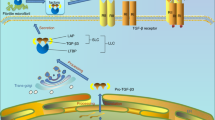
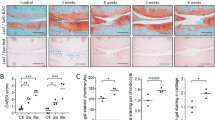
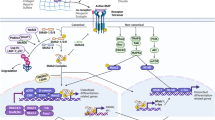
Abstract
Regulated fibroblast growth factor (FGF) signalling is a prerequisite for the correct development and homeostasis of articular cartilage, as evidenced by the fact that aberrant FGF signalling contributes to the maldevelopment of joints and to the onset and progression of osteoarthritis. Of the four FGF receptors (FGFRs 1–4), FGFR1 and FGFR3 are strongly implicated in osteoarthritis, and FGFR1 antagonists, as well as agonists of FGFR3, have shown therapeutic efficacy in mouse models of spontaneous and surgically induced osteoarthritis. FGF18, a high affinity ligand for FGFR3, is the only FGF-based drug currently in clinical trials for osteoarthritis. This Review covers the latest advances in our understanding of the molecular mechanisms that regulate FGF signalling during normal joint development and in the pathogenesis of osteoarthritis. Strategies for FGF signalling-based treatment of osteoarthritis and for cartilage repair in animal models and clinical trials are also introduced. An improved understanding of FGF signalling from a structural biology perspective, and of its roles in skeletal development and diseases, could unlock new avenues for discovery of modulators of FGF signalling that can slow or stop the progression of osteoarthritis.
Key points
-
Fibroblast growth factor (FGF) signalling pathways have important roles in the development and homeostasis of articular cartilage.
-
Aberrant FGF activity contributes to joint deformity and to the onset and progression of osteoarthritis.
-
Structural studies have provided important mechanistic insights into the regulation of FGF signalling.
-
Advances in understanding FGF signalling suggest potential approaches to FGF-based therapeutics for the treatment of osteoarthritis and cartilage injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huey, D. J., Hu, J. C. & Athanasiou, K. A. Unlike bone, cartilage regeneration remains elusive. Science 338, 917–921 (2012).
Zhang, Y. & Jordan, J. M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 26, 355–369 (2010).
Allen, K. D. & Golightly, Y. M. State of the evidence. Curr. Opin. Rheumatol. 27, 276–283 (2015).
Wallace, I. J. et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl Acad. Sci. USA 114, 9332–9336 (2017).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Roemer, F. W. et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809 (2011).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 (2011).
Kwon, H. et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 (2019).
Tiku, M. L. & Sabaawy, H. E. Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 7, 76–87 (2015).
Mastbergen, S. C., Saris, D. B. & Lafeber, F. P. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat. Rev. Rheumatol. 9, 277–290 (2013).
Chijimatsu, R. & Saito, T. Mechanisms of synovial joint and articular cartilage development. Cell. Mol. Life Sci. 76, 3939–3952 (2019).
Ellman, M. B. et al. Fibroblast growth factor control of cartilage homeostasis. J. Cell. Biochem. 114, 735–742 (2013).
Su, N., Jin, M. & Chen, L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2, 14003 (2014).
Ornitz, D. M. & Marie, P. J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 29, 1463–1486 (2015).
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug. Discov. 8, 235–253 (2009).
Itoh, N. & Ornitz, D. M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121–130 (2011).
Goetz, R. et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell Biol. 27, 3417–3428 (2007).
Belov, A. A. & Mohammadi, M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb. Perspect. Biol. 5, a015958 (2013).
Plotnikov, A. N., Schlessinger, J., Hubbard, S. R. & Mohammadi, M. Structural basis for FGF receptor dimerization and activation. Cell 98, 641–650 (1999).
Mohammadi, M., Olsen, S. K. & Ibrahimi, O. A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 16, 107–137 (2005).
Stauber, D. J., DiGabriele, A. D. & Hendrickson, W. A. Structural interactions of fibroblast growth factor receptor with its ligands. Proc. Natl Acad. Sci. USA 97, 49–54 (2000).
Kalinina, J. et al. The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition. Structure 20, 77–88 (2012).
Olsen, S. K. et al. Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc. Natl Acad. Sci. USA 101, 935–940 (2004).
Fantl, W. J., Johnson, D. E. & Williams, L. T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 62, 453–481 (1993).
Givol, D. & Yayon, A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 6, 3362–3369 (1992).
Ornitz, D. M. et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 (1996).
