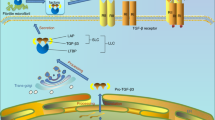
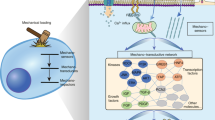
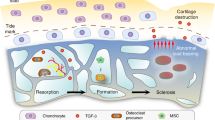
Abstract
Mechanical stimuli have fundamental roles in articular cartilage during health and disease. Chondrocytes respond to the physical properties of the cartilage extracellular matrix (ECM) and the mechanical forces exerted on them during joint loading. In osteoarthritis (OA), catabolic processes degrade the functional ECM and the composition and viscoelastic properties of the ECM produced by chondrocytes are altered. The abnormal loading environment created by these alterations propagates cell dysfunction and inflammation. Chondrocytes sense their physical environment via an array of mechanosensitive receptors and channels that activate a complex network of downstream signalling pathways to regulate several cell processes central to OA pathology. Advances in understanding the complex roles of specific mechanosignalling mechanisms in healthy and OA cartilage have highlighted molecular processes that can be therapeutically targeted to interrupt pathological feedback loops. The potential for combining these mechanosignalling targets with the rapidly expanding field of smart mechanoresponsive biomaterials and delivery systems is an emerging paradigm in OA treatment. The continued advances in this field have the potential to enable restoration of healthy mechanical microenvironments and signalling through the development of precision therapeutics, mechanoregulated biomaterials and drug systems in the near future.
Key points
-
Mechanical forces are a critical environmental factor for maintaining joint homeostasis, determining cell phenotype, inflammatory responses and the tightly regulated anabolic–catabolic signalling axis essential for cartilage homeostasis.
-
Chondrocytes sense their mechanical environment through numerous direct and indirect mechanisms that regulate cell function in health and degenerative diseases, such as osteoarthritis.
-
Targeted inhibition of mechanoinflammatory signalling pathways or restoration of functional chondroprotective extracellular matrix environments in osteoarthritis could prevent extracellular matrix degradation and promote reparative anabolic processes.
-
Development of self-regulating and mechanically responsive biomaterials and drug delivery systems offer advanced ‘on-demand’ therapeutic approaches for the treatment of osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vincent, T. L. & Wann, A. K. Mechanoadaptation: articular cartilage through thick and thin. J. Physiol. 597, 1271–1281 (2019).
Chang, S. H. et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat. Commun. 10, 1442 (2019).
Goldring, S. R. & Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 12, 632–644 (2016).
Pap, T. & Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis — two unequal siblings. Nat. Rev. Rheumatol. 11, 606–615 (2015).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Collins, K. H. et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl Acad. Sci. USA 118, e2021096118 (2021).
Watt, F. E. Posttraumatic osteoarthritis: what have we learned to advance osteoarthritis? Curr. Opin. Rheumatol. 33, 74–83 (2021).
Greene, M. A. & Loeser, R. F. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23, 1966–1971 (2015).
McNulty, M. A. et al. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthritis Cartilage 20, 949–956 (2012).
Lotz, M. & Loeser, R. F. Effects of aging on articular cartilage homeostasis. Bone 51, 241–248 (2012).
Loeser, R. F. et al. Microarray analysis reveals age‐related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 64, 705–717 (2012).
Thomas, A. C., Hubbard-Turner, T., Wikstrom, E. A. & Palmieri-Smith, R. M. Epidemiology of posttraumatic osteoarthritis. J. Athlet. Train. 52, 491–496 (2017).
Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Burleigh, A. et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 64, 2278–2288 (2012).
Ismail, H. M. et al. Interleukin‐1 acts via the JNK‐2 signaling pathway to induce aggrecan degradation by human chondrocytes. Arthritis Rheumatol. 67, 1826–1836 (2015).
Zhang, M. et al. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J. Clin. Invest. 126, 2893–2902 (2016).
Gilbert, S. J. & Blain, E. J. in Mechanobiology in Health and Disease (ed. Verbruggen, S. W.) 99–126 (Elsevier, 2018).
Guilak, F., Nims, R. J., Dicks, A., Wu, C.-L. & Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 71–72, 40–50 (2018).
Vincent, T. L. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr. Opin. Pharmacol. 13, 449–454 (2013).
