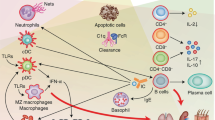
Abstract
Interactions between lymphocytes and stromal cells have an important role in immune cell development and responses. During inflammation, stromal cells contribute to inflammation, from induction to chronicity or resolution, through direct cell interactions and through the secretion of pro-inflammatory and anti-inflammatory mediators. Stromal cells are imprinted with tissue-specific phenotypes and contribute to site-specific lymphocyte recruitment. During chronic inflammation, the modified pro-inflammatory microenvironment leads to changes in the stromal cells, which acquire a pathogenic phenotype. At the site of inflammation, infiltrating B cells and T cells interact with stromal cells. These interactions induce a plasma cell-like phenotype in B cells and T cells, associated with secretion of immunoglobulins and inflammatory cytokines, respectively. B cells and T cells also influence the stromal cells, inducing cell proliferation, molecular changes and cytokine production. This positive feedback loop contributes to disease chronicity. This Review describes the importance of these cell interactions in chronic inflammation, with a focus on human disease, using three selected autoimmune and inflammatory diseases: rheumatoid arthritis, psoriatic arthritis (and psoriasis) and systemic lupus erythematosus. Understanding the importance and disease specificity of these interactions could provide new therapeutic options.
Key points
-
During chronic inflammation, the pro-inflammatory environment leads to changes in stromal cells, and these altered phenotypes contribute to disease induction and chronicity.
-
At inflammatory sites, infiltrating B cells and T cells interact with pathogenic stromal cells, promoting inflammation that in turn induces and maintains the pathogenic phenotype of stromal cells.
-
Interactions with stromal cells induce accumulation, activation and survival of T lymphocytes and B lymphocytes, through direct cell contact and soluble factors.
-
Despite common characteristics such as the involvement of the T helper 17 axis, inflammatory diseases differ, such as in affected sites and types and phenotypes of stromal cells.
-
These differences can affect immune cell–stromal cell interactions and their resulting effects on cell activation, proliferation and cytokine production.
-
A better understanding of lymphocyte–stromal cell interactions is needed to determine how heterogeneity among these cells, including pathogenic cell subsets, could be used to stratify patients and be targeted more specifically.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nagasawa, T. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 6, 107–116 (2006).
Takahama, Y., Ohigashi, I., Baik, S. & Anderson, G. Generation of diversity in thymic epithelial cells. Nat. Rev. Immunol. 17, 295–305 (2017).
Roozendaal, R. & Mebius, R. E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 29, 23–43 (2011).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
Aloisi, F. & Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217 (2006).
Garrood, T., Lee, L. & Pitzalis, C. Molecular mechanisms of cell recruitment to inflammatory sites: general and tissue-specific pathways. Rheumatology 45, 250–260 (2006).
Berg, E. L. et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174, 1461–1466 (1991).
Bevilacqua, M. P., Pober, J. S., Mendrick, D. L., Cotran, R. S. & Gimbrone, M. A. Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc. Natl Acad. Sci. USA 84, 9238–9242 (1987).
Parsonage, G. et al. A stromal address code defined by fibroblasts. Trends Immunol. 26, 150–156 (2005).
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Ayala, A., Chung, C. S., Grutkoski, P. S. & Song, G. Y. Mechanisms of immune resolution. Crit. Care Med. 31, S558–S571 (2003).
Strasser, A., Jost, P. J. & Nagata, S. The many rolesof FAS receptor signaling in the immune system. Immunity 30, 180–192 (2009).
Zhang, H. & Xu, X. Mutation-promoting molecular networks of uncontrolled inflammation. Tumour Biol. 39, 1010428317701310 (2017).
Ai, R. et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat. Commun. 9, 1921 (2018).
Nakano, K., Boyle, D. L. & Firestein, G. S. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J. Immunol. 190, 1297–1303 (2013).
Buckley, C. D. et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22, 199–204 (2001).
Brouty-Boye, D., Pottin-Clemenceau, C., Doucet, C., Jasmin, C. & Azzarone, B. Chemokines and CD40 expression in human fibroblasts. Eur. J. Immunol. 30, 914–919 (2000).
