Abstract
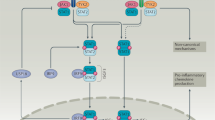
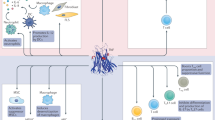
Interferon-γ (IFNγ) is a pleiotropic cytokine with multiple effects on the inflammatory response and on innate and adaptive immunity. Overproduction of IFNγ underlies several, potentially fatal, hyperinflammatory or immune-mediated diseases. Several data from animal models and/or from translational research in patients point to a role of IFNγ in hyperinflammatory diseases, such as primary haemophagocytic lymphohistiocytosis, various forms of secondary haemophagocytic lymphohistiocytosis, including macrophage activation syndrome, and cytokine release syndrome, all of which are often managed by rheumatologists or in consultation with rheumatologists. Given the effects of IFNγ on B cells and T follicular helper cells, a role for IFNγ in systemic lupus erythematosus pathogenesis is emerging. To improve our understanding of the role of IFNγ in human disease, IFNγ-related biomarkers that are relevant for the management of hyperinflammatory diseases are progressively being identified and studied, especially because circulating levels of IFNγ do not always reflect its overproduction in tissue. These biomarkers include STAT1 (specifically the phosphorylated form), neopterin and the chemokine CXCL9. IFNγ-neutralizing agents have shown efficacy in the treatment of primary haemophagocytic lymphohistiocytosis in clinical trials and initial promising results have been obtained in various forms of secondary haemophagocytic lymphohistiocytosis, including macrophage activation syndrome. In clinical practice, there is a growing body of evidence supporting the usefulness of circulating CXCL9 levels as a biomarker reflecting IFNγ production.
Key points
-
Hyperinflammation is characterized by excessive systemic inflammation resulting from an exaggerated immune response to physiological stimuli, which eventually leads to tissue damage.
-
Haemaphagocytic lymphohistiocytoses (HLHs) are hyperinflammatory diseases and are considered canonical cytokine release syndromes.
-
Studies of interferon-γ (IFNγ) biology have shed light on the role of this immune modulator in various hyperinflammatory diseases, such as the different forms of HLH, and also in other immune-mediated diseases.
-
IFNγ hyperproduction is a feature of the different forms of HLH, and IFNγ neutralization decreases disease severity and reverts lethality in mouse models of HLH.
-
As serum IFNγ levels do not always reflect IFNγ overproduction in tissue in patients, efforts are underway to identify biomarkers that are easily measurable in blood and reflect tissue IFNγ activity.
-
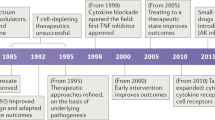
Therapeutic IFNγ neutralization shows promising results in early clinical trials in patients with various forms of HLH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kastner, D. L., Aksentijevich, I. & Goldbach-Mansky, R. Autoinflammatory disease reloaded: a clinical perspective. Cell 140, 784–790 (2010).
Martinez-Quiles, N. & Goldbach-Mansky, R. Updates on autoinflammatory diseases. Curr. Opin. Immunol. 55, 97–105 (2018).
Henderson, L. A. et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 72, 1059–1063 (2020).
Schoenborn, J. R. & Wilson, C. B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007).
Darwich, L. et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 126, 386–393 (2009).
Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673 (2012).
Suarez-Ramirez, J. E., Tarrio, M. L., Kim, K., Demers, D. A. & Biron, C. A. CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection. mBio 5, e01978-01914 (2014).
Kannan, Y. et al. IκBζ augments IL-12- and IL-18-mediated IFN-γ production in human NK cells. Blood 117, 2855–2863 (2011).
Nakanishi, K. Unique action of interleukin-18 on T cells and other immune cells. Front. Immunol. 9, 763 (2018).
de Weerd, N. A. & Nguyen, T. The interferons and their receptors — distribution and regulation. Immunol. Cell Biol. 90, 483–491 (2012).
Chistiakov, D. A., Myasoedova, V. A., Revin, V. V., Orekhov, A. N. & Bobryshev, Y. V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 223, 101–111 (2018).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Wu, C. et al. IFN-γ primes macrophage activation by increasing phosphatase and tensin homolog via downregulation of miR-3473b. J. Immunol. 193, 3036–3044 (2014).