Zhang, X. et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694–15700 (2006).
Schlessinger, J. et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6, 743–750 (2000).
Asada, M. et al. Glycosaminoglycan affinity of the complete fibroblast growth factor family. Biochim. Biophys. Acta 1790, 40–48 (2009).
Sarrazin, S., Lamanna, W. C. & Esko, J. D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952 (2011).
Goetz, R. et al. Conversion of a paracrine fibroblast growth factor into an endocrine fibroblast growth factor. J. Biol. Chem. 287, 29134–29146 (2012).
Goetz, R. & Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14, 166–180 (2013).
Itoh, N., Ohta, H. & Konishi, M. Endocrine FGFs: evolution, physiology, pathophysiology, and pharmacotherapy. Front. Endocrinol. 6, 154 (2015).
Beenken, A. & Mohammadi, M. The structural biology of the FGF19 subfamily. Adv. Exp. Med. Biol. 728, 1–24 (2012).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Goetz, R. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-klotho complex formation. Proc. Natl Acad. Sci. USA 107, 407–412 (2010).
Henrissat, B. & Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7, 637–644 (1997).
Chen, G. et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553, 461–466 (2018).
Ogawa, Y. et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007).
Kurosu, H. et al. Tissue-specific expression of βklotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007).
Makarenkova, H. P. et al. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55 (2009).
Zinkle, A. & Mohammadi, M. Structural biology of the FGF7 subfamily. Front. Genet. 10, 102 (2019).
Huang, Z. et al. Uncoupling the mitogenic and metabolic functions of FGF1 by tuning FGF1-FGF receptor dimer stability. Cell Rep. 20, 1717–1728 (2017).
Zinkle, A. & Mohammadi, M. A threshold model for receptor tyrosine kinase signaling specificity and cell fate determination. F1000Research 7, 872 (2018).
Scotet, E. & Houssaint, E. The choice between alternative IIIb and IIIc exons of the FGFR-3 gene is not strictly tissue-specific. Biochim. Biophys. Acta 1264, 238–242 (1995).
Murgue, B., Tsunekawa, S., Rosenberg, I., de Beaumont, M. & Podolsky, D. K. Identification of a novel variant form of fibroblast growth factor receptor 3 (FGFR3 IIIb) in human colonic epithelium. Cancer Res. 54, 5206–5211 (1994).
Plotnikov, A. N., Hubbard, S. R., Schlessinger, J. & Mohammadi, M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 101, 413–424 (2000).
Yeh, B. K. et al. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc. Natl Acad. Sci. USA 100, 2266–2271 (2003).
Olsen, S. K. et al. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 20, 185–198 (2006).
Liu, Y. et al. Regulation of receptor binding specificity of FGF9 by an autoinhibitory homodimerization. Structure 25, 1325–1336.e3 (2017).
Ornitz, D. M. & Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 (2015).
Bae, J. H. et al. Asymmetric receptor contact is required for tyrosine autophosphorylation of fibroblast growth factor receptor in living cells. Proc. Natl Acad. Sci. USA 107, 2866–2871 (2010).
Chen et al. Molecular basis for receptor tyrosine kinase A-loop tyrosine transphosphorylation. Nat. Chem. Biol. 16, 267–277 (2020).
Mohammadi, M., Schlessinger, J. & Hubbard, S. R. Structure of the FGF receptor tyrosine kinase domain reveals a novel autoinhibitory mechanism. Cell 86, 577–587 (1996).
Chen, H. et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol. Cell 27, 717–730 (2007).
Eswarakumar, V. P., Lax, I. & Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 16, 139–149 (2005).
Mohammadi, M. et al. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol. Cell Biol. 11, 5068–5078 (1991).
Peters, K. G., Werner, S., Chen, G. & Williams, L. T. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development 114, 233–243 (1992).
Newton, A. C. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 101, 2353–2364 (2001).
Rosse, C. et al. PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 11, 103–112 (2010).
Igumenova, T. I. Dynamics and membrane interactions of protein kinase C. Biochemistry 54, 4953–4968 (2015).
Turner, N. & Grose, R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 10, 116–129 (2010).
Shin, E. Y. et al. Basic fibroblast growth factor stimulates activation of Rac1 through a p85 βPIX phosphorylation-dependent pathway. J. Biol. Chem. 279, 1994–2004 (2004).