Pap, T., Muller-Ladner, U., Gay, R. E. & Gay, S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2, 361–367 (2000).
Buckley, C. D., Barone, F., Nayar, S., Benezech, C. & Caamano, J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu. Rev. Immunol. 33, 715–745 (2015).
Muller-Ladner, U., Ospelt, C., Gay, S., Distler, O. & Pap, T. Cells of the synovium in rheumatoid arthritis. synovial fibroblasts. Arthritis Res. Ther. 9, 223 (2007).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Dakin, S. G. et al. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat. Rev. Rheumatol. 14, 714–726 (2018).
Mohr, W., Beneke, G. & Mohing, W. Proliferation of synovial lining cells and fibroblasts. Ann. Rheum. Dis. 34, 219–224 (1975).
Yan, Y. et al. Comparative study of normal and rheumatoid arthritis fibroblast-like synoviocytes proliferation under cyclic mechanical stretch: role of prostaglandin E2. Connect. Tissue Res. 53, 246–254 (2011).
Baier, A., Meineckel, I., Gay, S. & Pap, T. Apoptosis in rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 274–279 (2003).
Korb, A., Pavenstadt, H. & Pap, T. Cell death in rheumatoid arthritis. Apoptosis 14, 447–454 (2009).
Nygaard, G. & Firestein, G. S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16, 316–333 (2020).
Tolboom, T. C. et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann. Rheum. Dis. 61, 975–980 (2002).
Firestein, G. S. et al. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 149, 2143–2151 (1996).
Firestein, G. S., Echeverri, F., Yeo, M., Zvaifler, N. J. & Green, D. R. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc. Natl Acad. Sci. USA 94, 10895–10900 (1997).
Franz, J. K. et al. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 43, 599–607 (2000).
Toh, M. L. et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS ONE 5, e13416 (2010).
Muller-Ladner, U. et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149, 1607–1615 (1996).
Lefevre, S. et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15, 1414–1420 (2009).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Orange, D. E. et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 70, 690–701 (2018).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Noack, M., Ndongo-Thiam, N. & Miossec, P. Interaction among activated lymphocytes and mesenchymal cells through podoplanin is critical for a high IL-17 secretion. Arthritis Res. Ther. 18, 148 (2016).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Humby, F. et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann. Rheum. Dis. 78, 761–772 (2019).
Nerviani, A. et al. A pauci-immune synovial pathotype predicts inadequate response to TNFalpha-blockade in rheumatoid arthritis patients. Front. Immunol. 11, 845 (2020).
Lewis, M. J. et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 28, 2455–2470.e5 (2019).
Humby, F. et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet 397, 305–317 (2021).
Yoshitomi, H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front. Immunol. 10, 1395 (2019).
Fox, D. A., Gizinski, A., Morgan, R. & Lundy, S. K. Cell-cell interactions in rheumatoid arthritis synovium. Rheum. Dis. Clin. North. Am. 36, 311–323 (2010).
Bradfield, P. F. et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 48, 2472–2482 (2003).
Buckley, C. D. et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J. Immunol. 165, 3423–3429 (2000).
McGettrick, H. M. et al. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 39, 113–125 (2009).
McGettrick, H. M., Buckley, C. D., Filer, A., Rainger, G. E. & Nash, G. B. Stromal cells differentially regulate neutrophil and lymphocyte recruitment through the endothelium. Immunology 131, 357–370 (2010).
Kobayashi, S. et al. TGF-β induces the differentiation of human CXCL13-producing CD4+ T cells. Eur. J. Immunol. 46, 360–371 (2016).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Petrasca, A. et al. Targeting bioenergetics prevents CD4 T cell-mediated activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology 59, 2816–2828 (2020).
Mori, M. et al. Cell-contact-dependent activation of CD4+ T cells by adhesion molecules on synovial fibroblasts. Mod. Rheumatol. 27, 448–456 (2017).
