Abstract
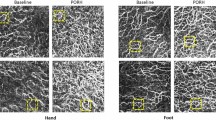
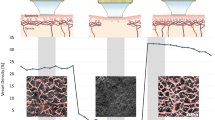
Morphological and functional analysis of the microcirculation are objective outcome measures that are recommended for use in the presence of clinical signs of altered peripheral blood flow (such as Raynaud phenomenon), which can occur in systemic sclerosis (SSc) and other autoimmune rheumatic diseases. Several advanced non-invasive tools are available for monitoring the microcirculation, including nailfold videocapillaroscopy, which is the best-studied and most commonly used method for distinguishing and quantifying microvascular morphological alterations in SSc. Nailfold videocapillaroscopy can also be used alongside laser Doppler techniques to assist in the early diagnosis and follow-up of patients with dermatomyositis or mixed connective tissue disease. Power Doppler ultrasonography, which has been used for many years to evaluate the vascularity of synovial tissue in rheumatoid arthritis, is another promising tool for the analysis of skin and nailbed capillary perfusion in other autoimmune rheumatic diseases. Other emerging methods include raster-scanning optoacoustic mesoscopy, which offers non-invasive high-resolution 3D visualization of capillaries and has been tested in psoriatic arthritis and SSc. The principle functions and operative characteristics of several non-invasive tools for analysing microvascular changes are outlined in this Review, and the clinical roles of validated or tested imaging methods are discussed for autoimmune rheumatic diseases.
Key points
-
Careful detection of the microvasculature can be performed using a variety of advanced imaging methods, which have also enabled new insights into the pathophysiology of several autoimmune rheumatic diseases.
-
Nailfold videocapillaroscopy is the gold standard method for distinguishing between primary and secondary Raynaud phenomenon, through the identification of a ‘scleroderma’ pattern, and for quantifying differences in microvascular morphology.
-
The presence of a ‘scleroderma-like’ nailfold videocapillaroscopy pattern supports the diagnosis of autoimmune rheumatic diseases such as dermatomyositis, mixed connective tissue disease and antisynthetase syndrome.
-
Several non-invasive methods, including laser Doppler techniques, enable the detection and quantification of characteristic alterations in peripheral blood perfusion in a number of autoimmune rheumatic diseases.
-
Imaging of the microcirculation is recommended at least twice a year for patients with persistent Raynaud phenomenon, and also for the follow-up of patients with selected autoimmune rheumatic diseases.
-
Almost all the most common techniques for morphological and functional microvascular evaluation can be used in combinations and managed by rheumatologists, bringing diagnostic power to the rheumatologist’s imaging armamentarium.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Murray, A. K. et al. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Rheum. 15, 1103–1111 (2009).
Cutolo, M., Sulli, A. & Smith, V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat. Rev. Rheumatol. 6, 578–587 (2010).
Ingegnoli, F. et al. An international survey on non-invasive techniques to assess the microcirculation in patients with Raynaud’s phenomenon (SUNSHINE survey). Rheumatol. Int. 37, 1879–1890 (2017).
Cutolo, M., Grassi, W. & Matucci Cerinic, M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 48, 3023–3030 (2003).
Herrick, A. L., Dinsdale, G. & Murray, A. New perspectives in the imaging of Raynaud’s phenomenon. Eur. J. Rheumatol. 7 (Suppl. 3), 212–221 (2020).
Cutolo, M., Soldano, S. & Smith, V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev. Clin. Immunol. 15, 753–764 (2019).
Cutolo, M., Sulli, A., Pizzorni, C. & Accardo, S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J. Rheumatol. 27, 155–160 (2000).
Lonzetti, L. S. et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 44, 735–736 (2001).
Hudson, M. et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin. Exp. Rheumatol. 25, 754–757 (2007).
Koenig, M. et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis. A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 58, 3902–3912 (2008).
Van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis. Arthritis Rheum. 65, 2737–2747 (2013).
Cutolo, M. & Sulli, A. Optimized treatment algorithms for digital vasculopathy in SSc. Nat. Rev. Rheumatol. 11, 569–571 (2015).