Lee, J. G. & Kay, E. P. FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Invest. Ophthalmol. Vis. Sci. 47, 1376–1386 (2006).
Schlessinger, J. Phospholipase Cγ activation and phosphoinositide hydrolysis are essential for embryonal development. Proc. Natl Acad. Sci. USA 94, 2798–2799 (1997).
Ellis, M. V. et al. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of PLCδ1. J. Biol. Chem. 273, 11650–11659 (1998).
Kolch, W. et al. Protein kinase Cα activates RAF-1 by direct phosphorylation. Nature 364, 249–252 (1993).
Bunney, T. D. et al. Structural and functional integration of the PLCγ interaction domains critical for regulatory mechanisms and signaling deregulation. Structure 20, 2062–2075 (2012).
Hajicek, N., Charpentier, T. H., Rush, J. R., Harden, T. K. & Sondek, J. Autoinhibition and phosphorylation-induced activation of phospholipase C-γ isozymes. Biochemistry 52, 4810–4819 (2013).
Poulin, B., Sekiya, F. & Rhee, S. G. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-γ1. Proc. Natl Acad. Sci. USA 102, 4276–4281 (2005).
Huang, Z. et al. Two FGF receptor kinase molecules act in concert to recruit and transphosphorylate phospholipase Cγ. Mol. Cell 61, 98–110 (2016).
Wang, J. K., Xu, H., Li, H. C. & Goldfarb, M. Broadly expressed SNT-like proteins link FGF receptor stimulation to activators of Ras. Oncogene 13, 721–729 (1996).
Kouhara, H. et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693–702 (1997).
Hadari, Y. R., Kouhara, H., Lax, I. & Schlessinger, J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell Biol. 18, 3966–3973 (1998).
Gotoh, N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 99, 1319–1325 (2008).
Xu, H., Lee, K. W. & Goldfarb, M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273, 17987–17990 (1998).
Ong, S. H. et al. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell Biol. 20, 979–989 (2000).
Cha, J. Y., Maddileti, S., Mitin, N., Harden, T. K. & Der, C. J. Aberrant receptor internalization and enhanced FRS2-dependent signaling contribute to the transforming activity of the fibroblast growth factor receptor 2 IIIb C3 isoform. J. Biol. Chem. 284, 6227–6240 (2009).
Dhalluin, C. et al. Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors. Mol. Cell 6, 921–929 (2000).
Chardin, P. et al. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260, 1338–1343 (1993).
Li, N. et al. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363, 85–88 (1993).
Simon, J. A. & Schreiber, S. L. Grb2 SH3 binding to peptides from Sos: evaluation of a general model for SH3-ligand interactions. Chem. Biol. 2, 53–60 (1995).
Schaeper, U. et al. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149, 1419–1432 (2000).
Ong, S. H. et al. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl Acad. Sci. USA 98, 6074–6079 (2001).
Lock, L. S., Royal, I., Naujokas, M. A. & Park, M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 275, 31536–31545 (2000).
Lamothe, B. et al. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol. Cell Biol. 24, 5657–5666 (2004).
Rodrigues, G. A., Falasca, M., Zhang, Z., Ong, S. H. & Schlessinger, J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell Biol. 20, 1448–1459 (2000).
Faes, S. & Dormond, O. PI3K and AKT: unfaithful partners in cancer. Int. J. Mol. Sci. 16, 21138–21152 (2015).
Birge, R. B., Kalodimos, C., Inagaki, F. & Tanaka, S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun. Signal. 7, 13 (2009).
Seo, J. H., Suenaga, A., Hatakeyama, M., Taiji, M. & Imamoto, A. Structural and functional basis of a role for CRKL in a fibroblast growth factor 8-induced feed-forward loop. Mol. Cell Biol. 29, 3076–3087 (2009).
Park, T. J. & Curran, T. Essential roles of Crk and CrkL in fibroblast structure and motility. Oncogene 33, 5121–5132 (2014).
Park, T., Koptyra, M. & Curran, T. Fibroblast growth requires CT10 regulator of kinase (Crk) and Crk-like (CrkL). J. Biol. Chem. 291, 26273–26290 (2016).
Collins, T. N. et al. Crk proteins transduce FGF signaling to promote lens fiber cell elongation. eLife 7, e32586 (2018).
Kao, S., Jaiswal, R. K., Kolch, W. & Landreth, G. E. Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 276, 18169–18177 (2001).
