Abstract
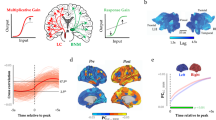
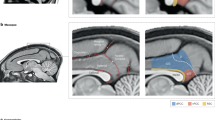
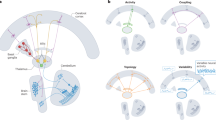
The locus coeruleus (LC), or ‘blue spot’, is a small nucleus located deep in the brainstem that provides the far-reaching noradrenergic neurotransmitter system of the brain. This phylogenetically conserved nucleus has proved relatively intractable to full characterization, despite more than 60 years of concerted efforts by investigators. Recently, an array of powerful new neuroscience tools have provided unprecedented access to this elusive nucleus, revealing new levels of organization and function. We are currently at the threshold of major discoveries regarding how this tiny brainstem structure exerts such varied and significant influences over brain function and behaviour. All LC neurons receive inputs related to autonomic arousal, but distinct subpopulations of those neurons can encode specific cognitive processes, presumably through more specific inputs from the forebrain areas. This ability, combined with specific patterns of innervation of target areas and heterogeneity in receptor distributions, suggests that activation of the LC has more specific influences on target networks than had initially been imagined.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Totah, N. K. B., Logothetis, N. K. & Eschenko, O. Noradrenergic ensemble-based modulation of cognition over multiple timescales. Brain Res. 1709, 50–66 (2019).
Likhtik, E. & Johansen, J. P. Neuromodulation in circuits of aversive emotional learning. Nat. Neurosci. 22, 1586–1597 (2019).
Chandler, D. J. et al. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. J. Neurosci. 39, 8239–8249 (2019).
Kebschull, J. M. et al. High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987 (2016).
Robertson, S. D., Plummer, N. W. & Jensen, P. Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain Res. 1641, 234–244 (2016).
Schwarz, L. A. et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Uematsu, A. et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611 (2017). This behavioural study in rats reveals a modular organization of LC with projection and behaviour-specific cell populations.
Plummer, N. W. et al. An intersectional viral-genetic method for fluorescent tracing of axon collaterals reveals details of noradrenergic locus coeruleus structure. eNeuro 7, ENEURO.0010-20.202 (2020).
Agster, K. L., Mejias-Aponte, C. A., Clark, B. D. & Waterhouse, B. D. Evidence for a regional specificity in the density and distribution of noradrenergic varicosities in rat cortex. J. Comp. Neurol. 521, 2195–2207 (2013).
Lewis, D. A. & Morrison, J. H. Noradrenergic innervation of monkey prefrontal cortex: a dopamine-β-hydroxylase immunohistochemical study. J. Comp. Neurol. 282, 317–330 (1989).
Morrison, J. H. & Foote, S. L. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J. Comp. Neurol. 243, 117–138 (1986).
Hirschberg, S., Li, Y., Randall, A., Kremer, E. J. & Pickering, A. E. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 6, e29808 (2017). This study reveals the modular organization of LC with projection and behaviour-specific cell populations.
Waterhouse, B. D. & Chandler, D. J. Heterogeneous organization and function of the central noradrenergic system. Brain Res. 1641, v–x (2016).
Chandler, D. J., Gao, W. J. & Waterhouse, B. D. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl Acad. Sci. USA 111, 6816–6821 (2014). This comprehensive study uses anatomical, molecular and electrophysiological approaches to demonstrate the heterogeneity of LC cell populations projecting to prefrontal or motor cortices.
Chandler, D. J., Waterhouse, B. D. & Gao, W. J. New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front. Neural Circuits 8, 53 (2014).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 (2019).
Feng, J. et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019).
Shipley, M. T., Fu, L., Ennis, M., Liu, W. L. & Aston-Jones, G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J. Comp. Neurol. 365, 56–68 (1996).
Aston-Jones, G., Zhu, Y. & Card, J. P. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J. Neurosci. 24, 2313–2321 (2004).
Breton-Provencher, V. & Sur, M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22, 218–228 (2019).
Aston-Jones, G., Ennis, M., Pieribone, V. A., Nickell, W. T. & Shipley, M. T. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234, 734–737 (1986).
Luppi, P. H., Aston-Jones, G., Akaoka, H., Chouvet, G. & Jouvet, M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience 65, 119–160 (1995).
Aston-Jones, G., Chen, S., Zhu, Y. & Oshinsky, M. L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 4, 732–738 (2001).