Agarwal, P. et al. A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat. Biomed. Eng. 5, 1472–1484 (2021).
Nims, R. J. et al. A synthetic mechanogenetic gene circuit for autonomous drug delivery in engineered tissues. Sci. Adv. 7, eabd9858 (2021).
Deng, Y. et al. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 9, 4564 (2018).
Eckstein, F. et al. Intra-articular sprifermin reduces cartilage loss in addition to increasing cartilage gain independent of location in the femorotibial joint: post-hoc analysis of a randomised, placebo-controlled phase II clinical trial. Ann. Rheum. Dis. 79, 525–528 (2020).
Peredo, A. P. et al. Mechano-activated biomolecule release in regenerating load-bearing tissue microenvironments. Biomaterials 265, 120255 (2021).
Nims, R. J., Pferdehirt, L. & Guilak, F. Mechanogenetics: harnessing mechanobiology for cellular engineering. Curr. Opin. Biotechnol. 73, 374–379 (2022).
Poole, A. R. et al. Composition and structure of articular cartilage: a template for tissue repair. Clin. Orthop. Relat. Res. 391, S26–S33 (2001).
Mow, V. C., Ratcliffe, A. & Poole, A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13, 67–97 (1992).
Schätti, O. R., Marková, M., Torzilli, P. A. & Gallo, L. M. Mechanical loading of cartilage explants with compression and sliding motion modulates gene expression of lubricin and catabolic enzymes. Cartilage 6, 185–193 (2015).
Melrose, J., Hayes, A. J., Whitelock, J. M. & Little, C. B. Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight‐bearing connective tissues. Bioessays 30, 457–469 (2008).
Poole, C. A. Articular cartilage chondrons: form, function and failure. J. Anat. 191, 1–13 (1997).
Schinagl, R. M., Gurskis, D., Chen, A. C. & Sah, R. L. Depth‐dependent confined compression modulus of full‐thickness bovine articular cartilage. J. Ortho Res. 15, 499–506 (1997).
Xia, Y., Moody, J. B., Alhadlaq, H. & Hu, J. Imaging the physical and morphological properties of a multi‐zone young articular cartilage at microscopic resolution. J. Mag. Reson. Imaging 17, 365–374 (2003).
Ratcliffe, A., Fryer, P. R. & Hardingham, T. E. The distribution of aggregating proteoglycans in articular cartilage: comparison of quantitative immunoelectron microscopy with radioimmunoassay and biochemical analysis. J. Histochem. Cytochem. 32, 193–201 (1984).
Maroudas, A., Muir, H. & Wingham, J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim. Biophys. Acta 177, 492–500 (1969).
Wilusz, R. E., Zauscher, S. & Guilak, F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage 21, 1895–1903 (2013).
Chery, D. R. et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta Biomater. 111, 267–278 (2020).
Simon, W. H. Scale effects in animal joints. I. Articular cartilage thickness and compressive stress. Arthritis Rheum. 13, 244–255 (1970).
Loeser, R. F. Integrins and cell signaling in chondrocytes. Biorheology 39, 119–124 (2002).
Millward-Sadler, S. J. & Salter, D. M. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann. Biomed. Eng. 32, 435–446 (2004).
Ross, T. D. et al. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25, 613–618 (2013).
Blain, E. J. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int. J. Exp. Pathol. 90, 1–15 (2009).
Barrett-Jolley, R., Lewis, R., Fallman, R. & Mobasheri, A. The emerging chondrocyte channelome. Front. Physiol. 1, 135 (2010).
Matta, C., Zákány, R. & Mobasheri, A. Voltage-dependent calcium channels in chondrocytes: roles in health and disease. Curr. Rheumatol. Rep. 17, 43 (2015).
Mobasheri, A. et al. The chondrocyte channelome: a narrative review. Jt. Bone Spine 86, 29–35 (2019).
Ruhlen, R. & Marberry, K. The chondrocyte primary cilium. Osteoarthritis Cartilage 22, 1071–1076 (2014).
Tao, F., Jiang, T., Tao, H., Cao, H. & Xiang, W. Primary cilia: versatile regulator in cartilage development. Cell Prolif. 53, e12765 (2020).
Guilak, F. et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann. N. Y. Acad. Sci. 1068, 498–512 (2006).