Manser, E., Leung, T., Salihuddin, H., Zhao, Z. S. & Lim, L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 (1994).
Zugasti, O. et al. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol. Cell Biol. 21, 6706–6717 (2001).
Lu, W., Gong, D., Bar-Sagi, D. & Cole, P. A. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell 8, 759–769 (2001).
Araki, T., Nawa, H. & Neel, B. G. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J. Biol. Chem. 278, 41677–41684 (2003).
Dance, M., Montagner, A., Salles, J. P., Yart, A. & Raynal, P. The molecular functions of Shp2 in the Ras/mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 20, 453–459 (2008).
Hanafusa, H., Torii, S., Yasunaga, T. & Nishida, E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 4, 850–858 (2002).
Hanafusa, H., Torii, S., Yasunaga, T., Matsumoto, K. & Nishida, E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 279, 22992–22995 (2004).
Ornitz, D. M. & Marie, P. J. Fibroblast growth factors in skeletal development. Curr. Top. Dev. Biol. 133, 195–234 (2019).
Du, X., Xie, Y., Xian, C. J. & Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell. Physiol. 227, 3731–3743 (2012).
Muenke, M. et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat. Genet. 8, 269–274 (1994).
White, K. E. et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 76, 361–367 (2005).
Wilkie, A. O. et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat. Genet. 9, 165–172 (1995).
Meyers, G. A. et al. FGFR2 exon IIIa and IIIc mutations in Crouzon, Jackson-Weiss, and Pfeiffer syndromes: evidence for missense changes, insertions, and a deletion due to alternative RNA splicing. Am. J. Hum. Genet. 58, 491–498 (1996).
Park, W. J. et al. Novel FGFR2 mutations in Crouzon and Jackson-Weiss syndromes show allelic heterogeneity and phenotypic variability. Hum. Mol. Genet. 4, 1229–1233 (1995).
Pulleyn, L. J. et al. Spectrum of craniosynostosis phenotypes associated with novel mutations at the fibroblast growth factor receptor 2 locus. Eur. J. Hum. Genet. 4, 283–291 (1996).
Przylepa, K. A. et al. Fibroblast growth factor receptor 2 mutations in Beare-Stevenson cutis gyrata syndrome. Nat. Genet. 13, 492–494 (1996).
Merrill, A. E. et al. Bent bone dysplasia-FGFR2 type, a distinct skeletal disorder, has deficient canonical FGF signaling. Am. J. Hum. Genet. 90, 550–557 (2012).
Robertson, S. C. et al. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc. Natl Acad. Sci. USA 95, 4567–4572 (1998).
Muenke, M. et al. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am. J. Hum. Genet. 60, 555–564 (1997).
Tavormina, P. L. et al. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat. Genet. 9, 321–328 (1995).
Ye, X. et al. Mutation screening of candidate genes in patients with nonsyndromic sagittal craniosynostosis. Plast. Reconstr. Surg. 137, 952–961 (2016).
Barroso, E. et al. Mild isolated craniosynostosis due to a novel FGFR3 mutation, p.Ala334Thr. Am. J. Med. Genet. A 155A, 3050–3053 (2011).
Rousseau, F. et al. Missense FGFR3 mutations create cysteine residues in thanatophoric dwarfism type I (TD1). Hum. Mol. Genet. 5, 509–512 (1996).
Shiang, R. et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78, 335–342 (1994).
Bellus, G. A. et al. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat. Genet. 10, 357–359 (1995).
Wilkes, D. et al. A recurrent mutation, ala391glu, in the transmembrane region of FGFR3 causes Crouzon syndrome and acanthosis nigricans. J. Med. Genet. 33, 744–748 (1996).
Sarabipour, S. & Hristova, K. Mechanism of FGF receptor dimerization and activation. Nat. Commun. 7, 10262 (2016).
Sarabipour, S. & Hristova, K. FGFR3 unliganded dimer stabilization by the juxtamembrane domain. J. Mol. Biol. 427, 1705–1714 (2015).
Li, E., You, M. & Hristova, K. FGFR3 dimer stabilization due to a single amino acid pathogenic mutation. J. Mol. Biol. 356, 600–612 (2006).
Di Rocco, F. et al. FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum. Mol. Genet. 23, 2914–2925 (2014).
Kannan, K. & Givol, D. FGF receptor mutations: dimerization syndromes, cell growth suppression, and animal models. IUBMB Life 49, 197–205 (2000).
