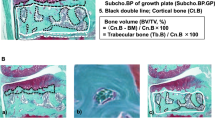
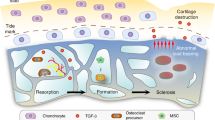
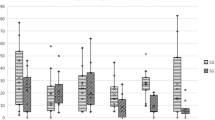
Abstract
Bone marrow lesions (BMLs), which are early signs of osteoarthritis (OA) that are associated with the presence, onset and severity of pain, represent an emerging imaging biomarker and clinical target. Little is known, however, regarding their early spatial and temporal development, structural relationships or aetiopathogenesis, because of the sparsity of human early OA imaging and paucity of relevant tissue samples. The use of animal models is a logical approach to fill the gaps in our knowledge, and it can be informed by appraising models in which BMLs and closely related subchondral cysts have already been reported, including in spontaneous OA and pain models. The utility of these models in OA research, their relevance to clinical BMLs and practical considerations for their optimal deployment can also inform medical and veterinary clinicians and researchers alike.
Key points
-
Bone marrow lesions (BMLs) are early signs of osteoarthritis (OA) that are associated with pain onset and severity, and they therefore represent potential biomarkers of OA and therapeutic targets.
-
Human studies of BMLs are limited by long imaging intervals and lack of joint tissue availability, particularly in early-stage OA or pre-OA, whereas animal studies have no such restrictions.
-
Available animal models exhibiting BMLs (or closely related subchondral cysts) include induced models (with induction by joint destabilization, loading or chemical treatment) and spontaneous models.
-
Animal BML models can represent both fast-onset traumatic and slower-onset ‘naturally occurring’ OA, and the occurrence of BMLs before and after cartilage lesion development.
-
Collectively, animal models provide opportunities for exploring BML aetiopathogenesis and bone–cartilage crosstalk, elucidating structural associations longitudinally and trialling therapeutic interventions before back-translating the results to humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Felson, D. T. et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann. Intern. Med. 134, 541–549 (2001).
Sowers, M. F. et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage 11, 387–393 (2003).
Felson, D. T. et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 56, 2986–2992 (2007).
Driban, J. B. et al. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker — longitudinal relationships with pain and structural changes: data from the Osteoarthritis Initiative. Arthritis Res. Ther. 15, R112 (2013).
Carrino, J. A., Blum, J., Parellada, J. A., Schweitzer, M. E. & Morrison, W. B. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 14, 1081–1085 (2006).
Hunter, D. J. et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 54, 1529–1535 (2006).
Kuttapitiya, A. et al. Microarray analysis of bone marrow lesions in osteoarthritis demonstrates upregulation of genes implicated in osteochondral turnover, neurogenesis and inflammation. Ann. Rheum. Dis. 76, 1764–1773 (2017).
Conaghan, P. G. et al. Impact and therapy of osteoarthritis: the Arthritis Care OA Nation 2012 survey. Clin. Rheumatol. 34, 1581–1588 (2015).
Xu, L., Hayashi, D., Roemer, F. W., Felson, D. T. & Guermazi, A. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin. Arthritis Rheum. 42, 105–118 (2012).
Wilson, A. J., Murphy, W. A., Hardy, D. C. & Totty, W. G. Transient osteoporosis: transient bone marrow edema? Radiology 167, 757–760 (1988).
Roemer, F. W. et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage 17, 1115–1131 (2009).
Zanetti, M., Bruder, E., Romero, J. & Hodler, J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 215, 835–840 (2000).
Koushesh, S. et al. The osteoarthritis bone score (OABS): a new histological scoring system for the characterisation of bone marrow lesions in osteoarthritis. Osteoarthritis Cartilage 30, 746–755 (2022).
Hunter, D. J. et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 19, 990–1002 (2011).
Peterfy, C. G. et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12, 177–190 (2004).
Bergman, A. G., Willen, H. K., Lindstrand, A. L. & Pettersson, H. T. Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal Radiol. 23, 445–448 (1994).
Kazakia, G. J. et al. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage 21, 94–101 (2013).
Zhang, Y. et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 63, 691–699 (2011).
Sun, Y. et al. Effects of a phosphocitrate analogue on osteophyte, subchondral bone advance, and bone marrow lesions in Hartley guinea pigs. Bone Joint Res. 7, 157–165 (2018).
Crema, M. D. et al. Contrast-enhanced MRI of subchondral cysts in patients with or at risk for knee osteoarthritis: the MOST study. Eur. J. Radiol. 75, e92–e96 (2010).