Youn, I., Choi, J., Cao, L., Setton, L. & Guilak, F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage 14, 889–897 (2006).
Martin, J., Miller, B., Scherb, M., Lembke, L. & Buckwalter, J. Co-localization of insulin-like growth factor binding protein 3 and fibronectin in human articular cartilage. Osteoarthritis Cartilage 10, 556–563 (2002).
Vincent, T., Hermansson, M., Bolton, M., Wait, R. & Saklatvala, J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc. Natl Acad. Sci. USA 99, 8259–8264 (2002).
Vincent, T. L., Hermansson, M. A., Hansen, U. N., Amis, A. A. & Saklatvala, J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 50, 526–533 (2004).
Vincent, T. L., McLean, C. J., Full, L. E., Peston, D. & Saklatvala, J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 15, 752–763 (2007).
Vincent, T. L. Fibroblast growth factor 2: good or bad guy in the joint? Arthritis Res. Ther. 13, 127 (2011).
Xie, Y., Zinkle, A., Chen, L. & Mohammadi, M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat. Rev. Rheumatol. 16, 547–564 (2020).
Makarenkova, H. P. et al. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55 (2009).
Eckstein, F., Wirth, W., Guermazi, A., Maschek, S. & Aydemir, A. Brief report: intraarticular sprifermin not only increases cartilage thickness, but also reduces cartilage loss: location‐independent post hoc analysis using magnetic resonance imaging. Arthritis Rheumatol. 67, 2916–2922 (2015).
Lohmander, L. S. et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double‐blind, placebo‐controlled trial. Arthritis Rheumatol. 66, 1820–1831 (2014).
Hochberg, M. C. et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA 322, 1360–1370 (2019).
Zhen, G. et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 12, 1706 (2021).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Seetharaman, S. & Etienne‐Manneville, S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell 110, 49–64 (2018).
Cantini, M., Donnelly, H., Dalby, M. J. & Salmeron‐Sanchez, M. The plot thickens: the emerging role of matrix viscosity in cell mechanotransduction. Adv. Healthc. Mater. 9, 1901259 (2020).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Puklin-Faucher, E. & Sheetz, M. P. The mechanical integrin cycle. J. Cell Sci. 122, 179–186 (2009).
Elosegui-Artola, A. et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 18, 540–548 (2016).
Elosegui-Artola, A. et al. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 13, 631–637 (2014).
Franz, F., Daday, C. & Gräter, F. Advances in molecular simulations of protein mechanical properties and function. Curr. Opin. Struct. Biol. 61, 132–138 (2020).
Gouttenoire, J. et al. BMP-2 and TGF-β1 differentially control expression of type II procollagen and α10 and α11 integrins in mouse chondrocytes. Eur. J. Cell Biol. 89, 307–314 (2010).
Salter, D., Hughes, D., Simpson, R. & Gardner, D. Integrin expression by human articular chondrocytes. Rheumatology 31, 231–234 (1992).
Zhang, W.-M. et al. Analysis of the human integrin α11 gene (ITGA11) and its promoter. Matrix Biol. 21, 513–523 (2002).
Loeser, R. F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 39, 11–16 (2014).
Orazizadeh, M. et al. CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res. Ther. 10, R4 (2008).
Ostergaard, K. et al. Expression of α and β subunits of the integrin superfamily in articular cartilage from macroscopically normal and osteoarthritic human femoral heads. Ann. Rheum. Dis. 57, 303–308 (1998).
Lucchinetti, E., Bhargava, M. M. & Torzilli, P. A. The effect of mechanical load on integrin subunits α5 and β1 in chondrocytes from mature and immature cartilage explants. Cell Tissue Res. 315, 385–391 (2004).
Millward-Sadler, S. et al. Integrin-regulated secretion of interleukin 4: a novel pathway of mechanotransduction in human articular chondrocytes. J. Cell Biol. 145, 183–189 (1999).
Millward‐Sadler, S., Wright, M., Davies, L., Nuki, G. & Salter, D. Mechanotransduction via integrins and interleukin‐4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 43, 2091–2099 (2000).
Steward, A. et al. Cell–matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater. 8, 2153–2159 (2012).
