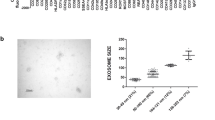
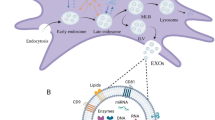
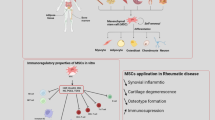
Abstract
The incidence of rheumatic diseases such as rheumatoid arthritis and osteoarthritis and injuries to articular cartilage that lead to osteochondral defects is predicted to rise as a result of population ageing and the increase in high-intensity physical activities among young and middle-aged people. Current treatments focus on the management of pain and joint functionality to improve the patient’s quality of life, but curative strategies are greatly desired. In the past two decades, the therapeutic value of mesenchymal stromal cells (MSCs) has been evaluated because of their regenerative potential, which is mainly attributed to the secretion of paracrine factors. Many of these factors are enclosed in extracellular vesicles (EVs) that reproduce the main functions of parental cells. MSC-derived EVs have anti-inflammatory, anti-apoptotic as well as pro-regenerative activities. Research on EVs has gained considerable attention as they are a potential cell-free therapy with lower immunogenicity and easier management than whole cells. MSC-derived EVs can rescue the pathogenetic phenotypes of chondrocytes and exert a protective effect in animal models of rheumatic disease. To facilitate the therapeutic use of EVs, appropriate cell sources for the production of EVs with the desired biological effects in each disease should be identified. Production and isolation of EVs should be optimized, and pre-isolation and post-isolation modifications should be considered to maximize the disease-modifying potential of the EVs.
Key points
-
Extracellular vesicles (EVs) have important roles in intercellular communication and convey many components at their surface or in their cargo that reproduce the functions of parental cells.
-
Mesenchymal stromal cell (MSC)-derived EVs can regulate and prevent symptoms in non-clinical models of osteoarthritis and rheumatoid arthritis, notably by targeting local and systemic inflammation, and restoring a healthy chondrocyte phenotype.
-
Improvement of isolation yields of MSC-EVs and functionality optimization by pre-isolation or post-isolation engineering are still required.
-
Clinical translation of MSC-EVs for treatment of rheumatic diseases is ongoing, suggesting future potential for the treatment of these painful and disabling diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
05 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41584-024-01114-8
References
Giorgino, R. et al. Knee osteoarthritis: epidemiology, pathogenesis, and mesenchymal stem cells: what else is new? an update. Int. J. Mol. Sci. 24, 6405 (2023).
Zhang, Z. & Schon, L. The current status of clinical trials on biologics for cartilage repair and osteoarthritis treatment: an analysis of clinicaltrials.gov data. Cartilage 13, 19476035221093065 (2022).
Muthu, S. et al. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: a meta-analysis. World J. Orthop. 14, 23–41 (2023).
Jeyaraman, M. et al. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp. Cell Res. 418, 113274 (2022).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Radler, J., Gupta, D., Zickler, A. & Andaloussi, S. E. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol. Ther. 31, 1231–1250 (2023).
Harding, C., Heuser, J. & Stahl, P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 35, 256–263 (1984).
van Niel, G. et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022).
Arya, S. B., Chen, S., Jordan-Javed, F. & Parent, C. A. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat. Cell Biol. 24, 1019–1028 (2022).
Barman, B. et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev. Cell 57, 974–994.e978 (2022).
Jeppesen, D. K., Zhang, Q., Franklin, J. L. & Coffey, R. J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 33, 667–681 (2023).
Zhang, Q., Jeppesen, D. K., Higginbotham, J. N., Franklin, J. L. & Coffey, R. J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat. Protoc. 18, 1462–1487 (2023).
Buzas, E. I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 23, 236–250 (2023).
Collino, F. et al. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev. 13, 226–243 (2017).
Cosenza, S. et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8, 1399–1410 (2018).
Dixson, A. C., Dawson, T. R., Di Vizio, D. & Weaver, A. M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476 (2023).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445.e418 (2019).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977 (2016).
Villatoro, A. J. et al. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 208, 6–15 (2019).