Takeuchi, T. et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016).
Castren, E., Thoenen, H. & Lindholm, D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience 64, 71–80 (1995).
Conner, J. M., Lauterborn, J. C., Yan, Q., Gall, C. M. & Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 17, 2295–2313 (1997).
Koylu, E. O., Smith, Y., Couceyro, P. R. & Kuhar, M. J. CART peptides colocalize with tyrosine hydroxylase neurons in rat locus coeruleus. Synapse 31, 309–311 (1999).
Simpson, K. L., Waterhouse, B. D. & Lin, R. C. Origin, distribution, and morphology of galaninergic fibers in the rodent trigeminal system. J. Comp. Neurol. 411, 524–534 (1999).
Xu, Z. Q., Shi, T. J. & Hokfelt, T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J. Comp. Neurol. 392, 227–251 (1998).
Devoto, P., Flore, G., Saba, P., Fa, M. & Gessa, G. L. Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci. 6, 31 (2005).
Devoto, P., Flore, G., Pani, L. & Gessa, G. L. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatry 6, 657–664 (2001). This study is an early demonstration that LC axonal terminals can co-release dopamine and noradrenaline.
Perez, S. E., Wynick, D., Steiner, R. A. & Mufson, E. J. Distribution of galaninergic immunoreactivity in the brain of the mouse. J. Comp. Neurol. 434, 158–185 (2001).
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Wang, H., Jing, M. & Li, Y. Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Curr. Opin. Neurobiol. 50, 171–178 (2018).
Beas, B. S. et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 21, 963–973 (2018).
Kempadoo, K. A., Mosharov, E. V., Choi, S. J., Sulzer, D. & Kandel, E. R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl Acad. Sci. USA 113, 14835–14840 (2016).
Wagatsuma, A. et al. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl Acad. Sci. USA 115, E310–E316 (2018).
Pomrenze, M. B. et al. Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Rep. 29, 13–21.e14 (2019).
Tillage, R. P. et al. Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Struct. Funct. 225, 785–803 (2020).
Sonneborn, A. & Greene, R. W. The norepinephrine transporter regulates dopamine-dependent synaptic plasticity in the mouse dorsal hippocampus. Preprint at bioRxiv https://doi.org/10.1101/793265 (2019).
Berridge, C. W. & Abercrombie, E. D. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience 93, 1263–1270 (1999).
Florin-Lechner, S. M., Druhan, J. P., Aston-Jones, G. & Valentino, R. J. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 742, 89–97 (1996).
Venton, B. J. & Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 145, 1158–1168 (2020).
Bucher, E. S. & Wightman, R. M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. 8, 239–261 (2015).
Schmidt, K. T. & McElligott, Z. A. Dissecting the catecholamines: how new approaches will facilitate the distinction between noradrenergic and dopaminergic systems. ACS Chem. Neurosci. 10, 1872–1874 (2019).
Roberts, J. G. & Sombers, L. A. Fast-scan cyclic voltammetry: chemical sensing in the brain and beyond. Anal. Chem. 90, 490–504 (2018).
Liberzon, I. et al. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry 71, 1174–1182 (2014).
McCune, S. K. & Hill, J. M. Ontogenic expression of two α-1 adrenergic receptor subtypes in the rat brain. J. Mol. Neurosci. 6, 51–62 (1995).
MacDonald, E. & Scheinin, M. Distribution and pharmacology of α2-adrenoceptors in the central nervous system. J. Physiol. Pharmacol. 46, 241–258 (1995).
Scheinin, M. et al. Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol. Brain Res. 21, 133–149 (1994).
Civantos Calzada, B. & Aleixandre de Artinano, A. α-Adrenoceptor subtypes. Pharmacol. Res. 44, 195–208 (2001).
Molinoff, P. B. α- and β-Adrenergic receptor subtypes properties, distribution and regulation. Drugs 28, 1–15 (1984).
Hertz, L., Chen, Y., Gibbs, M. E., Zang, P. & Peng, L. Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr. Drug. Targets CNS Neurol. Disord. 3, 239–267 (2004).
Nalepa, I., Kreiner, G., Bielawski, A., Rafa-Zablocka, K. & Roman, A. α1-Adrenergic receptor subtypes in the central nervous system: insights from genetically engineered mouse models. Pharmacol. Rep. 65, 1489–1497 (2013).