Asano, M., Nakane, A. & Minagawa, T. Endogenous gamma interferon is essential in granuloma formation induced by glycolipid-containing mycolic acid in mice. Infect. Immun. 61, 2872–2878 (1993).
Jaczewska, J. et al. TNF-α and IFN-γ promote lymphocyte adhesion to endothelial junctional regions facilitating transendothelial migration. J. Leukoc. Biol. 95, 265–274 (2014).
Metzemaekers, M., Vanheule, V., Janssens, R., Struyf, S. & Proost, P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front. Immunol. 8, 1970 (2017).
Pearl, J. E., Saunders, B., Ehlers, S., Orme, I. M. & Cooper, A. M. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol. 211, 43–50 (2001).
Swindle, E. J., Brown, J. M., Rådinger, M., DeLeo, F. R. & Metcalfe, D. D. Interferon-γ enhances both the anti-bacterial and the pro-inflammatory response of human mast cells to Staphylococcus aureus. Immunology 146, 470–485 (2015).
Lee, S. K. et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 37, 880–892 (2012).
Fang, D. et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. J. Exp. Med. 215, 2705–2714 (2018).
Jackson, S. W. et al. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J. Exp. Med. 213, 733–750 (2016).
Peng, S. L., Szabo, S. J. & Glimcher, L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl Acad. Sci. USA 99, 5545–5550 (2002).
Canna, S. W. & Marsh, R. A. Pediatric hemophagocytic lymphohistiocytosis. Blood 135, 1332–1343 (2020).
Bhatt, N. S., Oshrine, B. & An Talano, J. Hemophagocytic lymphohistiocytosis in adults. Leuk. Lymphoma 60, 19–28 (2019).
Henter, J. I. et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 48, 124–131 (2007).
Emile, J. F. et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 127, 2672–2681 (2016).
Grom, A. A., Horne, A. & De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 12, 259–268 (2016).
Bracaglia, C., Prencipe, G. & De Benedetti, F. Macrophage activation syndrome: different mechanisms leading to a one clinical syndrome. Pediatr. Rheumatol. Online J. 15, 5 (2017).
Ravelli, A. et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann. Rheum. Dis. 75, 481–489 (2016).
Bergsten, E. et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood 130, 2728–2738 (2017).
Henter, J. I. et al. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood 78, 2918–2922 (1991).
Imashuku, S. et al. Biomarker and morphological characteristics of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis. Med. Pediatr. Oncol. 31, 131–137 (1998).
Schneider, E. M. et al. Hemophagocytic lymphohistiocytosis is associated with deficiencies of cellular cytolysis but normal expression of transcripts relevant to killer-cell-induced apoptosis. Blood 100, 2891–2898 (2002).
Billiau, A. D., Roskams, T., Van Damme-Lombaerts, R., Matthys, P. & Wouters, C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood 105, 1648–1651 (2005).
Xu, X. J. et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J. Pediatr. 160, 984–990.e1 (2012).
Prencipe, G. et al. The interferon-gamma pathway is selectively up-regulated in the liver of patients with secondary hemophagocytic lymphohistiocytosis. PLoS ONE 14, e0226043 (2019).
Yang, S. L. et al. Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine 85, 14–17 (2016).
Buatois, V. et al. Use of a mouse model to identify a blood biomarker for IFNγ activity in pediatric secondary hemophagocytic lymphohistiocytosis. Transl. Res. 180, 37–52.e2 (2017).
Bracaglia, C. et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 76, 166–172 (2017).
Jordan, M. B., Hildeman, D., Kappler, J. & Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 104, 735–743 (2004).
Rood, J. E. et al. ST2 contributes to T-cell hyperactivation and fatal hemophagocytic lymphohistiocytosis in mice. Blood 127, 426–435 (2016).
Kögl, T. et al. Hemophagocytic lymphohistiocytosis in syntaxin-11-deficient mice: T-cell exhaustion limits fatal disease. Blood 121, 604–613 (2013).
Pachlopnik Schmid, J. et al. Neutralization of IFNγ defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol. Med. 1, 112–124 (2009).