Poon, I. K. H. et al. Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell. Vesicles 8, 1608786 (2019).
Zhang, X. et al. Proteomic analysis of MSC-derived apoptotic vesicles identifies Fas inheritance to ameliorate haemophilia a via activating platelet functions. J. Extracell. Vesicles 11, e12240 (2022).
Liu, J. et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 11, 507 (2020).
Fafian-Labora, J. A., Morente-Lopez, M. & Arufe, M. C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cell 11, 337–346 (2019).
Boulestreau, J., Maumus, M., Jorgensen, C. & Noel, D. Extracellular vesicles from mesenchymal stromal cells: therapeutic perspectives for targeting senescence in osteoarthritis. Adv. Drug. Deliv. Rev. 175, 113836 (2021).
Wolf, M. et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell. Vesicles 11, e12207 (2022).
Buzas, E. I. Opportunities and challenges in studying the extracellular vesicle corona. Nat. Cell Biol. 24, 1322–1325 (2022).
Toth, E. A. et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 10, e12140 (2021).
Kamei, N. et al. Comparative study of commercial protocols for high recovery of high-purity mesenchymal stem cell-derived extracellular vesicle isolation and their efficient labeling with fluorescent dyes. Nanomedicine 35, 102396 (2021).
Mol, E. A., Goumans, M. J., Doevendans, P. A., Sluijter, J. P. G. & Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 13, 2061–2065 (2017).
Ratajczak, M. Z., Ratajczak, D. & Pedziwiatr, D. Extracellular microvesicles (ExMVs) in cell to cell communication: a role of telocytes. Adv. Exp. Med. Biol. 913, 41–49 (2016).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Tkach, M. et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 36, 3012–3028 (2017).
Goncalves, E. et al. Systematic analysis of transcriptional and post-transcriptional regulation of metabolism in yeast. PLoS Comput. Biol. 13, e1005297 (2017).
Costa Verdera, H., Gitz-Francois, J. J., Schiffelers, R. M. & Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Rel. 266, 100–108 (2017).
Feng, D. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687 (2010).
Fitzner, D. et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 (2011).
Shi, M. M. et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 10, e12134 (2021).
Bertolino, G. M., Maumus, M., Jorgensen, C. & Noel, D. Recent advances in extracellular vesicle-based therapies using induced pluripotent stem cell-derived mesenchymal stromal cells. Biomedicines 10, 2281 (2022).
Wang, M. et al. Molecular crosstalk between articular cartilage, meniscus, synovium, and subchondral bone in osteoarthritis. Bone Jt Res. 11, 862–872 (2022).
Sellam, J. & Berenbaum, F. Is osteoarthritis a metabolic disease? Jt Bone Spine 80, 568–573 (2013).
Van Spil, W. E., Kubassova, O., Boesen, M., Bay-Jensen, A. C. & Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 165, 41–48 (2019).
Vonk, L. A. et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 8, 906–920 (2018).
Yan, L., Liu, G. & Wu, X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 11, e255 (2021).
Tan, F., Wang, D. & Yuan, Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation 43, 1498–1509 (2020).
Liu, Y. et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 17, 2411–2422 (2018).
Liu, Y. et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 475, 3629–3638 (2018).
Li, S., Liu, J., Liu, S., Jiao, W. & Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnology 19, 194 (2021).
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C. & Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7, 16214 (2017).
He, L. et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 11, 276 (2020).
Li, S. et al. hBMSC-derived extracellular vesicles attenuate IL-1beta-induced catabolic effects on OA-chondrocytes by regulating pro-inflammatory signaling pathways. Front. Bioeng. Biotechnol. 8, 603598 (2020).
Cavallo, C. et al. Small extracellular vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci. Rep. 11, 1053 (2021).
Chen, X. et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 22, 256 (2020).
Xia, Q., Wang, Q., Lin, F. & Wang, J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered 12, 11225–11238 (2021).
Kong, R. et al. Synovial mesenchymal stem cell-derived exosomal miR-320c enhances chondrogenesis by targeting ADAM19. Future Med. Chem. 14, 81–96 (2022).
Mao, G. et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9, 247 (2018).