Naski, M. C., Wang, Q., Xu, J. & Ornitz, D. M. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat. Genet. 13, 233–237 (1996).
Agochukwu, N. B., Solomon, B. D., Doherty, E. S. & Muenke, M. Palatal and oral manifestations of Muenke syndrome (FGFR3-related craniosynostosis). J. Craniofac. Surg. 23, 664–668 (2012).
Grillo, L. et al. Increased FGF3 and FGF4 gene dosage is a risk factor for craniosynostosis. Gene 534, 435–439 (2014).
Hardelin, J. P. & Dode, C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex. Dev. 2, 181–193 (2008).
Larsson, T. et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J. Clin. Endocrinol. Metab. 90, 2424–2427 (2005).
Rodriguez-Zabala, M. et al. FGF9 mutation causes craniosynostosis along with multiple synostoses. Hum. Mutat. 38, 1471–1476 (2017).
Rohmann, E. et al. Mutations in different components of FGF signaling in LADD syndrome. Nat. Genet. 38, 414–417 (2006).
Tang, L. et al. A point mutation in Fgf9 impedes joint interzone formation leading to multiple synostoses syndrome. Hum. Mol. Genet. 26, 1280–1293 (2017).
Tekin, M. et al. Homozygous mutations in fibroblast growth factor 3 are associated with a new form of syndromic deafness characterized by inner ear agenesis, microtia, and microdontia. Am. J. Hum. Genet. 80, 338–344 (2007).
Wu, X. L. et al. Multiple synostoses syndrome is due to a missense mutation in exon 2 of FGF9 gene. Am. J. Hum. Genet. 85, 53–63 (2009).
Chefetz, I. et al. A novel homozygous missense mutation in FGF23 causes familial tumoral calcinosis associated with disseminated visceral calcification. Hum. Genet. 118, 261–266 (2005).
Araya, K. et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J. Clin. Endocrinol. Metab. 90, 5523–5527 (2005).
Abbasi, F. et al. A new missense mutation in FGF23 gene in a male with hyperostosis-hyperphosphatemia syndrome (HHS). Gene 542, 269–271 (2014).
ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Gribaa, M. et al. An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J. Bone Miner. Metab. 28, 111–115 (2010).
Shimada, T. et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 (2002).
Ornitz, D. M. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 16, 205–213 (2005).
Long, F. & Ornitz, D. M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 5, a008334 (2013).
Sheeba, C. J., Andrade, R. P., Duprez, D. & Palmeirim, I. Comprehensive analysis of fibroblast growth factor receptor expression patterns during chick forelimb development. Int. J. Dev. Biol. 54, 1517–1526 (2010).
Orr-Urtreger, A., Givol, D., Yayon, A., Yarden, Y. & Lonai, P. Developmental expression of two murine fibroblast growth factor receptors, flg and bek. Development 113, 1419–1434 (1991).
Moon, A. M., Boulet, A. M. & Capecchi, M. R. Normal limb development in conditional mutants of Fgf4. Development 127, 989–996 (2000).
Mariani, F. V., Ahn, C. P. & Martin, G. R. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 453, 401–405 (2008).
Jin, L., Wu, J., Bellusci, S. & Zhang, J.-S. Fibroblast growth factor 10 and vertebrate limb development. Front. Genet. 9, 705 (2019).
Delezoide, A. L. et al. Spatio-temporal expression of FGFR 1, 2 and 3 genes during human embryo-fetal ossification. Mech. Dev. 77, 19–30 (1998).
Eswarakumar, V. P. et al. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development 129, 3783–3793 (2002).
Jacob, A. L., Smith, C., Partanen, J. & Ornitz, D. M. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev. Biol. 296, 315–328 (2006).
Lazarus, J. E., Hegde, A., Andrade, A. C., Nilsson, O. & Baron, J. Fibroblast growth factor expression in the postnatal growth plate. Bone 40, 577–586 (2007).
Partanen, J. et al. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 10, 1347–1354 (1991).
Cool, S., Jackson, R., Pincus, P., Dickinson, I. & Nurcombe, V. Fibroblast growth factor receptor 4 (FGFR4) expression in newborn murine calvaria and primary osteoblast cultures. Int. J. Dev. Biol. 46, 519–523 (2002).