Maricq, H. R., Weinberger, A. B. & LeRoy, E. C. Early detection of scleroderma-spectrum disorders by in vivo capillary microscopy. J. Rheumatol. 9, 289–291 (1982).
Cracowski, J. L. & Roustit, M. Human skin microcirculation. Compr. Physiol. 10, 1105–1154 (2020).
Anderson, M. E. et al. Computerized nailfold video capillaroscopy — a new tool for assessment of Raynaud’s phenomenon. J. Rheumatol. 32, 841–848 (2005).
Herrick, A. L. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat. Rev. Rheumatol. 8, 469–479 (2012).
Smith, V. et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 19, 102458 (2020).
Cutolo, M., Paolino, S. & Smith, V. Nailfold capillaroscopy in rheumatology: ready for the daily use but with care in terminology. Clin. Rheumatol. 38, 2293–2297 (2019).
Sulli, A., Secchi, M. E., Pizzorni, C. & Cutolo, M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann. Rheum. Dis. 67, 885–887 (2008).
Smith, V. et al. Fast track algorithm: how to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun. Rev. 18, 102394 (2019).
Murray, A. K. et al. Preliminary clinical evaluation of semi-automated nailfold capillaroscopy in the assessment of patients with Raynaud’s phenomenon. Microcirculation 18, 440–447 (2011).
Gronenschild, E. H. et al. Semi-automatic assessment of skin capillary density: proof of principle and validation. Microvasc. Res. 90, 192–198 (2013).
Berks, M. A. et al. An automated system for detecting and measuring nailfold capillaries. Med. Image Comput. Comput. Assist. Interv. 17, 658–665 (2014).
Cheng, C., Lee, C. W. & Daskalakis, C. A reproducible computerized method for quantitation of capillary density using nailfold capillaroscopy. J. Vis. Exp. 27, e53088 (2015).
Cutolo, M. et al. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: proof of principle and validation in systemic sclerosis. Microcirculation 25, e12447 (2018).
Emrani, Z., Karbalaie, A., Fatemi, A., Etehadtavakol, M. & Björn-Erlandsson, E. Capillary density: an important parameter in nailfold capillaroscopy. Microvasc. Res. 10, 7–18 (2017).
Herrick, A. L., Berks, M. & Taylor, C. Quantitative nailfold capillaroscopy — update and possible next steps. Rheumatology 60, 2054–2065 (2021).
Dinsdale, G. et al. The assessment of nailfold capillaries: comparison of dermoscopy and nailfold videocapillaroscopy. Rheumatology 5, 1115–1116 (2018).
Hughes, M. et al. A study comparing videocapillaroscopy and dermoscopy in the assessment of nailfold capillaries in patients with systemic sclerosis-spectrum disorders. Rheumatology 54, 1435–1442 (2015).
Radic, M. et al. Consensus-based evaluation of dermatoscopy versus nailfold videocapillaroscopy in Raynaud’s phenomenon linking USA and Europe: a European League Against Rheumatism study group on microcirculation in rheumatic diseases project. Clin. Exp. Rheumatol. 38 (Suppl. 125), 132–136 (2020).
Berks, M. et al. Comparison between low cost USB nailfold capillaroscopy and videocapillaroscopy: a pilot study. Rheumatology 60, 3862–3867 (2021).
Masthoff, M. et al. Multispectral optoacoustic tomography of systemic sclerosis. J. Biophotonics 11, e201800155 (2018).
Nitkunanantharajah, S. et al. Three-dimensional optoacoustic imaging of nailfold capillaries in systemic sclerosis and its potential for disease differentiation using deep learning. Sci. Rep. 10, 16444 (2020).
Daoudi, K. et al. Photoacoustic and high-frequency ultrasound imaging of systemic sclerosis patients. Arthritis Res. Ther. 23, 22 (2021).
Abignano, G. et al. Virtual skin biopsy by optical coherence tomography: the first quantitative imaging biomarker for scleroderma. Ann. Rheum. Dis. 72, 1845–1851 (2013).