Qi, H. et al. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitr. Cell Dev. Biol. Anim. 55, 203–210 (2019).
Li, X. et al. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 45, 2096–2106 (2021).
Lin, T. et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR1405poverexpressing human dental pulp stem cells. Int. J. Mol. Med. 47, 7 (2021).
Guillen, M. I., Tofino-Vian, M., Silvestre, A., Castejon, M. A. & Alcaraz, M. J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 30, 61–69 (2021).
Tang, S. et al. Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem. 123, 151796 (2021).
Wu, J. et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100 (2019).
Jin, Y. et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 25, 9281–9294 (2021).
Wen, T. et al. Bone mesenchymal stem cell-derived extracellular vesicles promote the repair of intervertebral disc degeneration by transferring microRNA-199a. Cell Cycle 20, 256–270 (2021).
Xu, H. & Xu, B. BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-κB p65 to chondrocytes. Mediators Inflamm. 2021, 9972805 (2021).
Jiang, K., Jiang, T., Chen, Y. & Mao, X. Mesenchymal stem cell-derived exosomes modulate chondrocyte glutamine metabolism to alleviate osteoarthritis progression. Mediators Inflamm. 2021, 2979124 (2021).
Tofino-Vian, M., Guillen, M. I., Perez Del Caz, M. D., Silvestre, A. & Alcaraz, M. J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol. Biochem. 47, 11–25 (2018).
Li, K. et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnology 20, 38 (2022).
Zhang, J., Rong, Y., Luo, C. & Cui, W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging 12, 25138–25152 (2020).
Pan, C. et al. LncRNA Malat-1 from MSCs-derived extracellular vesicles suppresses inflammation and cartilage degradation in osteoarthritis. Front. Bioeng. Biotechnol. 9, 772002 (2021).
Ragni, E. et al. Interaction hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 10, 109 (2019).
Aletaha, D. et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
van der Woude, D. & van der Helm-van Mil, A. H. M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 32, 174–187 (2018).
Conley, B. et al. What are the core recommendations for rheumatoid arthritis care? Systematic review of clinical practice guidelines. Clin. Rheumatol. https://doi.org/10.1007/s10067-023-06654-0 (2023).
Lopez-Santalla, M., Fernandez-Perez, R. & Garin, M. I. Mesenchymal stem/stromal cells for rheumatoid arthritis treatment: an update on clinical applications. Cells 9, 1852 (2020).
Skalska, U. & Kontny, E. Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity 49, 124–131 (2016).
Skalska, U. et al. Articular and subcutaneous adipose tissues of rheumatoid arthritis patients represent equal sources of immunoregulatory mesenchymal stem cells. Autoimmunity 50, 441–450 (2017).
Fu, Y. et al. Umbilical cord mesenchymal stem cell-derived exosomes alleviate collagen-induced arthritis by balancing the population of Th17 and regulatory T cells. FEBS Lett. 596, 2668–2677 (2022).
Tian, X. et al. Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell Mol. Med. 26, 693–708 (2022).
Xu, K. et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Mol. Immunol. 135, 36–44 (2021).
Ma, D. et al. Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int. Immunopharmacol. 74, 105687 (2019).
Huang, Y. et al. miR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages. Mol. Immunol. 143, 68–76 (2022).
Kay, A. G. et al. Therapeutic effects of hypoxic and pro-inflammatory priming of mesenchymal stem cell-derived extracellular vesicles in inflammatory arthritis. Int. J. Mol. Sci. 23, 126 (2021).
Chen, Z., Wang, H., Xia, Y., Yan, F. & Lu, Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J. Immunol. 201, 2472–2482 (2018).
Meng, Q. & Qiu, B. Exosomal microRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression. Front. Physiol. 11, 441 (2020).
Li, G. Q. et al. MicroRNA-21 from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis. Ther. Adv. Chronic Dis. 12, 20406223211007369 (2021).
Su, Y. et al. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway. J. Orthop. Surg. Res. 16, 116 (2021).