Jablonski, C. L., Ferguson, S., Pozzi, A. & Clark, A. L. Integrin α1β1 participates in chondrocyte transduction of osmotic stress. Biochem. Biophys. Res. Commun. 445, 184–190 (2014).
Wright, M. et al. Hyperpolarisation of cultured human chondrocytes following cyclical pressure‐induced strain: evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J. Ortho Res. 15, 742–747 (1997).
Camper, L., Hellman, U. & Lundgren-Åkerlund, E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 273, 20383–20389 (1998).
Bengtsson, T., Camper, L., Schneller, M. & Lundgren-Åkerlund, E. Characterization of the mouse integrin subunit α10 gene and comparison with its human homologue: genomic structure, chromosomal localization and identification of splice variants. Matrix Biol. 20, 565–576 (2001).
Lehnert, K. et al. Cloning, sequence analysis, and chromosomal localization of the novel human integrin α11 subunit (ITGA11). Genomics 60, 179–187 (1999).
Varas, L. et al. α10 integrin expression is up-regulated on fibroblast growth factor-2-treated mesenchymal stem cells with improved chondrogenic differentiation potential. Stem Cell Dev. 16, 965–978 (2007).
Delco, M. L. et al. Integrin α10β1-selected mesenchymal stem cells mitigate the progression of osteoarthritis in an equine talar impact model. Am. J. Sports Med. 48, 612–623 (2020).
Hirose, N. et al. Protective effects of cilengitide on inflammation in chondrocytes under excessive mechanical stress. Cell Biol. Int. 44, 966–974 (2020).
Chao, P. H., West, A. C. & Hung, C. T. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am. J. Physiol. Cell Physiol. 291, 718–725 (2006).
Erickson, G. R., Northrup, D. L. & Guilak, F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage 11, 187–197 (2003).
Grodzinsky, A. J., Levenston, M. E., Jin, M. & Frank, E. H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2, 691–713 (2000).
Guilak, F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J. Biomech. 28, 1529–1541 (1995).
Blain, E. J., Mason, D. J. & Duance, V. C. The effect of thymosin β4 on articular cartilage chondrocyte matrix metalloproteinase expression. Biochem. Soc. Trans. 30, 879–882 (2002).
Fioravanti, A., Nerucci, F., Annefeld, M., Collodel, G. & Marcolongo, R. Morphological and cytoskeletal aspects of cultivated normal and osteoarthritic human articular chondrocytes after cyclical pressure: a pilot study. Clin. Exp. Rheumatol. 21, 739–746 (2003).
Fioravanti, A., Benetti, D., Coppola, G. & Collodel, G. Effect of continuous high hydrostatic pressure on the morphology and cytoskeleton of normal and osteoarthritic human chondrocytes cultivated in alginate gels. Clin. Exp. Rheumatol. 23, 847–853 (2005).
Isermann, P. & Lammerding, J. Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol. 23, R1113–R1121 (2013).
Khilan, A. A., Al-Maslamani, N. A. & Horn, H. F. Cell stretchers and the LINC complex in mechanotransduction. Arch. Biochem. Biophys. 702, 108829 (2021).
Lee, D. A. et al. Chondrocyte deformation within compressed agarose constructs at the cellular and sub-cellular levels. J. Biomech. 33, 81–95 (2000).
Irianto, J. et al. Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys. J. 104, 759–769 (2013).
Hopewell, B. & Urban, J. P. Adaptation of articular chondrocytes to changes in osmolality. Biorheology 40, 73–77 (2003).
Hung, C. T. et al. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology 40, 61–72 (2003).
Killaars, A. R., Walker, C. J. & Anseth, K. S. Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling. Proc. Natl Acad. Sci. USA 117, 21258 (2020).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410 (2017).
Delco, M. L. & Bonassar, L. J. Targeting calcium-related mechanotransduction in early OA. Nat. Rev. Rheumatol. 17, 445–446 (2021).
O’Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B. & Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl Acad. Sci. USA 111, 1316–1321 (2014).
Phan, M. N. et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 60, 3028–3037 (2009).
Clark, A. L., Votta, B. J., Kumar, S., Liedtke, W. & Guilak, F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 62, 2973–2983 (2010).
O’Conor, C. J. et al. Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Sci. Rep. 6, 29053 (2016).