Karolak, M. R., Yang, X. & Elefteriou, F. FGFR1 signaling in hypertrophic chondrocytes is attenuated by the Ras-GAP neurofibromin during endochondral bone formation. Hum. Mol. Genet. 24, 2552–2564 (2015).
Hung, I. H., Yu, K., Lavine, K. J. & Ornitz, D. M. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev. Biol. 307, 300–313 (2007).
Karuppaiah, K. et al. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development 143, 1811–1822 (2016).
Ohbayashi, N. et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 16, 870–879 (2002).
Longobardi, L. et al. Synovial joints: from development to homeostasis. Curr. Osteoporos. Rep. 13, 41–51 (2015).
Lovinescu, I., Koyama, E. & Pacifici, M. Roles of FGF-10 on the development of diathrodial limb joints. Penn Dent. J. 103, 5–9 (2003).
Yan, D. et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res. Ther. 13, R130 (2011).
Chen, T. M., Chen, Y. H., Sun, H. S. & Tsai, S. J. Fibroblast growth factors: potential novel targets for regenerative therapy of osteoarthritis. Chin. J. Physiol. 62, 2–10 (2019).
Hagan, A. S. et al. Generation and validation of novel conditional flox and inducible Cre alleles targeting fibroblast growth factor 18 (Fgf18). Dev. Dyn. 248, 882–893 (2019).
Malemud, C. J. Growth hormone, VEGF and FGF: involvement in rheumatoid arthritis. Clin. Chim. Acta 375, 10–19 (2007).
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Loeser, R. F., Collins, J. A. & Diekman, B. O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420 (2016).
Daouti, S. et al. Development of comprehensive functional genomic screens to identify novel mediators of osteoarthritis. Osteoarthr. Cartilage 13, 508–518 (2005).
Im, H. J. et al. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cδ pathways in human adult articular chondrocytes. J. Biol. Chem. 282, 11110–11121 (2007).
Weng, T. et al. Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis Rheum. 64, 3982–3992 (2012).
Xu, W. et al. A novel fibroblast growth factor receptor 1 inhibitor protects against cartilage degradation in a murine model of osteoarthritis. Sci. Rep. 6, 24042 (2016).
Tang, J. et al. Fibroblast growth factor receptor 3 inhibits osteoarthritis progression in the knee joints of adult mice. Arthritis Rheumatol. 68, 2432–2443 (2016).
Zhou, S. et al. Conditional deletion of Fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Sci. Rep. 6, 24039 (2016).
Kuang, L. et al. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann. Rheum. Dis. 79, 112–122 (2020).
Klag, K. A. & Horton, W. A. Advances in treatment of achondroplasia and osteoarthritis. Hum. Mol. Genet. 25, R2–R8 (2016).
Kisand, K., Tamm, A. E., Lintrop, M. & Tamm, A. O. New insights into the natural course of knee osteoarthritis: early regulation of cytokines and growth factors, with emphasis on sex-dependent angiogenesis and tissue remodeling. A pilot study. Osteoarthr. Cartilage 26, 1045–1054 (2018).
Im, H. J. et al. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J. Cell. Physiol. 215, 452–463 (2008).
Vincent, T. L., McLean, C. J., Full, L. E., Peston, D. & Saklatvala, J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthr. Cartilage 15, 752–763 (2007).
Muddasani, P., Norman, J. C., Ellman, M., van Wijnen, A. J. & Im, H. J. Basic fibroblast growth factor activates the MAPK and NFκB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J. Biol. Chem. 282, 31409–31421 (2007).
Nummenmaa, E., Hamalainen, M., Moilanen, T., Vuolteenaho, K. & Moilanen, E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand. J. Rheumatol. 44, 321–330 (2015).
Chong, K. W. et al. Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum. 65, 2346–2355 (2013).
Sawaji, Y., Hynes, J., Vincent, T. & Saklatvala, J. Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage. Arthritis Rheum. 58, 3498–3509 (2008).
Tang, Z. F. & Li, H. Y. Effects of fibroblast growth factors 2 and low intensity pulsed ultrasound on the repair of knee articular cartilage in rabbits. Eur. Rev. Med. Pharmacol. Sci. 22, 2447–2453 (2018).
Cuevas, P., Burgos, J. & Baird, A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem. Biophys. Res. Commun. 156, 611–618 (1988).
Chia, S. L. et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 60, 2019–2027 (2009).