Zhang, J. et al. Therapeutic potential of exosomal circRNA derived from synovial mesenchymal cells via targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF functional module in rheumatoid arthritis. Int. J. Nanomed. 16, 7977–7994 (2021).
Huang, Y., Chen, L., Chen, D., Fan, P. & Yu, H. Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1. Mol. Med. 28, 36 (2022).
Lu, R. Q. et al. SGK1, a critical regulator of immune modulation and fibrosis and a potential therapeutic target in chronic graft-versus-host disease. Front. Immunol. 13, 822303 (2022).
Meng, H. Y., Chen, L. Q. & Chen, L. H. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet. Disord. 21, 150 (2020).
Chang, L. & Kan, L. Mesenchymal stem cell-originated exosomal circular RNA circFBXW7 attenuates cell proliferation, migration and inflammation of fibroblast-like synoviocytes by targeting miR-216a-3p/HDAC4 in rheumatoid arthritis. J. Inflamm. Res. 14, 6157–6171 (2021).
Smith, L. J., Nerurkar, N. L., Choi, K. S., Harfe, B. D. & Elliott, D. M. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model. Mech. 4, 31–41 (2011).
DiStefano, T. J. et al. Extracellular vesicles as an emerging treatment option for intervertebral disc degeneration: therapeutic potential, translational pathways, and regulatory considerations. Adv. Healthc. Mater. 11, e2100596 (2022).
Cheng, X. et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell Mol. Med. 22, 261–276 (2018).
Liao, Z. et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9, 4084–4100 (2019).
Hingert, D., Ekstrom, K., Aldridge, J., Crescitelli, R. & Brisby, H. Extracellular vesicles from human mesenchymal stem cells expedite chondrogenesis in 3D human degenerative disc cell cultures. Stem Cell Res. Ther. 11, 323 (2020).
Zhu, L. et al. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 19, 1727–1739 (2020).
Xia, C. et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free. Radic. Biol. Med. 143, 1–15 (2019).
Sozen, T., Ozisik, L. & Basaran, N. C. An overview and management of osteoporosis. Eur. J. Rheumatol. 4, 46–56 (2017).
Martins, M., Ribeiro, D., Martins, A., Reis, R. L. & Neves, N. M. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Rep. 6, 284–291 (2016).
Xu, R. et al. Identification of the canonical and noncanonical role of miR-143/145 in estrogen-deficient bone loss. Theranostics 11, 5491–5510 (2021).
Hu, Y. et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 95, 93–101 (2019).
Zhang, L., Wang, Q., Su, H. & Cheng, J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J. Biosci. Bioeng. 131, 671–678 (2021).
Behera, J., Kumar, A., Voor, M. J. & Tyagi, N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics 11, 7715–7734 (2021).
Yan, L. & Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 36, 165–178 (2020).
Najrana, T. et al. Mechanical stretch regulates the expression of specific miRNA in extracellular vesicles released from lung epithelial cells. J. Cell Physiol. 235, 8210–8223 (2020).
Bister, N. et al. Hypoxia and extracellular vesicles: a review on methods, vesicular cargo and functions. J. Extracell. Vesicles 10, e12002 (2020).
Wang, J., Bonacquisti, E. E., Brown, A. D. & Nguyen, J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 9, 660 (2020).
Boker, K. O. et al. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol. Ther. 26, 634–647 (2018).
Sinha, S. et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell Biol. 214, 197–213 (2016).
Shyong, Y. J., Chang, K. C. & Lin, F. H. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloids Surf. B Biointerfaces 171, 391–397 (2018).
Pascucci, L. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control. Rel. 192, 262–270 (2014).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Rankin-Turner, S. et al. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug. Deliv. Rev. 173, 479–491 (2021).
Hettich, B. F., Bader, J. J. & Leroux, J. C. Encapsulation of hydrophilic compounds in small extracellular vesicles: loading capacity and impact on vesicle functions. Adv. Healthc. Mater. 11, e2100047 (2022).
Feng, K. et al. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J. Extracell. Vesicles 10, e12160 (2021).
You, D. G. et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 7, eabe0083 (2021).
Lee, E. S. et al. Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 128, 462–473 (2021).
