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Active-control group
-
The group assigned to receive a treatment that is known to have physiological effects.
- Balanced-placebo
-
The balanced-placebo design refers to a research methodology used in placebo-controlled studies to differentiate between the pharmacological effects of a treatment and the psychological effects of believing that one is receiving the treatment. In this design, participants are divided into groups, and each group receives a combination of active treatment, placebo and information about which they have received.
- Bias
-
Bias in research refers to an aspect of the study design, data collection or analysis of data that can lead to incorrect interpretation and/or conclusions about the results of a study.
- Control groups
-
The groups assigned to receive either no treatment or a placebo, enabling comparison to determine the effectiveness of the experimental treatment.
- Dose-extending placebo
-
A classic conditioning placebo-related procedure used to extend the effect of the active treatment. After repeated pairing of the active full-dose treatment with a conditioning stimulus, exposure to the conditioned stimulus, either alone or together with a lower dose of the active treatment, mimics the therapeutic effect of the active treatment or extends its effect.
- Double-blind versus deceptive
-
Comparison between a double-blind and a deceptive design. In the double-blind design neither the participants nor the researchers administering the treatments know who is receiving the active treatment and who is receiving the placebo. This helps to reduce bias and ensures objective evaluation of the treatment’s effects. The deceptive design in the context of clinical trials or experiments refers to intentionally misleading participants or withholding information about the nature of the treatment or intervention that they are receiving.
- Expectancy
-
Implicit expectancies are those that are present without full awareness or conscious intent. As opposed to expectations, expectancies are difficult to formally measure and quantify.
- Expectations
-
Expectations refer to the belief or anticipation that a certain outcome will occur, which can be both conscious and unconscious. Expectations can be measured using validated scales and questionnaires to assess how strongly participants expect a certain outcome to occur in clinical trials and other studies.
- Hawthorne effects
-
Hawthorne effects refer to the phenomenon whereby individuals modify their behaviours or responses in research or clinical settings because of the awareness of being observed or studied.
- Natural history
-
The natural history of a condition refers to the expected course and outcome of a particular medical condition in the absence of any intervention or treatment.
- No-treatment group
-
The group randomly assigned to receive no treatment who provide information about the natural history of the condition in the absence of the intervention.
- Open–hidden treatment
-
A research design where some participants are aware of the treatment they are receiving (open treatment), whereas others are unaware or are kept in the dark about the nature of their treatment or the time of administration (hidden treatment). This design enables the investigation of how participants’ knowledge or lack thereof about their treatment influences treatment outcomes and placebo responses.
- Open-label placebo
-
An adjuvant treatment given along with the treatment-as-usual to elicit placebo effects. Participants and researchers are aware that the treatment being given is a placebo and not an active treatment.
- Placebo
-
A substance or treatment that is physically inert and has no therapeutic effect on a person’s health condition. Non-physical placebos do not involve any tangible substances and encompass a wide range of interventions, such as sham procedures, psychological interventions and imagined treatments.
- Placebo effects
-
Placebo effects refer specifically to short-lasting improvements in symptoms that occur because of physiological changes.
- Placebo responses
-
Placebo responses refer to the individual variability in response to placebos given in the context of clinical trials and other settings.
- Regression to the mean
-
The phenomenon where extreme results obtained by chance after a first measurement tend to move closer to the mean on repeated measurements, often seen when patients with high-activity disease flares are enrolled in trials and experience reduction of disease activity that is falsely attributed to the treatment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neogi, T., Colloca, L. Placebo effects in osteoarthritis: implications for treatment and drug development. Nat Rev Rheumatol 19, 613–626 (2023). https://doi.org/10.1038/s41584-023-01021-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01021-4