Abignano, G., Laws, P., Del Galdo, F., Marzo-Ortega, H. & McGonagle, D. Three-dimensional nail imaging by optical coherence tomography: a novel biomarker of response to therapy for nail disease in psoriasis and psoriatic arthritis. Clin. Exp. Dermatol. 44, 462–465 (2019).
Abignano, G. & Del Galdo, F. Biomarkers as an opportunity to stratify for outcome in systemic sclerosis. Eur. J. Rheumatol. 7 (Suppl. 3), 193–202 (2020).
Ranjbar, M. et al. Evaluation of choroidal substructure perfusion in patients affected by systemic sclerosis: an optical coherence tomography angiography study. Scand. J. Rheumatol. 49, 141–145 (2020).
Rommel, F., Prangel, D., Prasuhn, M., Grisanti, S. & Ranjbar, M. Correlation of retinal and choroidal microvascular impairment in systemic sclerosis. Orphanet J. Rare Dis. 16, 27–32 (2021).
Clark, S. et al. Comparison of thermography and laser Doppler imaging in the assessment of Raynaud’s phenomenon. Microvasc. Res. 66, 73–76 (2003).
Chojnowski, M. Infrared thermal imaging in connective tissue diseases. Reumatologia 5, 38–43 (2017).
Pauling, J. D., Shipley, J. A., Harris, N. D. & McHugh, N. J. Use of infrared thermography as an endpoint in therapeutic trials of Raynaud’s phenomenon and systemic sclerosis. Clin. Exp. Rheumatol. 30 (Suppl. 71), 103–115 (2012).
Hughes, M. et al. Thermographic abnormalities are associated with future digital ulcers and death in patients with systemic sclerosis. J. Rheumatol. 43, 1519–1522 (2016).
Murray, A. K., Herrick, A. L. & King, T. A. Laser Doppler imaging: a developing technique for application in the rheumatic diseases. Rheumatology 43, 1210–1218 (2004).
Cutolo, M. et al. Peripheral blood perfusion correlates with microvascular abnormalities in systemic sclerosis: a laser-Doppler and nailfold videocapillaroscopy study. J. Rheumatol. 37, 1174–1180 (2010).
Melsens, K., Paolino, S., Vanhaecke, A., Cutolo, M. & Smith, V. The preliminary validation of laser Doppler flowmetry in systemic sclerosis in accordance with the OMERACT filter: a systematic review. Semin. Arthritis Rheum. 50, 321–328 (2020).
Sulli, A., Ruaro, B. & Cutolo, M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann. Rheum. Dis. 73, 2059–2061 (2014).
Ruaro, B. et al. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann. Rheum. Dis. 73, 1181–1185 (2014).
Cutolo, M. et al. Is laser speckle contrast analysis (LASCA) the new kid on the block in systemic sclerosis? A systematic literature review and pilot study to evaluate reliability of LASCA to measure peripheral blood perfusion in scleroderma patients. Autoimmun. Rev. 17, 775–780 (2018).
Pauling, J. D., Shipley, J. A., Hart, D. J., McGrogan, A. & McHugh, N. J. Use of laser speckle contrast imaging to assess digital microvascular function in primary Raynaud phenomenon and systemic sclerosis: a comparison using the Raynaud Condition Score Diary. J. Rheumatol. 42, 1163–1168 (2015).
Lambrecht, V. et al. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann. Rheum. Dis. 75, 1263–1264 (2016).
Ruaro, B., Paolino, S., Pizzorni, C., Cutolo, M. & Sulli, A. Assessment of treatment effects on digital ulcer and blood perfusion by laser speckle contrast analysis in a patient affected by systemic sclerosis. Reumatismo 69, 134–136 (2017).
Gigante, A., Villa, A. & Rosato, E. Laser speckle contrast analysis predicts major vascular complications and mortality of patients with systemic sclerosis. Rheumatology 60, 1850–1857 (2021).
Iagnocco, A. et al. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology 54, 1890–1896 (2015).