Tao, S. C. et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195 (2017).
Wang, Z. et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 37, 85–96 (2021).
Liang, Y. et al. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater. Sci. 10, 4095–4106 (2022).
Colombini, A. et al. Adipose-derived mesenchymal stromal cells treated with interleukin 1 β produced chondro-protective vesicles able to fast penetrate in cartilage. Cells 10, 1180 (2021).
Kim, M., Shin, D. I., Choi, B. H. & Min, B. H. Exosomes from IL-1β-primed mesenchymal stem cells inhibited IL-1β- and TNF-α-mediated inflammatory responses in osteoarthritic SW982. Cells Tissue Eng. Regen. Med. 18, 525–536 (2021).
Wang, R., Xu, B. & Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17, 2756–2765 (2018).
Li, S. et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 12, 252 (2021).
Xu, C. et al. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J. Nanobiotechnology 20, 123 (2022).
Shao, L. T., Luo, L., Qiu, J. H. & Deng, D. Y. B. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res. Ther. 24, 96 (2022).
Liu, C. et al. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine 15, 273–288 (2020).
Rong, Y. et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 122, 325–342 (2021).
Zhang, B., Tian, X., Qu, Z., Hao, J. & Zhang, W. Hypoxia-preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes 12, 225 (2022).
Chen, P. et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9, 2439–2459 (2019).
Sang, X. et al. Thermosensitive hydrogel loaded with primary chondrocyte-derived exosomes promotes cartilage repair by regulating macrophage polarization in osteoarthritis. Tissue Eng. Regen. Med. 19, 629–642 (2022).
Zhu, Y. et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8, 64 (2017).
Fazaeli, H. et al. A comparative study on the effect of exosomes secreted by mesenchymal stem cells derived from adipose and bone marrow tissues in the treatment of osteoarthritis-induced mouse model. Biomed. Res. Int. 2021, 9688138 (2021).
Liu, Y. et al. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of vegfa in a rat model. Am. J. Sports Med. 50, 1088–1105 (2022).
Wan, S. et al. Extracellular vesicles from hypoxic pretreated urine-derived stem cells enhance the proliferation and migration of chondrocytes by delivering miR-26a-5p. Cartilage 13, 19476035221077401 (2022).
Liu, X. et al. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 14, 470 (2019).
Zhang, Y. et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J. Nanobiotechnology 20, 56 (2022).
Pieters, B. C. H. et al. Bovine milk-derived extracellular vesicles inhibit catabolic and inflammatory processes in cartilage from osteoarthritis patients. Mol. Nutr. Food Res. 66, e2100764 (2022).
Da-Wa, Z. X. et al. Exosomes derived from M2 macrophages exert a therapeutic effect via inhibition of the PI3K/AKT/mTOR pathway in rats with knee osteoarthritic. Biomed. Res. Int. 2021, 7218067 (2021).
Kato, T. et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 16, R163 (2014).
Wu, X., Crawford, R., Xiao, Y., Mao, X. & Prasadam, I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells 10, 251 (2021).
Yang, R. Z. et al. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging 13, 4647–4662 (2021).
Chen, P. et al. Synovial tissue-derived extracellular vesicles induce chondrocyte inflammation and degradation via NF-κB signalling pathway: an in vitro study. J. Cell Mol. Med. 26, 2038–2048 (2022).
Simmonds, M. C. et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann. Intern. Med. 158, 877–889 (2013).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 20, 101–124 (2021).
Maumus, M., Rozier, P., Boulestreau, J., Jorgensen, C. & Noel, D. Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front. Bioeng. Biotechnol. 8, 997 (2020).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed substantially to discussion of the content, and to writing the article. D.N. and G.M.B. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks M. J. Alcaraz; M. Peffers, who co-reviewed with E.-J. Clarke; and M. Ullah for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bertolino, G.M., Maumus, M., Jorgensen, C. et al. Therapeutic potential in rheumatic diseases of extracellular vesicles derived from mesenchymal stromal cells. Nat Rev Rheumatol 19, 682–694 (2023). https://doi.org/10.1038/s41584-023-01010-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01010-7