Page, G. et al. Plasma cell-like morphology of Th1-cytokine-producing cells associated with the loss of CD3 expression. Am. J. Pathol. 164, 409–417 (2004).
Sawai, H., Park, Y. W., He, X., Goronzy, J. J. & Weyand, C. M. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 56, 3215–3225 (2007).
Nanki, T. et al. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 46, 2878–2883 (2002).
Lebre, M. C. et al. Synovial IL-21/TNF-producing CD4+ T cells induce joint destruction in rheumatoid arthritis by inducing matrix metalloproteinase production by fibroblast-like synoviocytes. J. Leukoc. Biol. 101, 775–783 (2017).
Nanki, T. et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 165, 6590–6598 (2000).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010).
Chabaud, M., Page, G. & Miossec, P. Enhancing effect of IL-1, IL-17, and TNF-α on macrophage inflammatory protein-3α production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J. Immunol. 167, 6015–6020 (2001).
Tanida, S. et al. CCL20 produced in the cytokine network of rheumatoid arthritis recruits CCR6+ mononuclear cells and enhances the production of IL-6. Cytokine 47, 112–118 (2009).
Mitra, A., Raychaudhuri, S. K. & Raychaudhuri, S. P. Functional role of IL-22 in psoriatic arthritis. Arthritis Res. Ther. 14, R65 (2012).
Tran, C. N. et al. Molecular interactions between T cells and fibroblast-like synoviocytes: role of membrane tumor necrosis factor-alpha on cytokine-activated T cells. Am. J. Pathol. 171, 1588–1598 (2007).
Bombara, M. P. et al. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J. Leukoc. Biol. 54, 399–406 (1993).
Nguyen, H. N. et al. Autocrine loop involving IL-6 family member LIF, LIF receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity 46, 220–232 (2017).
van Hamburg, J. P. et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 63, 73–83 (2011).
Tak, P. P. et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 40, 217–225 (1997).
Dechanet, J., Merville, P., Durand, I., Banchereau, J. & Miossec, P. The ability of synoviocytes to support terminal differentiation of activated B cells may explain plasma cell accumulation in rheumatoid synovium. J. Clin. Invest. 95, 456–463 (1995).
Wang, Q., Ma, Y., Liu, D., Zhang, L. & Wei, W. The roles of B cells and their interactions with fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis. Int. Arch. Allergy Immunol. 155, 205–211 (2011).
Burger, J. A., Zvaifler, N. J., Tsukada, N., Firestein, G. S. & Kipps, T. J. Fibroblast-like synoviocytes support B-cell pseudoemperipolesis via a stromal cell-derived factor-1- and CD106 (VCAM-1)-dependent mechanism. J. Clin. Invest. 107, 305–315 (2001).
Ohata, J. et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J. Immunol. 174, 864–870 (2005).
Rochas, C. et al. Transmembrane BAFF from rheumatoid synoviocytes requires interleukin-6 to induce the expression of recombination-activating gene in B lymphocytes. Arthritis Rheum. 60, 1261–1271 (2009).
Bombardieri, M. et al. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Ann. Rheum. Dis. 70, 1857–1865 (2011).
Schett, G. Autoimmunity as a trigger for structural bone damage in rheumatoid arthritis. Mod. Rheumatol. 27, 193–197 (2017).
Kang, Y. M. et al. LIGHT up-regulated on B lymphocytes and monocytes in rheumatoid arthritis mediates cellular adhesion and metalloproteinase production by synoviocytes. Arthritis Rheum. 56, 1106–1117 (2007).
Schmitt, V. et al. Interleukin-36 receptor mediates the crosstalk between plasma cells and synovial fibroblasts. Eur. J. Immunol. 47, 2101–2112 (2017).
Frey, S. et al. The novel cytokine interleukin-36alpha is expressed in psoriatic and rheumatoid arthritis synovium. Ann. Rheum. Dis. 72, 1569–1574 (2013).
Boehncke, W. H. & Schon, M. P. Psoriasis. Lancet 386, 983–994 (2015).