Lee, S. I., Lee, S. Y. & Yoo, W. H. The usefulness of power Doppler ultrasonography in differentiating primary and secondary Raynaud’s phenomenon. Clin. Rheumatol. 25, 814–818 (2006).
Schioppo, T. et al. Evidence of macro- and micro-angiopathy in scleroderma: an integrated approach combining 22-MHz power Doppler ultrasonography and video-capillaroscopy. Microvasc. Res. 12, 125–130 (2019).
Eisenbrey, J. R., Stanczak, M., Forsberg, F., Mendoza-Ballesteros, F. A. & Lyshchik, A. Photoacoustic oxygenation quantification in patients with Raynaud’s: first-in-human results. Ultrasound Med. Biol. 44, 2081–2088 (2018).
Werner, S. G. et al. Indocyanine green-enhanced fluorescence optical imaging in patients with early and very early arthritis: a comparative study with magnetic resonance imaging. Arthritis Rheum. 65, 3036–3044 (2013).
Fischer, T. et al. Detection of rheumatoid arthritis using non-specific contrast enhanced fluorescence imaging. Acad. Radiol. 17, 375–378 (2010).
Friedrich, S. et al. Disturbed microcirculation in the hands of patients with systemic sclerosis detected by fluorescence optical imaging: a pilot study. Arthritis Res. Ther. 19, 87–92 (2017).
Deschepper, E. et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology 55, 883–890 (2016).
Cutolo, M. et al. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatology 57, 757–759 (2018).
Cutolo, M., Pizzorni, C. & Sulli, A. Identification of transition from primary Raynaud’s phenomenon to secondary Raynaud’s phenomenon by nailfold videocapillaroscopy. Arthritis Rheum. 56, 2102–2103 (2007).
Smith, V. et al. May capillaroscopy be a candidate tool in future algorithms for SSc-ILD: are we looking for the holy grail? A systematic review. Autoimmun. Rev. 19, 102619 (2020).
Sulli, A. et al. Progression of nailfold capillaroscopic patterns and correlation with organ involvement in systemic sclerosis: a 12 year study. Rheumatology 59, 1051–1058 (2020).
Smith, V. et al. Nailfold capillaroscopy: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann. Rheum. Dis. 70, 180–183 (2011).
Smith, V. E. et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann. Rheum. Dis. 71, 1636–1639 (2012).
Trombetta, A. C. et al. Quantitative alterations of capillary diameter have a predictive value for development of the capillaroscopic systemic sclerosis pattern. J. Rheumatol. 43, 599–606 (2016).
Cutolo, M. et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheumatol. 6, 527–539 (2016).
Sulli, A. et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum. 64, 821–825 (2012).
Caetano, J., Paula, F. S., Amaral, M., Oliveira, S. & Alves, J. D. Nailfold videocapillaroscopy changes are associated with the presence and severity of systemic sclerosis-related interstitial lung disease. J. Clin. Rheumatol. 25, e12–e15 (2019).
Gheorghiu, A. M. et al. Capillary loss reflects disease activity and prognosis in patients with systemic sclerosis. Exp. Ther. Med. 20, 3438–3443 (2020).
Berks, M. et al. Automated structure and flow measurement — a promising tool in nailfold capillaroscopy. Microvasc. Res. 118, 173–177 (2018).
Sternbersky, J., Tichy, M. & Zapletalova, J. Infrared thermography and capillaroscopy in the diagnosis of Raynaud’s phenomenon. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 165, 90–98 (2021).
Pavlov-Dolijanovic, S. et al. Relationship between 99mTc-pertechnetate hand perfusion scintigraphy and nailfold capillaroscopy in systemic sclerosis patients: a pilot study. Arch. Rheumatol. 35, 321–327 (2020).
Pauling, J. D. & Christopher-Stine, L. The aetiopathogenic significance, clinical relevance and therapeutic implications of vasculopathy in idiopathic inflammatory myopathy. Rheumatology 60, 1593–1607 (2021).
Bertolazzi, C., Cutolo, M., Smith, V. & Gutierrez, M. State of the art on nailfold capillaroscopy in dermatomyositis and polymyositis. Semin. Arthritis Rheum. 47, 432–444 (2017).