Drexler, S. K., Wann, A. K. T. & Vincent, T. L. Are cellular mechanosensors potential therapeutic targets in osteoarthritis. Int. J. Clin. Rheumatol. 9, 155–167 (2014).
Lee, W., Guilak, F. & Liedtke, W. Role of Piezo channels in joint health and injury. Curr. Top. Membr. 79, 263–273 (2017).
Sun, Y. et al. Mechanism of abnormal chondrocyte proliferation induced by Piezo1-siRNA exposed to mechanical stretch. BioMed. Res. Int. 2020, 8538463 (2020).
Lee, W. et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl Acad. Sci. USA 111, E5114–E5122 (2014).
Gnanasambandam, R. et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys. J. 112, 31–45 (2017).
Suchyna, T. M. Piezo channels and GsMTx4: two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Biol. 130, 244–253 (2017).
Xiao, W. F., Li, Y. S., Deng, A., Yang, Y. T. & He, M. Functional role of hedgehog pathway in osteoarthritis. Cell Biochem. Funct. 38, 122–129 (2020).
McGlashan, S. R., Cluett, E. C., Jensen, C. G. & Poole, C. A. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev. Dyn. 237, 2013–2020 (2008).
McGlashan, S. R., Jensen, C. G. & Poole, C. A. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 54, 1005–1014 (2006).
Chang, C. F., Ramaswamy, G. & Serra, R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage 20, 152–161 (2012).
Irianto, J., Ramaswamy, G., Serra, R. & Knight, M. M. Depletion of chondrocyte primary cilia reduces the compressive modulus of articular cartilage. J. Biomech. 47, 579–582 (2014).
Wann, A. K. T. et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 26, 1663–1671 (2012).
Shao, Y. Y., Wang, L., Welter, J. F. & Ballock, R. T. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50, 79–84 (2012).
Pingguan‐Murphy, B., El‐Azzeh, M., Bader, D. & Knight, M. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate‐and frequency‐dependent manner. J. Cell Physiol. 209, 389–397 (2006).
Zhang, J. et al. Connexin43 hemichannels mediate small molecule exchange between chondrocytes and matrix in biomechanically-stimulated temporomandibular joint cartilage. Osteoarthritis Cartilage 22, 822–830 (2014).
Garcia, M. & Knight, M. M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 28, 510–515 (2010).
Chowdhury, T. & Knight, M. Purinergic pathway suppresses the release of NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J. Cell Physiol. 209, 845–853 (2006).
Huang, C., Holfeld, J., Schaden, W., Orgill, D. & Ogawa, R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 19, 555–564 (2013).
Thompson, W. R., Scott, A., Loghmani, M. T., Ward, S. R. & Warden, S. J. Understanding mechanobiology: physical therapists as a force in mechanotherapy and musculoskeletal regenerative rehabilitation. Phys. Ther. 96, 560–569 (2016).
Dell’Accio, F., De Bari, C., Eltawil, N. M., Vanhummelen, P. & Pitzalis, C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 58, 1410–1421 (2008).
Loeser, R. F., Erickson, E. A. & Long, D. L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 20, 581–586 (2008).
Fanning, P. J. et al. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J. Biol. Chem. 278, 50940–50948 (2003).
Forsyth, C. B., Pulai, J. & Loeser, R. F. Fibronectin fragments and blocking antibodies to α2β1 and α5β1 integrins stimulate mitogen‐activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 46, 2368–2376 (2002).
Im, H.-J. et al. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J. Biol. Chem. 278, 25386–25394 (2003).
Loeser, R. F., Forsyth, C. B., Samarel, A. M. & Im, H.-J. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J. Biol. Chem. 278, 24577–24585 (2003).
Pulai, J. I. et al. NF-κB mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 174, 5781–5788 (2005).
Del Carlo, M., Schwartz, D., Erickson, E. A. & Loeser, R. F. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free. Radic. Biol. Med. 42, 1350–1358 (2007).
Long, D. L., Willey, J. S. & Loeser, R. F. Rac1 is required for matrix metalloproteinase 13 production by chondrocytes in response to fibronectin fragments. Arthritis Rheum. 65, 1561–1568 (2013).
Gemba, T., Valbracht, J., Alsalameh, S. & Lotz, M. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J. Biol. Chem. 277, 907–911 (2002).