Ritchlin, C. T., Colbert, R. A. & Gladman, D. D. Psoriatic arthritis. N. Engl. J. Med. 376, 2095–2096 (2017).
Veale, D. J. & Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 391, 2273–2284 (2018).
Priestley, G. C. & Adams, L. W. Hyperactivity of fibroblasts cultured from psoriatic skin: I. Faster proliferation and effect of serum withdrawal. Br. J. Dermatol. 109, 149–156 (1983).
Saiag, P., Coulomb, B., Lebreton, C., Bell, E. & Dubertret, L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science 230, 669–672 (1985).
Quan, C. et al. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 6, 890–903 (2015).
Gunther, C., Carballido-Perrig, N., Kaesler, S., Carballido, J. M. & Biedermann, T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J. Invest. Dermatol. 132, 626–634 (2012).
Dustin, M. L., Singer, K. H., Tuck, D. T. & Springer, T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J. Exp. Med. 167, 1323–1340 (1988).
Veale, D. et al. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. 36, 893–900 (1993).
Homey, B. et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157–165 (2002).
Noack, M., Ndongo-Thiam, N. & Miossec, P. Role of podoplanin in the high interleukin-17A secretion resulting from interactions between activated lymphocytes and psoriatic skin-derived mesenchymal cells. Clin. Exp. Immunol. 186, 64–74 (2016).
Schirmer, C., Klein, C., von Bergen, M., Simon, J. C. & Saalbach, A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 116, 1715–1725 (2010).
Piskin, G., Sylva-Steenland, R. M., Bos, J. D. & Teunissen, M. B. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J. Immunol. 176, 1908–1915 (2006).
Orlik, C. et al. Keratinocytes costimulate naive human T cells via CD2: a potential target to prevent the development of proinflammatory Th1 cells in the skin. Cell Mol. Immunol. 17, 380–394 (2019).
Lowes, M. A. et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128, 1207–1211 (2008).
Blauvelt, A. & Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 55, 379–390 (2018).
Noack, M., Beringer, A. & Miossec, P. Additive or synergistic interactions between IL-17A or IL-17F and TNF or IL-1beta depend on the cell type. Front. Immunol. 10, 1726 (2019).
Chiricozzi, A., Romanelli, P., Volpe, E., Borsellino, G. & Romanelli, M. Scanning the immunopathogenesis of psoriasis. Int. J. Mol. Sci. 19, 179 (2018).
Ma, W. Y., Jia, K. & Zhang, Y. IL-17 promotes keratinocyte proliferation via the downregulation of C/EBPalpha. Exp. Ther. Med. 11, 631–636 (2016).
Kim, T. G. et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J. Invest. Dermatol. 134, 1462–1465 (2014).
Furue, K., Ito, T., Tsuji, G., Nakahara, T. & Furue, M. The CCL20 and CCR6 axis in psoriasis. Scand. J. Immunol. 91, e12846 (2019).
Wolk, K. et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36, 1309–1323 (2006).
Tohyama, M. et al. Bcl-3 induced by IL-22 via STAT3 activation acts as a potentiator of psoriasis-related gene expression in epidermal keratinocytes. Eur. J. Immunol. 48, 168–179 (2018).
Pfaff, C. M., Marquardt, Y., Fietkau, K., Baron, J. M. & Luscher, B. The psoriasis-associated IL-17A induces and cooperates with IL-36 cytokines to control keratinocyte differentiation and function. Sci. Rep. 7, 15631 (2017).
Carrier, Y. et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 131, 2428–2437 (2011).
Albanesi, C., Madonna, S., Gisondi, P. & Girolomoni, G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front. Immunol. 9, 1549 (2018).
Frieder, J., Kivelevitch, D., Haugh, I., Watson, I. & Menter, A. Anti-IL-23 and anti-IL-17 biologic agents for the treatment of immune-mediated inflammatory conditions. Clin. Pharmacol. Ther. 103, 88–101 (2018).
Belasco, J. et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 67, 934–944 (2015).
van Kuijk, A. W. & Tak, P. P. Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy. Curr. Rheumatol. Rep. 13, 353–359 (2011).