Miossi, R., de Souza, F. H. C. & Shinjo, S. K. Nailfold capillary changes in adult new-onset dermatomyositis: a prospective cross-sectional study. Clin. Rheumatol. 38, 2319–2326 (2019).
Pizzorni, C. et al. Long-term follow-up of nailfold videocapillaroscopic changes in dermatomyositis versus systemic sclerosis patients. Clin. Rheumatol. 37, 2723–2729 (2018).
Cavagna, L. et al. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine 94, e1144 (2015).
Cassone, G. et al. Nailfold videocapillaroscopy in antisynthetase syndrome. Reumatismo 70, 257–258 (2018).
Sebastiani, M. et al. Nailfold capillaroscopy characteristics of antisynthetase syndrome and possible clinical associations: results of a multicenter international study. J. Rheumatol. 46, 279–284 (2019).
Candela, M. et al. Nailfold capillary microscopy in patients with antiphospholipid syndrome. Recenti Prog. Med. 89, 444–449 (1998).
Pyrpasopoulou, A., Triantafyllou, A., Anyfanti, P., Douma, S. & Aslanidis, S. Capillaroscopy as a screening test for clinical antiphospholipid syndrome. Eur. J. Intern. Med. 22, e158–e159 (2011).
Aslanidis, S. et al. Association of capillaroscopic microhaemorrhages with clinical and immunological antiphospholipid syndrome. Clin. Exp. Rheumatol. 29, 307–309 (2011).
Sulli, A., Pizzorni, C. & Cutolo, M. Nailfold videocapillaroscopy abnormalities in patients with antiphospholipid antibodies. J. Rheumatol. 27, 1574–1576 (2000).
Bernardino, V., Rodrigues, A., Lladó, A. & Panarra, A. Nailfold capillaroscopy and autoimmune connective tissue diseases in patients from a Portuguese nailfold capillaroscopy clinic. Rheumatol. Int. 40, 295–301 (2020).
Nagy, Y. & Czirjak, L. Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud’s disease. J. Eur. Acad. Dermatol. Venereol. 18, 62–68 (2004).
Paolino, S. et al. Long-term follow-up of nailfold videocapillaroscopic microvascular parameters in mixed connective tissue disease versus systemic sclerosis patients: a retrospective cohort study. Clin. Exp. Rheumatol. 37 (Suppl. 119), 102–107 (2019).
Cutolo, M. et al. Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun. Rev. 17, 344–352 (2018).
Schonenberg-Meinema, D. et al. Capillaroscopy in childhood-onset systemic lupus erythematosus: a first systematic review. Clin. Exp. Rheumatol. 38, 350–354 (2020).
Schonenberg-Meinema, D. et al. Nailfold capillary abnormalities in childhood-onset systemic lupus erythematosus: a cross-sectional study compared with healthy controls. Lupus 30, 818–827 (2021).
Ruaro, B. et al. Peripheral blood perfusion in patients with systemic lupus erythematosus and in primary Raynaud’s phenomenon. Eur. J. Rheumatol. 8, 7–11 (2021).
Capobianco, K. G., Xavier, R. M., Bredemeier, M., Restelli, V. G. & Brenol, J. C. Nailfold capillaroscopic findings in primary Sjögren’s syndrome: clinical and serological correlations. Clin. Exp. Rheumatol. 23, 789–794 (2005).
Szabo, N. et al. Functional and morphological evaluation of hand microcirculation with nailfold capillaroscopy and laser Doppler imaging in Raynaud’s and Sjögren’s syndrome and poly/dermatomyositis. Scand. J. Rheumatol. 37, 23–29 (2008).
Melsens, K. et al. Nailfold capillaroscopy in Sjögren’s syndrome: a systematic literature review and standardised interpretation. Clin. Exp. Rheumatol. 38 (Suppl. 126), 150–157 (2020).
Hendriks, A. G. et al. Whole field laser Doppler imaging of the microcirculation in psoriasis and clinically unaffected skin. J. Dermatolog. Treat. 25, 18–21 (2014).