Plummer, N. W., Scappini, E. L., Smith, K. G., Tucker, C. J. & Jensen, P. Two subpopulations of noradrenergic neurons in the locus coeruleus complex distinguished by expression of the dorsal neural tube marker Pax7. Front. Neuroanat. 11, 60 (2017).
Hirsch, M. R., Tiveron, M. C., Guillemot, F., Brunet, J. F. & Goridis, C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125, 599–608 (1998).
Brunet, J. F. & Pattyn, A. Phox2 genes — from patterning to connectivity. Curr. Opin. Genet. Dev. 12, 435–440 (2002).
Holm, P. C. et al. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development 130, 3535–3545 (2003).
Shi, M. et al. Notch–Rbpj signaling is required for the development of noradrenergic neurons in the mouse locus coeruleus. J. Cell Sci. 125, 4320–4332 (2012).
Goridis, C. & Rohrer, H. Specification of catecholaminergic and serotonergic neurons. Nat. Rev. Neurosci. 3, 531–541 (2002).
Li, S. et al. Conversion of astrocytes and fibroblasts into functional noradrenergic neurons. Cell Rep. 28, 682–697.e687 (2019).
Marshall, K. C., Christie, M. J., Finlayson, P. G. & Williams, J. T. Developmental aspects of the locus coeruleus–noradrenaline system. Prog. Brain Res. 88, 173–185 (1991).
Nakamura, S., Kimura, F. & Sakaguchi, T. Postnatal development of electrical activity in the locus ceruleus. J. Neurophysiol. 58, 510–524 (1987).
Debiec, J. & Sullivan, R. M. The neurobiology of safety and threat learning in infancy. Neurobiol. Learn. Mem. 143, 49–58 (2017).
Caldji, C. et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl Acad. Sci. USA 95, 5335–5340 (1998).
Hassani, O. K. et al. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: implications for development and Parkinson’s disease. Neurobiol. Aging 85, 22–37 (2020).
Christie, M. J. Generators of synchronous activity of the locus coeruleus during development. Semin. Cell Dev. Biol. 8, 29–34 (1997).
Bezin, L., Marcel, D., Desgeorges, S., Pujol, J. F. & Weissmann, D. Singular subsets of locus coeruleus neurons may recover tyrosine hydroxylase phenotype transiently expressed during development. Mol. Brain Res. 76, 275–281 (2000).
Williams, J. T. & Marshall, K. C. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J. Neurosci. 7, 3687–3694 (1987).
Ennis, M. & Aston-Jones, G. Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res. 374, 299–305 (1986).
Williams, J. T., North, R. A., Shefner, S. A., Nishi, S. & Egan, T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience 13, 137–156 (1984).
Bouret, S. & Sara, S. J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005). The authors develop the hypothesis that NA released in forebrain structures in response to prediction error promotes resetting of cortical networks and cognitive flexibility.
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84 (2003).
Berridge, C. W., Schmeichel, B. E. & Espana, R. A. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev. 16, 187–197 (2012).
Alreja, M. & Aghajanian, G. K. Use of the whole-cell patch–clamp method in studies on the role of cAMP in regulating the spontaneous firing of locus coeruleus neurons. J. Neurosci. Methods 59, 67–75 (1995).
Wagner-Altendorf, T. A., Fischer, B. & Roeper, J. Axonal projection-specific differences in somatodendritic α2 autoreceptor function in locus coeruleus neurons. Eur. J. Neurosci. 50, 3772–3785 (2019).
Cadwell, C. R. et al. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat. Protoc. 12, 2531–2553 (2017).
Totah, N. K., Neves, R. M., Panzeri, S., Logothetis, N. K. & Eschenko, O. The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 99, 1055–1068.e1056 (2018).
Arnsten, A. F. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 69, e89–e99 (2011).
Spencer, R. C. & Berridge, C. W. Receptor and circuit mechanisms underlying differential procognitive actions of psychostimulants. Neuropsychopharmacology 44, 1820–1827 (2019).
Harley, C. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog. Brain Res. 88, 307–321 (1991).
Sara, S. J., Vankov, A. & Herve, A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull. 35, 457–465 (1994).
McGaugh, J. L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28 (2004).
Sara, S. J. & Segal, M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog. Brain Res. 88, 571–585 (1991). The study is one of the first demonstrations in a behaving animal of rapid responses of LC neurons to changes in reinforcement contingencies in a formal learning protocol.