Burt, P. M., Xiao, L., Doetschman, T. & Hurley, M. M. Ablation of low-molecular-weight FGF2 isoform accelerates murine osteoarthritis while loss of high-molecular-weight FGF2 isoforms offers protection. J. Cell. Physiol. 234, 4418–4431 (2019).
Li, R. et al. Upregulation of fibroblast growth factor 1 in the synovial membranes of patients with late stage osteoarthritis. Genet. Mol. Res. 14, 11191–11199 (2015).
El-Seoudi, A. et al. Catabolic effects of FGF-1 on chondrocytes and its possible role in osteoarthritis. J. Cell Commun. Signal. 11, 255–263 (2017).
Uchii, M. et al. Role of fibroblast growth factor 8 (FGF8) in animal models of osteoarthritis. Arthritis Res. Ther. 10, R90 (2008).
Rockel, J. S. et al. Hedgehog inhibits β-catenin activity in synovial joint development and osteoarthritis. J. Clin. Invest. 126, 1649–1663 (2016).
Mori, Y. et al. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J. Biol. Chem. 289, 10192–10200 (2014).
Ellsworth, J. L. et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthr. Cartilage 10, 308–320 (2002).
Moore, E. E. et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartilage 13, 623–631 (2005).
Zhou, S. et al. Exogenous fibroblast growth factor 9 attenuates cartilage degradation and aggravates osteophyte formation in post-traumatic osteoarthritis. Osteoarthr. Cartilage 24, 2181–2192 (2016).
Sun, Y. et al. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet. Disord. 11, 19 (2010).
Li, Z. C. et al. Fibroblast growth factor-21 concentration in serum and synovial fluid is associated with radiographic bone loss of knee osteoarthritis. Scand. J. Clin. Lab. Invest. 75, 121–125 (2015).
Bianchi, A. et al. Fibroblast growth factor 23 drives MMP13 expression in human osteoarthritic chondrocytes in a Klotho-independent manner. Osteoarthr. Cartilage 24, 1961–1969 (2016).
Meo Burt, P., Xiao, L. & Hurley, M. M. FGF23 regulates Wnt/β-catenin signaling-mediated osteoarthritis in mice overexpressing high-molecular-weight FGF2. Endocrinology 159, 2386–2396 (2018).
Kim, J. H., Lee, M. C., Seong, S. C., Park, K. H. & Lee, S. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng. Part A 17, 991–1002 (2011).
Li, J. & Pei, M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng. Part A 17, 703–712 (2011).
Chen, X. et al. Integration capacity of human induced pluripotent stem cell-derived cartilage. Tissue Eng. Part A 25, 437–445 (2018).
Gigout, A. et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr. Cartilage 25, 1858–1867 (2017).
Meloni, G. R. et al. Recombinant human FGF18 preserves depth-dependent mechanical inhomogeneity in articular cartilage. Eur. Cell Mater. 38, 23–34 (2019).
Sennett, M. L. et al. Sprifermin treatment enhances cartilage integration in an in vitro repair model. J. Orthop. Res. 36, 2648–2656 (2018).
Barr, L., Getgood, A., Guehring, H., Rushton, N. & Henson, F. M. The effect of recombinant human fibroblast growth factor-18 on articular cartilage following single impact load. J. Orthop. Res. 32, 923–927 (2014).
Yao, X. et al. Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol. Res. 139, 314–324 (2019).
Howard, D., Wardale, J., Guehring, H. & Henson, F. Delivering rhFGF-18 via a bilayer collagen membrane to enhance microfracture treatment of chondral defects in a large animal model. J. Orthop. Res. 33, 1120–1127 (2015).
Power, J., Hernandez, P., Guehring, H., Getgood, A. & Henson, F. Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J. Orthop. Res. 32, 669–676 (2014).
Eckstein, F., Wirth, W., Guermazi, A., Maschek, S. & Aydemir, A. Brief report: intraarticular sprifermin not only increases cartilage thickness, but also reduces cartilage loss: location-independent post hoc analysis using magnetic resonance imaging. Arthritis Rheumatol. 67, 2916–2922 (2015).
Lohmander, L. S. et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 1820–1831 (2014).
Hochberg, M. C. et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA 322, 1360–1370 (2019).
Sanghani, A., Chimutengwende-Gordon, M., Adesida, A. & Khan, W. Applications of stem cell therapy for physeal injuries. Curr. Stem Cell Res. Ther. 8, 451–455 (2013).