Gorbachova, T. et al. Nomenclature of subchondral nonneoplastic bone lesions. AJR Am. J. Roentgenol. 213, 963–982 (2019).
Resnick, D., Niwayama, G. & Coutts, R. D. Subchondral cysts (geodes) in arthritic disorders: pathologic and radiographic appearance of the hip joint. AJR Am. J. Roentgenol. 128, 799–806 (1977).
Crema, M. D. et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with MR imaging — the MOST study. Radiology 256, 855–862 (2010).
Link, T. M. et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 226, 373–381 (2003).
Hayes, C. W. et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology 237, 998–1007 (2005).
Mennan, C. et al. The use of technology in the subcategorisation of osteoarthritis: a Delphi study approach. Osteoarthr. Cartil. Open. 2, 100081 (2020).
Roemer, F. W., Crema, M. D., Trattnig, S. & Guermazi, A. Advances in imaging of osteoarthritis and cartilage. Radiology 260, 332–354 (2011).
Barr, A. J. et al. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 17, 228–228 (2015).
Dore, D. et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res. Ther. 12, R222 (2010).
Davies-Tuck, M. L. et al. Development of bone marrow lesions is associated with adverse effects on knee cartilage while resolution is associated with improvement–a potential target for prevention of knee osteoarthritis: a longitudinal study. Arthritis Res. Ther. 12, R10 (2010).
Bowes, M. A. et al. Osteoarthritic bone marrow lesions almost exclusively colocate with denuded cartilage: a 3D study using data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 75, 1852–1857 (2016).
Kumar, D. et al. Association of cartilage defects, and other MRI findings with pain and function in individuals with mild–moderate radiographic hip osteoarthritis and controls. Osteoarthritis Cartilage 21, 1685–1692 (2013).
Lim, Y. Z. et al. Association of obesity and systemic factors with bone marrow lesions at the knee: a systematic review. Semin. Arthritis Rheum. 43, 600–612 (2014).
Hayashi, D. et al. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage 20, 1227–1233 (2012).
Wang, Y. et al. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology 49, 997–1004 (2010).
Lim, Y. Z. et al. Are biomechanical factors, meniscal pathology, and physical activity risk factors for bone marrow lesions at the knee? A systematic review. Semin. Arthritis Rheum. 43, 187–194 (2013).
Beckwee, D. et al. The influence of joint loading on bone marrow lesions in the knee: a systematic review with meta-analysis. Am. J. Sports Med. 43, 3093–3107 (2015).
Burnett, W. D. et al. Knee osteoarthritis patients with more subchondral cysts have altered tibial subchondral bone mineral density. BMC Musculoskelet. Disord. 20, 14–14 (2019).
Audrey, H. X., Abd Razak, H. R. & Andrew, T. H. The truth behind subchondral cysts in osteoarthritis of the knee. Open. Orthop. J. 8, 7–10 (2014).
Wildi, L. M. et al. Relationship between bone marrow lesions, cartilage loss and pain in knee osteoarthritis: results from a randomised controlled clinical trial using MRI. Ann. Rheum. Dis. 69, 2118–2124 (2010).
Madan-Sharma, R. et al. Do MRI features at baseline predict radiographic joint space narrowing in the medial compartment of the osteoarthritic knee 2 years later? Skeletal Radiol. 37, 805–811 (2008).
Roemer, F. W. et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology 252, 772–780 (2009).
Kubota, M. et al. A longitudinal study of the relationship between the status of bone marrow abnormalities and progression of knee osteoarthritis. J. Orthop. Sci. 15, 641–646 (2010).
Driban, J. B. et al. Bone marrow lesion volume reduction is not associated with improvement of other periarticular bone measures: data from the Osteoarthritis Initiative. Arthritis Res. Ther. 15, R153 (2013).
Deveza, L. A. et al. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 25, 1926–1941 (2017).
Karsdal, M. A. et al. Osteoarthritis — a case for personalized health care? Osteoarthritis Cartilage 22, 7–16 (2014).
Holla, J. F. M. et al. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: a 5-year follow-up study. Ann. Rheum. Dis. 73, 1369 (2014).
Nicholls, E., Thomas, E., van der Windt, D. A., Croft, P. R. & Peat, G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage 22, 2041–2050 (2014).
Walsh, D. A., Sofat, N., Guermazi, A. & Hunter, D. J. Osteoarthritis bone marrow lesions. Osteoarthritis Cartilage 31, 11–17 (2023).