Campbell, J. J., O’Connell, D. J. & Wurbel, M. A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 178, 3358–3362 (2007).
Flytlie, H. A. et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine 49, 24–29 (2010).
Colucci, S. et al. Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J. Pathol. 212, 47–55 (2007).
Mahmoud, F. et al. Elevated B-lymphocyte levels in lesional tissue of non-arthritic psoriasis. J. Dermatol. 26, 428–433 (1999).
Lu, J., Ding, Y., Yi, X. & Zheng, J. CD19+ B cell subsets in the peripheral blood and skin lesions of psoriasis patients and their correlations with disease severity. Braz. J. Med. Biol. Res. 49, e5374 (2016).
Hayashi, M. et al. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J. Dermatol. Sci. 81, 93–100 (2015).
Kahlert, K. et al. Aberrant B-cell subsets and immunoglobulin levels in patients with moderate-to-severe psoriasis. Acta Derm. Venereol. 99, 226–227 (2019).
Samoud-El Kissi, S. et al. BAFF is elevated in serum of patients with psoriasis: association with disease activity. Br. J. Dermatol. 159, 765–768 (2008).
Gerhard, N. et al. IgVH-genes analysis from psoriatic arthritis shows involvement of antigen-activated synovial B-lymphocytes. Z. Rheumatol. 61, 718–727 (2002).
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2, 16039 (2016).
Steinmetz, O. M. et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 74, 448–457 (2008).
Da, Z. et al. CXCL13 promotes proliferation of mesangial cells by combination with CXCR5 in SLE. J. Immunol. Res. 2016, 2063985 (2016).
Karrar, S. & Cunninghame Graham, D. S. Abnormal B cell development in systemic lupus erythematosus: what the genetics tell us. Arthritis Rheumatol. 70, 496–507 (2018).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Chu, V. T. et al. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum. 60, 2083–2093 (2009).
Iwata, S. & Tanaka, Y. B-cell subsets, signaling and their roles in secretion of autoantibodies. Lupus 25, 850–856 (2016).
Vincent, F. B., Morand, E. F., Schneider, P. & Mackay, F. The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol. 10, 365–373 (2014).
Collins, C. E. et al. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res. Ther. 8, R6 (2006).
Sun, L. Y. et al. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 16, 121–128 (2007).
Menard, C. et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: standardization of immune quality controls. Stem Cell Dev. 22, 1789–1801 (2013).
Che, N. et al. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J. Immunol. 193, 5306–5314 (2014).
Fan, L. et al. Interaction between mesenchymal stem cells and B-cells. Int. J. Mol. Sci. 17, 650 (2016).
Rosado, M. M. et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cell Dev. 24, 93–103 (2015).
Suarez-Fueyo, A., Bradley, S. J. & Tsokos, G. C. T cells in systemic lupus erythematosus. Curr. Opin. Immunol. 43, 32–38 (2016).
Katsuyama, T., Tsokos, G. C. & Moulton, V. R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 9, 1088 (2018).
Robert, M. & Miossec, P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus 29, 6–14 (2020).
Koga, T., Ichinose, K. & Tsokos, G. C. T cells and IL-17 in lupus nephritis. Clin. Immunol. 185, 95–99 (2017).
Zickert, A. et al. IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol. 16, 7 (2015).
Alunno, A. et al. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin. Dev. Immunol. 2012, 823085 (2012).
Gu, Z. et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging 8, 1102–1114 (2016).
Geng, L. et al. Reduced Let-7f in bone marrow-derived mesenchymal stem cells triggers Treg/Th17 imbalance in patients with systemic lupus erythematosus. Front. Immunol. 11, 233 (2020).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Yasuda, S. Emerging targets for the treatment of lupus erythematosus: there is no royal road to treating lupus. Mod. Rheumatol. 29, 60–69 (2019).
Rendon, A. & Schakel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 20, 1475 (2019).
Smeets, T. J., Kraan, M. C., van Loon, M. E. & Tak, P. P. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 48, 2155–2162 (2003).