Aston-Jones, G., Rajkowski, J. & Kubiak, P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80, 697–715 (1997).
Jahn, C. I. et al. Dual contributions of noradrenaline to behavioural flexibility and motivation. Psychopharmacology 235, 2687–2702 (2018).
Weinshenker, D. & Schroeder, J. P. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32, 1433–1451 (2007).
Waterhouse, B. D. & Navarra, R. L. The locus coeruleus–norepinephrine system and sensory signal processing: A historical review and current perspectives. Brain Res. 1709, 1–15 (2019).
Sara, S. J. & Bouret, S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012).
Foote, S. L., Freedman, R. & Oliver, A. P. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 86, 229–242 (1975). This is the first demonstration in a behaving animal (in the awake monkey) that NA modulates signal to noise ratios in a sensory cortex.
Rogawski, M. A. & Aghajanian, G. K. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature 287, 731–734 (1980).
Manunta, Y. & Edeline, J. M. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J. Neurophysiol. 92, 1445–1463 (2004).
Devilbiss, D. M., Page, M. E. & Waterhouse, B. D. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J. Neurosci. 26, 9860–9872 (2006).
McCormick, D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221 (1989).
Vazey, E. M., Moorman, D. E. & Aston-Jones, G. Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl Acad. Sci. USA 115, E9439–E9448 (2018).
Lecas, J. C. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur. J. Neurosci. 19, 2519–2530 (2004).
Bouret, S. & Sara, S. J. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur. J. Neurosci. 16, 2371–2382 (2002).
McLean, J. & Waterhouse, B. D. Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res. 667, 83–97 (1994).
Waterhouse, B. D., Azizi, S. A., Burne, R. A. & Woodward, D. J. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 514, 276–292 (1990).
Escanilla, O., Arrellanos, A., Karnow, A., Ennis, M. & Linster, C. Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur. J. Neurosci. 32, 458–468 (2010).
Martins, A. R. & Froemke, R. C. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 18, 1483–1492 (2015).
Navarra, R. L., Clark, B. D., Gargiulo, A. T. & Waterhouse, B. D. Methylphenidate enhances early-stage sensory processing and rodent performance of a visual signal detection task. Neuropsychopharmacology 42, 1326–1337 (2017).
Devilbiss, D. M. & Waterhouse, B. D. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J. Neurosci. 24, 10773–10785 (2004).
Devilbiss, D. M. & Waterhouse, B. D. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37, 273–282 (2000).
Gelbard-Sagiv, H., Magidov, E., Sharon, H., Hendler, T. & Nir, Y. Noradrenaline modulates visual perception and late visually evoked activity. Curr. Biol. 28, 2239–2249.e2236 (2018).
McCarley, R. W. & Hobson, J. A. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science 189, 58–60 (1975).
Aston-Jones, G. & Bloom, F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886 (1981).
Carter, M. E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Lovett-Barron, M. et al. Ancestral circuits for the coordinated modulation of brain state. Cell 171, 1411–1423.e1417 (2017).
Hayat, H. et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 6, eaaz4232 (2020).
Joshi, S., Li, Y., Kalwani, R. M. & Gold, J. I. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 (2016).
Reimer, J. et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016).
Pettigrew, J. D. Pharmacologic control of cortical plasticity. Retina 2, 360–372 (1982).
Pettigrew, J. D. & Kasamatsu, T. Local perfusion of noradrenaline maintains visual cortical plasticity. Nature 271, 761–763 (1978).
Sullivan, R. M., Wilson, D. A. & Leon, M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J. Neurosci. 9, 3998–4006 (1989).
Neuman, R. S. & Harley, C. W. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 273, 162–165 (1983).
Stanton, P. K. & Sarvey, J. M. Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res. 361, 276–283 (1985).
Vankov, A., Herve-Minvielle, A. & Sara, S. J. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci. 7, 1180–1187 (1995).
Grella, S. L. et al. Locus coeruleus phasic, but not tonic, activation initiates global remapping in a familiar environment. J. Neurosci. 39, 445–455 (2019).
Hagena, H., Hansen, N. & Manahan-Vaughan, D. β-Adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and memory. Cereb. Cortex 26, 1349–1364 (2016).
Lemon, N., Aydin-Abidin, S., Funke, K. & Manahan-Vaughan, D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb. Cortex 19, 2827–2837 (2009).