Hendriks, A. G. et al. Clearing of psoriasis documented by laser Doppler perfusion imaging contrasts remaining elevation of dermal expression levels of CD31. Skin Res. Technol. 21, 340–345 (2015).
Cutolo, M. et al. Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J. Rheumatol. 40, 40–45 (2013).
Cutolo, M. et al. Longterm treatment with endothelin receptor antagonist bosentan and iloprost improves fingertip blood perfusion in systemic sclerosis. J. Rheumatol. 41, 881–386 (2014).
Bellando-Randone, S. et al. Combination therapy with bosentan and sildenafil improves Raynaud’s phenomenon and fosters the recovery of microvascular involvement in systemic sclerosis. Clin. Rheumatol. 35, 127–132 (2016).
Trombetta, A. C. et al. Effects of longterm treatment with bosentan and iloprost on nailfold absolute capillary number, fingertip blood perfusion, and clinical status in systemic sclerosis. J. Rheumatol. 43, 2033–2041 (2016).
Smith, V. et al. Stabilization of microcirculation in patients with early systemic sclerosis with diffuse skin involvement following rituximab treatment: an open-label study. J. Rheumatol. 43, 995–996 (2016).
Rotondo, C. et al. Evidence for increase in finger blood flow, evaluated by laser Doppler flowmetry, following iloprost infusion in patients with systemic sclerosis: a week-long observational longitudinal study. Scand. J. Rheumatol. 47, 311–318 (2018).
Ruaro, B. et al. Short-term follow-up of digital ulcers by laser speckle contrast analysis in systemic sclerosis patients. Microvas. Res. 101, 82–85 (2015).
Gaillard-Bigot, F. et al. Abnormal amplitude and kinetics of digital postocclusive reactive hyperemia in systemic sclerosis. Microvasc. Res. 94, 90–95 (2014).
Roustit, M. et al. On-demand sildenafil as a treatment for Raynaud phenomenon: a series of n-of-1 trials. Ann. Intern. Med. 169, 694–703 (2018).
Acknowledgements
The authors thank C. Pizzorni, A. Sulli and S. Paolino for their continuous support and S. De Gregorio and E. Gotelli for their involvement in figure definition. M.C. and V.S. are members of and/or chair the EULAR Study Group on Microcirculation in Rheumatic Diseases, which makes the dissemination of research projects on this topic possible.
Author information
Authors and Affiliations
Contributions
M.C. researched data for the article and wrote the article. V.S. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Disclaimer
V.S. is a senior clinical investigator of the Research Foundation Flanders, Belgium (FWO; 1.8.029.20 N). The FWO was not involved in the study design, collection, analysis and interpretation of data, writing of the report or in the decision to submit the manuscript for publication.
Peer review information
Nature Reviews Rheumatology thanks S. Lambova, J.-L. Cracowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Telangiectasias
-
Also named spider veins, these are small, dilated blood vessels that can occur near the surface of the skin or mucous membranes.
- Proximal–distal gradient
-
In healthy individuals, perfusion is typically high in the distal fingertips and low in the dorsum of the hands.
Rights and permissions
About this article
Cite this article
Cutolo, M., Smith, V. Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat Rev Rheumatol 17, 665–677 (2021). https://doi.org/10.1038/s41584-021-00685-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00685-0
This article is cited by
-
Nintedanib downregulates the profibrotic M2 phenotype in cultured monocyte-derived macrophages obtained from systemic sclerosis patients affected by interstitial lung disease
Arthritis Research & Therapy (2024)
-
Cardiovascular autonomic dysfunction in post-COVID-19 syndrome: a major health-care burden
Nature Reviews Cardiology (2024)
-
Microvascular status in juvenile Sjögren’s disease: the first nailfold videocapillaroscopy investigation
Clinical Rheumatology (2024)
-
A machine learning model identifies patients in need of autoimmune disease testing using electronic health records
Nature Communications (2023)
-
Involvement of the secosteroid vitamin D in autoimmune rheumatic diseases and COVID-19
Nature Reviews Rheumatology (2023)