Zoller, E. E. et al. Hemophagocytosis causes a consumptive anemia of inflammation. J. Exp. Med. 208, 1203–1214 (2011).
van Dommelen, S. L. et al. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity 25, 835–848 (2006).
Humblet-Baron, S. et al. IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis. J. Allergy Clin. Immunol. 143, 2215–2226.e7 (2019).
Burn, T. N. et al. Genetic deficiency of interferon-gamma reveals interferon-gamma-independent manifestations of murine hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 72, 335–347 (2020).
Tesi, B. et al. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-gamma receptor deficiency. J. Allergy Clin. Immunol. 135, 1638–1641 (2015).
Behrens, E. M. et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J. Clin. Invest. 121, 2264–2277 (2011).
Weaver, L. K., Chu, N. & Behrens, E. M. Brief report: interferon-γ-mediated immunopathology potentiated by toll-like receptor 9 activation in a murine model of macrophage activation syndrome. Arthritis Rheumatol. 71, 161–168 (2019).
Brisse, E. et al. Mouse cytomegalovirus infection in BALB/c mice resembles virus-associated secondary hemophagocytic lymphohistiocytosis and shows a pathogenesis distinct from primary hemophagocytic lymphohistiocytosis. J. Immunol. 196, 3124–3134 (2016).
Brisse, E. et al. Lytic viral replication and immunopathology in a cytomegalovirus-induced mouse model of secondary hemophagocytic lymphohistiocytosis. Virol. J. 14, 240 (2017).
de Benedetti, F. et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 34, 1158–1163 (1991).
De Benedetti, F. et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 367, 2385–2395 (2012).
Strippoli, R. et al. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 64, 1680–1688 (2012).
Prencipe, G. et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J. Allergy Clin. Immunol. 141, 1439–1449 (2018).
Weiss, E. S. et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 131, 1442–1455 (2018).
Yasin, S. et al. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology 59, 361–366 (2020).
Girard-Guyonvarc’h, C. et al. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood 131, 1430–1441 (2018).
Tsoukas, P. et al. Interleukin-18 and cytotoxic impairment are independent and synergistic causes of murine virus-induced hyperinflammation. Blood 136, 2162–2174 (2020).
Canna, S. W. et al. Proceedings from the 2nd next gen therapies for systemic juvenile idiopathic arthritis and macrophage activation syndrome symposium held on October 3–4, 2019. Pediatr. Rheumatol. Online J. 18, 53 (2020).
Schulert, G. S. et al. Systemic juvenile idiopathic arthritis-associated lung disease: characterization and risk factors. Arthritis Rheumatol. 71, 1943–1954 (2019).
Saper, V. E. et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann. Rheum. Dis. 78, 1722–1731 (2019).
Iriguchi, S. et al. T-cell-restricted T-bet overexpression induces aberrant hematopoiesis of myeloid cells and impairs function of macrophages in the lung. Blood 125, 370–382 (2015).
Gao, D. K. et al. IFN-γ is essential for alveolar macrophage driven pulmonary inflammation in macrophage activation syndrome. JCI Insight 6, 147593 (2021).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121, 5154–5157 (2013).
Brady, J. L. et al. Preclinical screening for acute toxicity of therapeutic monoclonal antibodies in a hu-SCID model. Clin. Transl. Immunol. 3, e29 (2014).
Oved, J. H., Barrett, D. M. & Teachey, D. T. Cellular therapy: immune-related complications. Immunol. Rev. 290, 114–126 (2019).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Wouters, C. H., Maes, A., Foley, K. P., Bertin, J. & Rose, C. D. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr. Rheumatol. Online J. 12, 33 (2014).
Takada, S. et al. Pluripotent stem cell models of Blau syndrome reveal an IFN-γ-dependent inflammatory response in macrophages. J. Allergy Clin. Immunol. 141, 339–349.e11 (2018).
Rosenzweig, H. L. et al. Nucleotide oligomerization domain-2 (NOD2)-induced uveitis: dependence on IFN-gamma. Invest. Ophthalmol. Vis. Sci. 50, 1739–1745 (2009).