Guggino, G. et al. Targeting IL-6 signalling in early rheumatoid arthritis is followed by Th1 and Th17 suppression and Th2 expansion. Clin. Exp. Rheumatol. 32, 77–81 (2014).
Noisette, A. & Hochberg, M. C. Abatacept for the treatment of adults with psoriatic arthritis: patient selection and perspectives. Psoriasis 8, 31–39 (2018).
Wang, G., Hu, P., Yang, J., Shen, G. & Wu, X. The effects of PDL-Ig on collagen-induced arthritis. Rheumatol. Int. 31, 513–519 (2011).
Kim, J. H. et al. Programmed cell death ligand 1 alleviates psoriatic inflammation by suppressing IL-17A production from programmed cell death 1-high T cells. J. Allergy Clin. Immunol. 137, 1466–1476 e1463 (2016).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Kumar, P., Saini, S. & Prabhakar, B. S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 64, 29–35 (2020).
Sawai, H. et al. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 52, 1392–1401 (2005).
Tanaka, Y. et al. Safety, pharmacokinetics, and efficacy of E6011, an antifractalkine monoclonal antibody, in a first-in-patient phase 1/2 study on rheumatoid arthritis. Mod. Rheumatol. 28, 58–65 (2018).
Quintanilla, M., Montero-Montero, L., Renart, J. & Martin-Villar, E. Podoplanin in inflammation and cancer. Int. J. Mol. Sci. 20, 707 (2019).
Krishnan, H. et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 109, 1292–1299 (2018).
Peters, A. et al. Podoplanin negatively regulates CD4+ effector T cell responses. J. Clin. Invest. 125, 129–140 (2015).
Caporali, R. & Zavaglia, D. Real-world experience with tofacitinib for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 37, 485–495 (2019).
Rosengren, S., Corr, M., Firestein, G. S. & Boyle, D. L. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann. Rheum. Dis. 71, 440–447 (2012).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Diller, M. et al. Targeting activated synovial fibroblasts in rheumatoid arthritis by peficitinib. Front. Immunol. 10, 541 (2019).
Hong, S. S. et al. PUMA gene delivery to synoviocytes reduces inflammation and degeneration of arthritic joints. Nat. Commun. 8, 146 (2017).
Bonaventura, P. et al. Protective effect of low dose intra-articular cadmium on inflammation and joint destruction in arthritis. Sci. Rep. 7, 2415 (2017).
Bonaventura, P., Lamboux, A., Albarede, F. & Miossec, P. Regulatory effects of zinc on cadmium-induced cytotoxicity in chronic inflammation. PLoS ONE 12, e0180879 (2017).
Viswanathan, S. et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 21, 1019–1024 (2019).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Rosengren, S., Boyle, D. L. & Firestein, G. S. Acquisition, culture, and phenotyping of synovial fibroblasts. Methods Mol. Med. 135, 365–375 (2007).
Acknowledgements
The work of the authors is supported in part by a grant from the Société Française de Rhumatologie.
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to discussions of content, wrote the article and researched data for the article. P.M. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks H. M. McGettrick, L. van Baarsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noack, M., Miossec, P. Importance of lymphocyte–stromal cell interactions in autoimmune and inflammatory rheumatic diseases. Nat Rev Rheumatol 17, 550–564 (2021). https://doi.org/10.1038/s41584-021-00665-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00665-4
This article is cited by
-
Post-transcriptional checkpoints in autoimmunity
Nature Reviews Rheumatology (2023)
-
Differential effects of interleukin-17A and 17F on cell interactions between immune cells and stromal cells from synovium or skin
Scientific Reports (2023)
-
The advent of immune stimulating CAFs in cancer
Nature Reviews Cancer (2023)
-
Elevated fatty acid β-oxidation by leptin contributes to the proinflammatory characteristics of fibroblast-like synoviocytes from RA patients via LKB1-AMPK pathway
Cell Death & Disease (2023)
-
Cell–cell interactions shape RA and PsA pathogenesis
Nature Reviews Rheumatology (2022)