National Institute for Health and Care Excellence. Osteoarthritis in over 16s: diagnosis and management (NG226) (2022).
Yusuf, E., Kortekaas, M. C., Watt, I., Huizinga, T. W. & Kloppenburg, M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann. Rheum. Dis. 70, 60–67 (2011).
Starr, A. M., Wessely, M. A., Albastaki, U., Pierre-Jerome, C. & Kettner, N. W. Bone marrow edema: pathophysiology, differential diagnosis, and imaging. Acta Radiol. 49, 771–786 (2008).
Kim, D. S. et al. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J. Neurosci. 32, 8977–8987 (2012).
Perry, T. A. et al. Association between bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthritis Cartilage 28, 316–323 (2020).
Zhou, F. et al. Associations of osteoclastogenesis and nerve growth in subchondral bone marrow lesions with clinical symptoms in knee osteoarthritis. J. Orthop. Transl. 32, 69–76 (2022).
Taljanovic, M. S. et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 37, 423–431 (2008).
Muratovic, D. et al. Bone matrix microdamage and vascular changes characterize bone marrow lesions in the subchondral bone of knee osteoarthritis. Bone 108, 193–201 (2018).
Burr, D. B. & Hooser, M. Alterations to the en bloc basic fuchsin staining protocol for the demonstration of microdamage produced in vivo. Bone 17, 431–433 (1995).
Hernandez, C. J., Lambers, F. M., Widjaja, J., Chapa, C. & Rimnac, C. M. Quantitative relationships between microdamage and cancellous bone strength and stiffness. Bone 66, 205–213 (2014).
Lambers, F. M., Bouman, A. R., Rimnac, C. M. & Hernandez, C. J. Microdamage caused by fatigue loading in human cancellous bone: relationship to reductions in bone biomechanical performance. PLoS One 8, e83662 (2013).
Chan, P. B., Wen, C., Yang, W. C., Yan, C. & Chiu, K. Is subchondral bone cyst formation in non-load-bearing region of osteoarthritic knee a vascular problem? Med. Hypotheses 109, 80–83 (2017).
McCoy, A. M. Animal models of osteoarthritis: comparisons and key considerations. Vet. Pathol. 52, 803–818 (2015).
Alliston, T., Hernandez, C. J., Findlay, D. M., Felson, D. T. & Kennedy, O. D. Bone marrow lesions in osteoarthritis: what lies beneath. J. Orthop. Res. 36, 1818–1825 (2018).
Norman, R., Boyde, A., Chenu, C., Sofat, N. & Pitsillides, A. A. Characterisation of subchondral cysts in a murine model of spontaneous osteoarthritis. J Bone Miner Res 5, e10499 (2021).
Findlay, D. M. & Kuliwaba, J. S. Bone–cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 4, 16028 (2016).
Segal, N. A. et al. The multicenter osteoarthritis study: opportunities for rehabilitation research. PM R. 5, 647–654 (2013).
Eriksen, E. F. Treatment of bone marrow lesions (bone marrow edema). Bonekey Rep. 4, 755 (2015).
Scher, C., Craig, J. & Nelson, F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol. 37, 609–617 (2008).
Baird, D. K. et al. Low-field magnetic resonance imaging of early subchondral cyst-like lesions in induced cranial cruciate ligament deficient dogs. Vet. Radiol. Ultrasound 39, 167–173 (1998).
Boileau, C. et al. Magnetic resonance imaging can accurately assess the long-term progression of knee structural changes in experimental dog osteoarthritis. Ann. Rheum. Dis. 67, 926–932 (2008).
d’Anjou, M. A. et al. Temporal assessment of bone marrow lesions on magnetic resonance imaging in a canine model of knee osteoarthritis: impact of sequence selection. Osteoarthritis Cartilage 16, 1307–1311 (2008).
Martig, S., Boisclair, J., Konar, M., Spreng, D. & Lang, J. MRI characteristics and histology of bone marrow lesions in dogs with experimentally induced osteoarthritis. Vet. Radiol. Ultrasound 48, 105–112 (2007).
Nolte-Ernsting, C. C., Adam, G., Buhne, M., Prescher, A. & Gunther, R. W. MRI of degenerative bone marrow lesions in experimental osteoarthritis of canine knee joints. Skeletal Radiol. 25, 413–420 (1996).
Raynauld, J. P. et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann. Rheum. Dis. 67, 683–688 (2008).