Ding, L., Guo, D. & Homandberg, G. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage 16, 1253–1262 (2008).
Ding, L., Guo, D. & Homandberg, G. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-src, NF-κB and protein kinase Cδ. Osteoarthritis Cartilage 17, 1385–1392 (2009).
Fitzgerald, J. B. et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J. Biol. Chem. 283, 6735–6743 (2008).
Zhang, J., Shen, B. & Lin, A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol. Sci. 28, 286–295 (2007).
González-Vázquez, A. et al. Accelerating bone healing in vivo by harnessing the age-altered activation of c-Jun N-terminal kinase 3. Biomaterials 268, 120540 (2021).
Agarwal, S. et al. A central role for the nuclear factor-κB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 17, 899–901 (2003).
Yang, Y. et al. Mechanical stress protects against osteoarthritis via regulation of the AMPK/NF-κB signaling pathway. J. Cell Physiol. 234, 9156–9167 (2019).
Vincent, T. L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 49, S36–S38 (2019).
Ismail, H. M., Didangelos, A., Vincent, T. L. & Saklatvala, J. Rapid activation of transforming growth factor β-activated kinase 1 in chondrocytes by phosphorylation and K(63)-linked polyubiquitination upon injury to animal articular cartilage. Arthritis Rheumatol. 69, 565–575 (2017).
Lee, W. et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc. Natl Acad. Sci. USA 118, e2001611118 (2021).
Nam, S. et al. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27(Kip1) signaling axis. Sci. Adv. 5, eaaw6171 (2019).
Lee, H. P., Gu, L., Mooney, D. J., Levenston, M. E. & Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243–1251 (2017).
Miller, J. R. The Wnts. Genome Biol. 3, reviews3001.1 (2001).
Blom, A. B. et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt‐induced signaling protein 1. Arthritis Rheum. 60, 501–512 (2009).
De Santis, M. et al. The role of Wnt pathway in the pathogenesis of OA and its potential therapeutic implications in the field of regenerative medicine. BioMed. Res. Int. 2018, 7402947 (2018).
Dell’Accio, F. et al. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res. Ther. 8, R139 (2006).
Bougault, C. et al. Protective role of frizzled-related protein B on matrix metalloproteinase induction in mouse chondrocytes. Arthritis Res. Ther. 16, R137 (2014).
Nalesso, G. et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 76, 218–226 (2017).
Wang, Y., Fan, X., Xing, L. & Tian, F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun. Signal. 17, 97 (2019).
Lories, R. J. & Monteagudo, S. Review article: is Wnt signaling an attractive target for the treatment of osteoarthritis? Rheumatol. Ther. 7, 259–270 (2020).
Monteagudo, S. & Lories, R. J. Cushioning the cartilage: a canonical Wnt restricting matter. Nat. Rev. Rheumatol. 13, 670–681 (2017).
Deshmukh, V. et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 26, 18–27 (2018).
Yazici, Y. et al. A phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthritis Cartilage 29, 654–666 (2021).
Deshmukh, V. et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthritis Cartilage 27, 1347–1360 (2019).
Monteagudo, S. et al. DOT1L safeguards cartilage homeostasis and protects against osteoarthritis. Nat. Commun. 8, 15889 (2017).
Cornelis, F. M. F. et al. Increased susceptibility to develop spontaneous and post-traumatic osteoarthritis in Dot1l-deficient mice. Osteoarthritis Cartilage 27, 513–525 (2019).
Castaño Betancourt, M. C. et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc. Natl Acad. Sci. USA 109, 8218–8223 (2012).
Deng, Y. et al. Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep. 14, 2224–2237 (2016).
Gumbiner, B. M. & Kim, N.-G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 127, 709–717 (2014).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Baker, B. M. & Chen, C. S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125, 3015–3024 (2012).
Caliari, S. R., Vega, S. L., Kwon, M., Soulas, E. M. & Burdick, J. A. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 103, 314–323 (2016).
Karystinou, A. et al. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res. Ther. 17, 147 (2015).
Mobasheri, A. et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13, 302–311 (2017).
Salinas, D., Mumey, B. M. & June, R. K. Physiological dynamic compression regulates central energy metabolism in primary human chondrocytes. Biomech. Model. Mechanobiol. 18, 69–77 (2019).