Janssen, C. E. et al. Morphologic and immunohistochemical characterization of granulomas in the nucleotide oligomerization domain 2-related disorders Blau syndrome and Crohn disease. J. Allergy Clin. Immunol. 129, 1076–1084 (2012).
Sarens, I. L. et al. Blau syndrome-associated uveitis: preliminary results from an international prospective interventional case series. Am. J. Ophthalmol. 187, 158–166 (2018).
Seery, J. P. et al. A central role for alpha beta T cells in the pathogenesis of murine lupus. J. Immunol. 162, 7241–7248 (1999).
Enghard, P., Langnickel, D. & Riemekasten, G. T cell cytokine imbalance towards production of IFN-gamma and IL-10 in NZB/W F1 lupus-prone mice is associated with autoantibody levels and nephritis. Scand. J. Rheumatol. 35, 209–216 (2006).
Jacob, C. O., van der Meide, P. H. & McDevitt, H. O. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J. Exp. Med. 166, 798–803 (1987).
Ozmen, L. et al. Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferon-gamma receptor inhibits the onset of glomerulonephritis. Eur. J. Immunol. 25, 6–12 (1995).
Haas, C. & Ryffel, B. & Le Hir, M. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW)F1 mice. J. Immunol. 160, 3713–3718 (1998).
Balomenos, D., Rumold, R. & Theofilopoulos, A. N. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J. Clin. Invest. 101, 364–371 (1998).
Domeier, P. P. et al. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J. Exp. Med. 213, 715–732 (2016).
Rana, A. et al. Gene expression of cytokines (TNF-α, IFN-γ), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus 21, 1105–1112 (2012).
Lyn-Cook, B. D. et al. Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol. Immunol. 61, 38–43 (2014).
Kokic, V. et al. Relationship between vitamin D, IFN-γ, and E2 levels in systemic lupus erythematosus. Lupus 25, 282–288 (2016).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Chiche, L., Jourde-Chiche, N., Pascual, V. & Chaussabel, D. Current perspectives on systems immunology approaches to rheumatic diseases. Arthritis Rheum. 65, 1407–1417 (2013).
Zhu, J., Huang, X. & Yang, Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. 16, 1300–1307 (2008).
Fava, A. et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 5, e138345 (2020).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Furie, R. A. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 1, e208–e219 (2019).
Lortat-Jacob, H., Baltzer, F. & Grimaud, J. A. Heparin decreases the blood clearance of interferon-gamma and increases its activity by limiting the processing of its carboxyl-terminal sequence. J. Biol. Chem. 271, 16139–16143 (1996).
Rutenfranz, I. & Kirchner, H. Pharmacokinetics of recombinant murine interferon-gamma in mice. J. Interferon Res. 8, 573–580 (1988).
Pascarella, A. et al. Monocytes from patients with macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis are hyperresponsive to interferon gamma. Front. Immunol. 12, 801 (2021).
Hu, X., Li, W. P., Meng, C. & Ivashkiv, L. B. Inhibition of IFN-γ signaling by glucocorticoids. J. Immunol. 170, 4833–4839 (2003).
Ibarra, M. F. et al. Serum neopterin levels as a diagnostic marker of hemophagocytic lymphohistiocytosis syndrome. Clin. Vaccin. Immunol. 18, 609–614 (2011).
Wada, T. et al. Cytokine profiles in children with primary Epstein-Barr virus infection. Pediatr. Blood Cancer 60, E46–E48 (2013).
Shimizu, M. et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology 49, 1645–1653 (2010).
Mizuta, M. et al. Comparison of serum cytokine profiles in macrophage activation syndrome complicating different background rheumatic diseases in children. Rheumatology https://doi.org/10.1093/rheumatology/keaa299 (2020).
de Matteis, A. et al. Traditional laboratory parameters and new biomarkers in macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 72 (suppl 4). https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis/ (2020).
Lim, K. L., Jones, A. C., Brown, N. S. & Powell, R. J. Urine neopterin as a parameter of disease activity in patients with systemic lupus erythematosus: comparisons with serum sIL-2R and antibodies to dsDNA, erythrocyte sedimentation rate, and plasma C3, C4, and C3 degradation products. Ann. Rheum. Dis. 52, 429–435 (1993).