Bouchgua, M. et al. Use of routine clinical multimodality imaging in a rabbit model of osteoarthritis — part I. Osteoarthritis Cartilage 17, 188–196 (2009).
Jia, L. et al. Magnetic resonance imaging of osteophytic, chondral, and subchondral structures in a surgically-induced osteoarthritis rabbit model. PLoS One 9, e113707–e113707 (2014).
Tsai, P. H. et al. Sequential change in T2* values of cartilage, meniscus, and subchondral bone marrow in a rat model of knee osteoarthritis. PLoS One 8, e76658 (2013).
Chavhan, G. B., Babyn, P. S., Thomas, B., Shroff, M. M. & Haacke, E. M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 29, 1433–1449 (2009).
McErlain, D. D. et al. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res. Ther. 14, R26 (2012).
Zhen, G. et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 (2013).
Beamer, W. G., Donahue, L. R., Rosen, C. J. & Baylink, D. J. Genetic variability in adult bone density among inbred strains of mice. Bone 18, 397–403 (1996).
Brimmo, O. A. et al. Development of a novel canine model for posttraumatic osteoarthritis of the knee. J. Knee Surg. 29, 235–241 (2016).
Brimmo, O. A. et al. Subchondroplasty for the treatment of post-traumatic bone marrow lesions of the medial femoral condyle in a pre-clinical canine model. J. Orthop. Res. 36, 2709–2717 (2018).
Poulet, B. et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthritis Cartilage 23, 940–948 (2015).
Matheny, J. B. et al. An in vivo model of a mechanically-induced bone marrow lesion. J. Biomech. 64, 258–261 (2017).
Bowen, A. et al. Animal models of bone marrow lesions in osteoarthritis. J Bone Miner. Res. 6, e10609 (2022).
Ramme, A. J., Lendhey, M., Raya, J. G., Kirsch, T. & Kennedy, O. D. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthritis Cartilage 24, 1776–1785 (2016).
Ko, F. C. et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65, 1569–1578 (2013).
Christiansen, B. A. et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 20, 773–782 (2012).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Ter Heegde, F. et al. Noninvasive mechanical joint loading as an alternative model for osteoarthritic pain. Arthritis Rheumatol. 71, 1078–1088 (2019).
Christiansen, B. A. et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage 23, 1627–1638 (2015).
Vincent, T. L., Williams, R. O., Maciewicz, R., Silman, A. & Garside, P. Mapping pathogenesis of arthritis through small animal models. Rheumatology 51, 1931–1941 (2012).
Unger, M. D. et al. Clinical magnetic resonance-enabled characterization of mono-iodoacetate-induced osteoarthritis in a large animal species. PLoS One 13, e0201673 (2018).
Pitcher, T., Sousa-Valente, J. & Malcangio, M. The monoiodoacetate model of osteoarthritis pain in the mouse. J. Vis. Exp. 111, 53746 (2016).
Bendele, A. M. & Hulman, J. F. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 34, 1180–1184 (1991).
Wei, L., Svensson, O. & Hjerpe, A. Correlation of morphologic and biochemical changes in the natural history of spontaneous osteoarthrosis in guinea pigs. Arthritis Rheum. 40, 2075–2083 (1997).
Watson, P. J., Hall, L. D., Malcolm, A. & Tyler, J. A. Degenerative joint disease in the guinea pig. Use of magnetic resonance imaging to monitor progression of bone pathology. Arthritis Rheum. 39, 1327–1337 (1996).
Tessier, J. J. et al. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthritis Cartilage 11, 845–853 (2003).
de Bri, E., Jonsson, K., Reinholt, F. P. & Svensson, O. Focal destruction and remodeling in guinea pig arthrosis. Acta Orthop. Scand. 67, 498–504 (1996).
Chan, P. B., Yang, W., Wen, C., Yan, C. & Chiu, K. Spontaneously hypertensive rat as a novel model of co-morbid knee osteoarthritis. Osteoarthritis Cartilage 25, S319–S320 (2017).
Walton, M. Degenerative joint disease in the mouse knee; radiological and morphological observations. J. Pathol. 123, 97–107 (1977).
Staines, K. A., Poulet, B., Wentworth, D. N. & Pitsillides, A. A. The STR/ort mouse model of spontaneous osteoarthritis — an update. Osteoarthritis Cartilage 25, 802–808 (2017).