Lehtinen, M. K. et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125, 987–1001 (2006).
Niehoff, A. et al. Dynamic and static mechanical compression affects Akt phosphorylation in porcine patellofemoral joint cartilage. J. Orthop. Res. 26, 616–623 (2008).
Holledge, M. M., Millward-Sadler, S. J., Nuki, G. & Salter, D. M. Mechanical regulation of proteoglycan synthesis in normal and osteoarthritic human articular chondrocytes–roles for α5 and αVβ5 integrins. Biorheology 45, 275–288 (2008).
Delco, M. L., Bonnevie, E. D., Bonassar, L. J. & Fortier, L. A. Mitochondrial dysfunction is an acute response of articular chondrocytes to mechanical injury. J. Orthop. Res. 36, 739–750 (2018).
Waller, K. A., Zhang, L. X. & Jay, G. D. Friction-induced mitochondrial dysregulation contributes to joint deterioration in Prg4 knockout mice. Int. J. Mol. Sci. 18, 1252 (2017).
Bartell, L. R. et al. Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J. Orthop. Res. 38, 1257–1267 (2020).
Jutila, A. A. et al. Candidate mediators of chondrocyte mechanotransduction via targeted and untargeted metabolomic measurements. Arch. Biochem. Biophys. 545, 116–123 (2014).
Zignego, D. L., Jutila, A. A., Gelbke, M. K., Gannon, D. M. & June, R. K. The mechanical microenvironment of high concentration agarose for applying deformation to primary chondrocytes. J. Biomech. 47, 2143–2148 (2014).
Hodgkinson, T. et al. The use of nanovibration to discover specific and potent bioactive metabolites that stimulate osteogenic differentiation in mesenchymal stem cells. Sci. Adv. 7, eabb7921 (2021).
Bonnevie, E. D. et al. Microscale frictional strains determine chondrocyte fate in loaded cartilage. J. Biomech. 74, 72–78 (2018).
Irwin, R. M. et al. Distinct tribological endotypes of pathological human synovial fluid reveal characteristic biomarkers and variation in efficacy of viscosupplementation at reducing local strains in articular cartilage. Osteoarthritis Cartilage 28, 492–501 (2020).
Xie, R. et al. Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nat. Biomed. Eng. 5, 1189–1201 (2021).
Grither, W. R. & Longmore, G. D. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc. Natl Acad. Sci. USA 115, E7786–E7794 (2018).
Kumar, A., Choudhury, M. D., Ghosh, P. & Palit, P. Discoidin domain receptor 2: an emerging pharmacological drug target for prospective therapy against osteoarthritis. Pharmacol. Rep. 71, 399–408 (2019).
Occhetta, P. et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat. Biomed. Eng. 3, 545–557 (2019).
Lee, J. et al. Combinatorial screening of biochemical and physical signals for phenotypic regulation of stem cell-based cartilage tissue engineering. Sci. Adv. 6, eaaz5913 (2020).
Wang, J., Lü, D., Mao, D. & Long, M. Mechanomics: an emerging field between biology and biomechanics. Protein Cell 5, 518–531 (2014).
Gabriel, S. E., Crowson, C. S. & O’Fallon, W. M. Comorbidity in arthritis. J. Rheumatol. 26, 2475–2479 (1999).
Shi, S., Man, Z., Li, W., Sun, S. & Zhang, W. Silencing of Wnt5a prevents interleukin-1β-induced collagen type II degradation in rat chondrocytes. Exp. Ther. Med. 12, 3161–3166 (2016).
Yan, H. et al. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl Acad. Sci. USA 113, E6199–E6208 (2016).
Rai, M. F. et al. Applications of RNA interference in the treatment of arthritis. Transl. Res. 214, 1–16 (2019).
Cheleschi, S. et al. Hydrostatic pressure regulates microRNA expression levels in osteoarthritic chondrocyte cultures via the Wnt/β-catenin pathway. Int. J. Mol. Sci. 18, 133 (2017).
De Palma, A. et al. Hydrostatic pressure as epigenetic modulator in chondrocyte cultures: a study on miRNA-155, miRNA-181a and miRNA-223 expression levels. J. Biomech. 66, 165–169 (2018).
Yang, X. et al. Mechanical and IL-1β responsive miR-365 contributes to osteoarthritis development by targeting histone deacetylase 4. Int. J. Mol. Sci. 17, 436 (2016).