Whyte, M. P. et al. Unique variant of NOD2 pediatric granulomatous arthritis with severe 1,25-dihydroxyvitamin D-mediated hypercalcemia and generalized osteosclerosis. J. Bone Min. Res. 33, 2071–2080 (2018).
Groom, J. R. & Luster, A. D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011).
Mizuta, M., Shimizu, M., Inoue, N., Nakagishi, Y. & Yachie, A. Clinical significance of serum CXCL9 levels as a biomarker for systemic juvenile idiopathic arthritis associated macrophage activation syndrome. Cytokine 119, 182–187 (2019).
My, L. T. et al. Comprehensive analyses and characterization of haemophagocytic lymphohistiocytosis in Vietnamese children. Br. J. Haematol. 148, 301–310 (2010).
Takada, H. et al. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin. Exp. Immunol. 133, 448–453 (2003).
Han, J. H. et al. Elevated circulating levels of the interferon-γ-induced chemokines are associated with disease activity and cutaneous manifestations in adult-onset Still’s disease. Sci. Rep. 7, 46652 (2017).
Takakura, M. et al. Comparison of serum biomarkers for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Clin. Immunol. 208, 108252 (2019).
Schulert, G. S. et al. Monocyte and bone marrow macrophage transcriptional phenotypes in systemic juvenile idiopathic arthritis reveal TRIM8 as a mediator of IFN-γ hyper-responsiveness and risk for macrophage activation syndrome. Ann. Rheum. Dis. 80, 617–625 (2021).
Carvalheiro, T. et al. Tolerogenic versus inflammatory activity of peripheral blood monocytes and dendritic cells subpopulations in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 934161 (2012).
Ferreira, G. A., Teixeira, A. L. & Sato, E. I. Atorvastatin therapy reduces interferon-regulated chemokine CXCL9 plasma levels in patients with systemic lupus erythematosus. Lupus 19, 927–934 (2010).
Locatelli, F. et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med. 382, 1811–1822 (2020).
Lounder, D. T., Bin, Q., de Min, C. & Jordan, M. B. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 3, 47–50 (2019).
Bracaglia, C. et al. IFNg neutralization in patients with NRLC4- and CDC42-associated autoinflammatory disease characterized by recurrent and severe HLH. Pediatr. Blood Cancer 67 (Suppl 1), e28077 (2019).
Lam, M. T. et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 216, 2778–2799 (2019).
De Benedetti, F. et al. Emapalumab (anti-interferon-gamma monoclonal antibody) in patients with macrophage activation syndrome (MAS) complicating systemic juvenile idiopathic arthritis (sJIA). Ann. Rheum. Dis. 79, 180 (2020).
Gabr, J. B. et al. Successful treatment of secondary macrophage activation syndrome with emapalumab in a patient with newly diagnosed adult-onset Still’s disease: case report and review of the literature. Ann. Transl. Med. 8, 887 (2020).
Boedigheimer, M. J. et al. Safety, pharmacokinetics and pharmacodynamics of AMG 811, an anti-interferon-γ monoclonal antibody, in SLE subjects without or with lupus nephritis. Lupus Sci. Med. 4, e000226 (2017).
Chen, P. et al. Pharmacokinetic and pharmacodynamic relationship of AMG 811, an anti-IFN-gamma IgG1 monoclonal antibody, in patients with systemic lupus erythematosus. Pharm. Res. 32, 640–653 (2015).
Bozza, S. et al. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood 108, 3387–3396 (2006).
Presti, R. M., Pollock, J. L., Dal Canto, A. J., O’Guin, A. K. & Virgin, H. W. T. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188, 577–588 (1998).
Bao, S., Beagley, K. W., France, M. P., Shen, J. & Husband, A. J. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99, 464–472 (2000).
Fidan, I., Yesilyurt, E., Gurelik, F. C., Erdal, B. & Imir, T. Effects of recombinant interferon-gamma on cytokine secretion from monocyte-derived macrophages infected with Salmonella typhi. Comp. Immunol. Microbiol. Infect. Dis. 31, 467–475 (2008).