Louka, P., Orriss, I. R. & Pitsillides, A. A. High bone mass in mice can be linked to lower osteoclast formation, resorptive capacity, and restricted in vitro sensitivity to inhibition by stable sulforaphane. Cell Biochem. Funct. 40, 683–693 (2022).
Hansen, B. D. Assessment of pain in dogs: veterinary clinical studies. ILAR J. 44, 197–205 (2003).
Deuis, J. R., Dvorakova, L. S. & Vetter, I. methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 10, 284–284 (2017).
Hartwig, V. et al. Biological effects and safety in magnetic resonance imaging: a review. Int. J. Environ. Res. Public Health 6, 1778–1798 (2009).
Ruder, T. D., Thali, M. J. & Hatch, G. M. Essentials of forensic post-mortem MR imaging in adults. Br. J. Radiol. 87, 20130567 (2014).
Muratovic, D. et al. Bone marrow lesions detected by specific combination of MRI sequences are associated with severity of osteochondral degeneration. Arthritis Res. Ther. 18, 54 (2016).
Muratovic, D. et al. Bone marrow lesions in knee osteoarthritis: regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthritis Cartilage 27, 1653–1662 (2019).
Campbell, T. M. et al. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol. 68, 1648–1659 (2016).
Bolen, G. E., Haye, D., Dondelinger, R. F., Massart, L. & Busoni, V. Impact of successive freezing-thawing cycles on 3-T magnetic resonance images of the digits of isolated equine limbs. Am. J. Vet. Res. 72, 780–790 (2011).
van der Made, A. D., Maas, M., Beenen, L. F., Oostra, R. J. & Kerkhoffs, G. M. Postmortem imaging exposed: an aid in MR imaging of musculoskeletal structures. Skeletal Radiol. 42, 467–472 (2013).
Alanen, A. M. et al. The effects of the method of death and lapsed time on proton relaxation time T1 in autopsied muscle samples. Invest. Radiol. 28, 529–532 (1993).
Ruder, T. D. et al. The influence of body temperature on image contrast in post mortem MRI. Eur. J. Radiol. 81, 1366–1370 (2012).
Roemer, F. W., Hunter, D. J. & Guermazi, A. MRI-based semiquantitative assessment of subchondral bone marrow lesions in osteoarthritis research. Osteoarthritis Cartilage 17, 414–415 (2009).
Wang, H. H. et al. Pitfalls in interpreting rat knee joint magnetic resonance images and their histological correlation. Acta Radiol. 50, 1042–1048 (2009).
Rothbauer, M. et al. Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. Lab Chip 20, 1461–1471 (2020).
Occhetta, P. et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat. Biomed. Eng. 3, 545–557 (2019).
Torisawa, Y.-s et al. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11, 663–669 (2014).
Paggi, C. A., Teixeira, L. M., Le Gac, S. & Karperien, M. Joint-on-chip platforms: entering a new era of in vitro models for arthritis. Nat. Rev. Rheumatol. 18, 217–231 (2022).
Cope, P. J., Ourradi, K., Li, Y. & Sharif, M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage 27, 230–239 (2019).
Bellido, T. & Delgado-Calle, J. Ex vivo organ cultures as models to study bone biology. J Bone Miner. Res. 14, 10 (2020).
Amado, I. A novel osteochondral explant model to study bone and cartilage responses to damage in PTOA. Osteoarthritis Cartilage 29, S199–S200 (2021).
Amado, I., Hodgkinson, T., Murphy, C. & Kennedy, O. The effect of chemical and mechanical damage in a novel osteochondral explant for post-traumatic osteoarthritis. Orthop. Proc. 103-B, 26 (2021).
Wang, J. et al. Association of patellar bone marrow lesions with knee pain, patellar cartilage defect and patellar cartilage volume loss in older adults: a cohort study. Osteoarthritis Cartilage 23, 1330–1336 (2015).
Staines, K. A., Brown, G. & Farquharson, C. The ex vivo organ culture of bone. Methods Mol. Biol. 1914, 199–215 (2019).
Meeson, R. L., Todhunter, R. J., Blunn, G., Nuki, G. & Pitsillides, A. A. Spontaneous dog osteoarthritis — a One Medicine vision. Nat. Rev. Rheumatol. 15, 273–287 (2019).
Styczynska-Soczka, K., Amin, A. K., Simpson, A. H. W. & Hall, A. C. Optimization and validation of a human ex vivo femoral head model for preclinical cartilage research and regenerative therapies. Cartilage 13, 386S–397S (2021).