Chung, R. & Xian, C. J. Recent research on the growth plate: mechanisms for growth plate injury repair and potential cell-based therapies for regeneration. J. Mol. Endocrinol. 53, T45–T61 (2014).
Henson, F. M., Bowe, E. A. & Davies, M. E. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthr. Cartilage 13, 537–544 (2005).
Cucchiarini, M., Schetting, S., Terwilliger, E. F., Kohn, D. & Madry, H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and α-SMA expression in human meniscal lesions. Gene Ther. 16, 1363–1372 (2009).
Schmidt, L., Taiyab, A., Melvin, V. S., Jones, K. L. & Williams, T. Increased FGF8 signaling promotes chondrogenic rather than osteogenic development in the embryonic skull. Dis. Model. Mech. 11, dmm.031526 (2018).
Dai, J. et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 33, 7699–7711 (2012).
Tan, Q. et al. A novel FGFR1-binding peptide attenuates the degeneration of articular cartilage in adult mice. Osteoarthr. Cartilage 26, 1733–1743 (2018).
Mobasheri, A. et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13, 302–311 (2017).
Makower, A. M., Wroblewski, J. & Pawlowski, A. Effects of IGF-I, rGH, FGF, EGF and NCS on DNA-synthesis, cell proliferation and morphology of chondrocytes isolated from rat rib growth cartilage. Cell Biol. Int. Rep. 13, 259–270 (1989).
Acknowledgements
M.M. and A.Z. acknowledge support provided by the National Institutes of Health (grant R01 DE13686 to M.M.). L.C. and X.Y. acknowledge support provided by the National Key Research and Development Program of China (grant 2018YFA0800802 to L.C.) and the National Natural Science Foundation of China (grants 81530071 and 81830075 to L.C.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Protomers
-
The smallest repeatable structural subunits of hetero-oligomeric protein complexes.
- Glycosyl hydrolase clan A
-
A group of glycosyl hydrolase families defined by its members containing one or two (β/α)8 triosephosphate isomerase barrel domains.
- Apo unphosphorylated kinase
-
A kinase that is unbound (apo) and inactive (unphosphorylated).
- Mesenchymal condensation
-
The first step in chondroinduction, in which the mesenchyme, the meshwork of embryonic connective tissue from which cartilage and bone are derived, condenses in one place.
- Growth plate
-
Cartilage layer lying between the epiphyses and metaphyses, the developmental centre for longitudinal bone growth through endochondral ossification, also known as the epiphyseal plate.
- Apical ectodermal ridge
-
The layer of surface ectodermal cells at the apex of the embryonic limb bud that acts as the signalling centre for the condensation of underlying mesenchyme and is necessary for limb outgrowth.
- Prechondrogenic condensation region
-
A region of prechondrogenic cells that precede the development of cartilage (cartilage anlagen).
- Perichondrium
-
A layer of dense fibrous connective tissue covering the surface of cartilage in developing bone.
- Periosteum
-
A layer of membranous connective tissue present in the surface of bone, containing bone stem (or progenitor) cells and possessing bone-forming potentialities.
- Hypertrophic chondrocytes
-
The terminally differentiated form of chondrocytes, which are enlarged and secrete large amounts of type X collagen.
- Superficial zone
-
The surface zone of articular cartilage that contains cells with stem or chondro-progenitor capacity and is characterized by elongated and flat-shaped cells oriented parallel to the articular surface.
Rights and permissions
About this article
Cite this article
Xie, Y., Zinkle, A., Chen, L. et al. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat Rev Rheumatol 16, 547–564 (2020). https://doi.org/10.1038/s41584-020-0469-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-0469-2
This article is cited by
-
Expression and Purification of FGFR1-Fc Fusion Protein and Its Effects on Human Lung Squamous Carcinoma
Applied Biochemistry and Biotechnology (2024)
-
Evaluating the Patterns of FAPI Uptake in the Shoulder Joint: a Preliminary Study Comparing with FDG Uptake in Oncological Studies
Molecular Imaging and Biology (2024)
-
Effects and action mechanisms of individual cytokines contained in PRP on osteoarthritis
Journal of Orthopaedic Surgery and Research (2023)
-
Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration
Journal of Nanobiotechnology (2023)
-
FGF19 induces the cell cycle arrest at G2-phase in chondrocytes
Cell Death Discovery (2023)