Stadnik, P. S. et al. Regulation of microRNA-221, -222, -21 and -27 in articular cartilage subjected to abnormal compressive forces. J. Physiol. 599, 143–155 (2021).
Dunn, W., DuRaine, G. & Reddi, A. H. Profiling microRNA expression in bovine articular cartilage and implications for mechanotransduction. Arthritis Rheum. 60, 2333–2339 (2009).
Iliopoulos, D., Malizos, K. N., Oikonomou, P. & Tsezou, A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS ONE 3, e3740 (2008).
Song, J. et al. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 3, 79–89 (2014).
Hecht, N., Johnstone, B., Angele, P., Walker, T. & Richter, W. Mechanosensitive MiRs regulated by anabolic and catabolic loading of human cartilage. Osteoarthritis Cartilage 27, 1208–1218 (2019).
Lolli, A., Colella, F., De Bari, C. & van Osch, G. J. V. M. Targeting anti-chondrogenic factors for the stimulation of chondrogenesis: a new paradigm in cartilage repair. J. Orthop. Res. 37, 12–22 (2019).
Mohanraj, B. et al. Mechanically activated microcapsules for “on-demand” drug delivery in dynamically loaded musculoskeletal tissues. Adv. Funct. Mater. 29, 1807909 (2019).
Cambré, I. et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 9, 4613 (2018).
Lin, X., Bai, Y., Zhou, H. & Yang, L. Mechano-active biomaterials for tissue repair and regeneration. J. Mater. Sci. Technol. 59, 227–233 (2020).
Zhang, Y., Yu, J., Bomba, H. N., Zhu, Y. & Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 116, 12536–12563 (2016).
Xiao, L. et al. Hyaluronic acid-based hydrogels containing covalently integrated drug depots: implication for controlling inflammation in mechanically stressed tissues. Biomacromolecules 14, 3808–3819 (2013).
Acknowledgements
T.H. is in receipt of a Marie Skłodowska-Curie Individual Fellowship from the European Commission through the Horizon 2020 project ChondroCONNECT (project ID: 894837). The work of D.C.K. is funded by Science Foundation Ireland under the European Regional Development Fund CÚRAM (13/RC/2073) and the Irish Research Council Government of Ireland Postgraduate Programme (GOIPG/2018/371). C.M.C. recognizes funding from the Irish Health Research Board (ILP-POR-2019-023 and ILP-POR-2017-032). F.J.O'B. acknowledges funding from the European Commission Horizon 2020 research and innovation programme under the European Research Council Advanced Grant (agreement ID: 788753, ReCaP) and from the Irish Health Research Board (ILP-POR-2017-032).
Author information
Authors and Affiliations
Contributions
T.H. and D.C.K. wrote the manuscript. All authors researched data for the article, made a substantial contribution to discussion of content and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks L. Bonassar, F. Guilak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Shear
-
A force or stress that is applied coplanar with the cross-section of the tissue.
- Stretch
-
Deformation resulting in the final length of a material being greater than the initial length.
- Pressure
-
A force applied acting inwards normal to any surface divided by the area of the surface.
- Strain
-
The deformation of a material from stress.
- F-actin rarefication
-
A process resulting in a reduction in F-actin abundance within a cell.
- Tribology
-
The science and engineering of interacting surfaces (wear, friction and lubrication) and how they behave in relative motion.
Rights and permissions
About this article
Cite this article
Hodgkinson, T., Kelly, D.C., Curtin, C.M. et al. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat Rev Rheumatol 18, 67–84 (2022). https://doi.org/10.1038/s41584-021-00724-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00724-w
This article is cited by
-
FTH1 protects against osteoarthritis by MAPK pathway inhibition of extracellular matrix degradation
BMC Musculoskeletal Disorders (2024)
-
Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway
Scientific Reports (2024)
-
Comparison studies identify mesenchymal stromal cells with potent regenerative activity in osteoarthritis treatment
npj Regenerative Medicine (2024)
-
Immediate and Delayed Effects of Joint Loading Activities on Knee and Hip Cartilage: A Systematic Review and Meta-analysis
Sports Medicine - Open (2023)
-
Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: a brief review
Biomaterials Research (2023)