Olive, J., d’Anjou, M. A., Cabassu, J., Chailleux, N. & Blond, L. Fast presurgical magnetic resonance imaging of meniscal tears and concurrent subchondral bone marrow lesions. Study of dogs with naturally occurring cranial cruciate ligament rupture. Vet. Comp. Orthop. Traumatol. 27, 1–7 (2014).
De Guio, C., Ségard-Weisse, E., Thomas-Cancian, A. & Schramme, M. Bone marrow lesions of the distal condyles of the third metacarpal bone are common and not always related to lameness in sports and pleasure horses. Vet. Radiol. Ultrasound 60, 167–175 (2019).
Olive, J., Mair, T. S. & Charles, B. Use of standing low-field magnetic resonance imaging to diagnose middle phalanx bone marrow lesions in horses. Equine Vet. Educ. 21, 116–123 (2009).
Biggi, M., Zani, D. D., De Zani, D. & Di Giancamillo, M. Magnetic resonance imaging findings of bone marrow lesions in the equine distal tarsus. Equine Vet. Educ. 24, 236–241 (2012).
Freund, E. The pathological significance of intra-articular pressure. Edinb. Med. J. 47, 192–203 (1940).
Rogers, W. M. & Gladstone, H. Vascular foramina and arterial supply of the distal end of the femur. J. Bone Joint Surg. Am. 32 a, 867–874 (1950).
Rhaney, K. & Lamb, D. W. The cysts of osteoarthritis of the hip; a radiological and pathological study. J. Bone Joint Surg. Br. 37-b, 663–675 (1955).
Pouders, C. et al. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. AJR Am. J. Roentgenol. 190, 17–21 (2008).
Hatton, R., Stimpel, M. & Chambers, T. J. Angiotensin II is generated from angiotensin I by bone cells and stimulates osteoclastic bone resorption in vitro. J. Endocrinol. 152, 5–10 (1997).
Zhang, Y. M., Wang, J. & Liu, X. G. Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Medicine 96, e7584 (2017).
Boyde, A., Felder, A. & Mills, D. New approach to increase information content in polarised light microscopy of skeletal and dental tissues. Proceedings of Microscience Microscopy Congress https://qmro.qmul.ac.uk/xmlui/handle/123456789/67119 (2019).
Kornaat, P. R. et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology 239, 811–817 (2006).
Laslett, L. L. et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann. Rheum. Dis. 71, 1322 (2012).
Pang, J. et al. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the Osteoarthritis Initiative. BMC Musculoskelet. Disord. 14, 3 (2013).
Jones, M. D. et al. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 62, 2726–2735 (2010).
Sowers, M., Karvonen-Gutierrez, C. A., Jacobson, J. A., Jiang, Y. & Yosef, M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J. Bone Joint Surg. Am. 93, 241–251 (2011).
d’Anjou, M. A. et al. Response to the Letter to the Editor by Roemer and collaborators entitled “MRI based semi-quantitative assessment of subchondral bone marrow lesions in osteoarthritis research” concerning the article published by d’Anjou et al. entitled “Temporal assessment of bone marrow lesions on magnetic resonance imaging in a canine model of knee osteoarthritis: impact of sequence selection”. Osteoarthritis Cartilage 17, 416–417 (2009).
Kornaat, P. R. et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS) — inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 34, 95–102 (2005).
Hunter, D. J. et al. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann. Rheum. Dis. 67, 206–211 (2008).
Berenbaum, F. The OARSI histopathology initiative — the tasks and limitations. Osteoarthritis Cartilage 18, S1 (2010).
Acknowledgements
The authors would like to thank B. Javaheri and P.M.B. Chan for their initial work in characterizing subchondral cysts in the STR/ort mouse, and A. Boyde of Queen Mary University, London, for producing polarized light microscopy images of subchondral cysts. We would also like to thank P. Louka and R. In’t Zandt of Lund University Bioimaging Centre for helping to guide appreciation of MRI. A.A.P. acknowledges research support from Versus Arthritis (grant number 21900).
Author information
Authors and Affiliations
Contributions
R.T.H. researched data for the article. A.A.P and R.T.H. contributed substantially to discussion of the content and wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Dao, C. Hernandez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hansen, R.T., Chenu, C., Sofat, N. et al. Bone marrow lesions: plugging the holes in our knowledge using animal models. Nat Rev Rheumatol 19, 429–445 (2023). https://doi.org/10.1038/s41584-023-00971-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-00971-z