Salat, J., Jelinek, J., Chmelar, J. & Kopecky, J. Efficacy of gamma interferon and specific antibody for treatment of microsporidiosis caused by Encephalitozoon cuniculi in SCID mice. Antimicrob. Agents Chemother. 52, 2169–2174 (2008).
Sumaria, N. et al. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol. Cell Biol. 87, 559–566 (2009).
Dorman, S. E. et al. Viral infections in interferon-gamma receptor deficiency. J. Pediatr. 135, 640–643 (1999).
Tran, D. Q. Susceptibility to mycobacterial infections due to interferon-gamma and interleukin-12 pathway defects. Allergy Asthma Proc. 26, 418–421 (2005).
Kampmann, B. et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J. Clin. Invest. 115, 2480–2488 (2005).
Wongkulab, P., Wipasa, J., Chaiwarith, R. & Supparatpinyo, K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS ONE 8, e76371 (2013).
Sologuren, I. et al. Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum. Mol. Genet. 20, 1509–1523 (2011).
Lammas, D. A., Casanova, J. L. & Kumararatne, D. S. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin. Exp. Immunol. 121, 417–425 (2000).
Doffinger, R. et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 38, e10–e14 (2004).
Poulin, S. et al. Fatal Mycobacterium colombiense/cytomegalovirus coinfection associated with acquired immunodeficiency due to autoantibodies against interferon gamma: a case report. BMC Infect. Dis. 13, 24 (2013).
Hommes, D. W. et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut 55, 1131–1137 (2006).
Reinisch, W. et al. Fontolizumab in moderate to severe Crohn’s disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm. Bowel Dis. 16, 233–242 (2010).
US Food and Drug Administration. Center for Drug Evaluation Research. BLA Multi-Disciplinary Review and Evaluation. BLA 761107. GAMIFANT(emapalumab), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761107Orig1s000MultidisciplineR.pdf (2018).
Tucci, F. et al. Treatment with emapalumab in an ADA-SCID patient with refractory hemophagocytic lymphohistiocytosis-related graft failure and disseminated BCGitis. Haematologica 106, 641–646 (2021).
Put, K. et al. Inflammatory gene expression profile and defective interferon-gamma and granzyme K in natural killer cells from systemic juvenile idiopathic arthritis patients. Arthritis Rheumatol. 69, 213–224 (2017).
Reinisch, W. et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon γ antibody, in patients with moderate to severe Crohn’s disease. Gut 55, 1138–1144 (2006).
Werth, V. P. et al. Brief report: pharmacodynamics, safety, and clinical efficacy of AMG 811, a human anti-interferon-gamma antibody, in patients with discoid lupus erythematosus. Arthritis Rheumatol. 69, 1028–1034 (2017).
Acknowledgements
The authors wish to thank Cristina de Min for her expert interèretation of the clinical trials with anti-IFNγ therapies and for critically revising the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
F.D.B. has received research grants from AbbVie, Novartis, Pfizer, Roche, Sanofi, Novimmune and Sobi. A.A.G. has served as a consultant for Sobi, AB2Bio, Juno and Novartis, and has received research support from Novartis, Sobi and AB2Bio. G.P., C.B. and E.M. declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks E. Brisse, who co-reviewed with P. Matthys, R. Planas, who co-reviewed with J. Pachlopnik Schmid, and R. Badolato for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Benedetti, F., Prencipe, G., Bracaglia, C. et al. Targeting interferon-γ in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol 17, 678–691 (2021). https://doi.org/10.1038/s41584-021-00694-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00694-z
This article is cited by
-
Trophoblast-derived miR-410-5p induces M2 macrophage polarization and mediates immunotolerance at the fetal-maternal interface by targeting the STAT1 signaling pathway
Journal of Translational Medicine (2024)
-
Recent advances and evolving concepts in Still’s disease
Nature Reviews Rheumatology (2024)
-
Interferon-γ and its response are determinants of antibody-mediated rejection and clinical outcomes in patients after renal transplantation
Genes & Immunity (2024)
-
Targeting disease with benzoxazoles: a comprehensive review of recent developments
Medicinal Chemistry Research (2024)
-
Immune cell signatures and inflammatory mediators: unraveling their genetic impact on chronic kidney disease through Mendelian randomization
Clinical and Experimental Medicine (2024)