Salgado, H., Kohr, G. & Trevino, M. Noradrenergic ‘tone’ determines dichotomous control of cortical spike-timing-dependent plasticity. Sci. Rep. 2, 417 (2012).
Poe, G. R., Walsh, C. M. & Bjorness, T. E. Both duration and timing of sleep are important to memory consolidation. Sleep 33, 1277–1278 (2010).
Mather, M., Clewett, D., Sakaki, M. & Harley, C. W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 39, e200 (2016).
Toussay, X., Basu, K., Lacoste, B. & Hamel, E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci. 33, 3390–3401 (2013).
O’Donnell, J., Ding, F. & Nedergaard, M. Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr. Sleep Med. Rep. 1, 1–8 (2015).
Oe, Y. et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun. 11, 471 (2020).
Porter-Stransky, K. A. et al. Noradrenergic transmission at α1-adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biol. Psychiatry 85, 237–247 (2019).
Kaufman, A. M., Geiller, T. & Losonczy, A. A role for the locus coeruleus in hippocampal CA1 place cell reorganization during spatial reward learning. Neuron 105, 1018–1026.e4 (2020).
Kitchigina, V., Vankov, A., Harley, C. & Sara, S. J. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci. 9, 41–47 (1997).
Hansen, N. & Manahan-Vaughan, D. Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by β-adrenoreceptors and the locus coeruleus. Hippocampus 25, 1285–1298 (2015).
Hansen, N. & Manahan-Vaughan, D. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cereb. Cortex 25, 1889–1896 (2015).
Sara, S. J. Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front. Behav. Neurosci. 4, 185 (2010).
Ferry, B., Roozendaal, B. & McGaugh, J. L. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J. Neurosci. 19, 5119–5123 (1999).
Clayton, E. C. & Williams, C. L. Posttraining inactivation of excitatory afferent input to the locus coeruleus impairs retention in an inhibitory avoidance learning task. Neurobiol. Learn. Mem. 73, 127–140 (2000).
Cahill, L. Neurobiological mechanisms of emotionally influenced, long-term memory. Prog. Brain Res. 126, 29–37 (2000).
Eschenko, O., Magri, C., Panzeri, S. & Sara, S. J. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up–down states during sleep. Cereb. Cortex 22, 426–435 (2012).
Sara, S. J. Locus coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 35, 87–94 (2015).
Bernabeu, R. et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl Acad. Sci. USA 94, 7041–7046 (1997).
O’Dell, T. J., Connor, S. A., Guglietta, R. & Nguyen, P. A. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 22, 461–471 (2015).
McGaughy, J., Ross, R. S. & Eichenbaum, H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153, 63–71 (2008).
Reynaud, A. J. et al. Atomoxetine improves attentional orienting in a predictive context. Neuropharmacology 150, 59–69 (2019).
Berridge, C. W. & Spencer, R. C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 1641, 189–196 (2016).
Bouret, S. & Sara, S. J. Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. Eur. J. Neurosci. 20, 791–802 (2004).
Xiang, L. et al. Behavioral correlates of activity of optogenetically identified locus coeruleus noradrenergic neurons in rats performing T-maze tasks. Sci. Rep. 9, 1361 (2019).
Aston-Jones, G., Rajkowski, J. & Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 (1999).
Devauges, V. & Sara, S. J. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav. Brain Res. 39, 19–28 (1990).
Tait, D. S. et al. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci. 25, 3719–3724 (2007).
Snyder, K., Wang, W. W., Han, R., McFadden, K. & Valentino, R. J. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37, 520–530 (2012).
Cope, Z. A., Vazey, E. M., Floresco, S. B. & Aston Jones, G. S. DREADD-mediated modulation of locus coeruleus inputs to mPFC improves strategy set-shifting. Neurobiol. Learn. Mem. 161, 1–11 (2019).
Tervo, D. G. R. et al. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell 159, 21–32 (2014).
Janitzky, K. et al. Optogenetic silencing of locus coeruleus activity in mice impairs cognitive flexibility in an attentional set-shifting task. Front. Behav. Neurosci. 9, 286 (2015).
von der Gablentz, J., Tempelmann, C., Munte, T. F. & Heldmann, M. Performance monitoring and behavioral adaptation during task switching: an fMRI study. Neuroscience 285, 227–235 (2015).
Hermans, E. J. et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153 (2011).
Bouret, S. & Richmond, B. J. Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci. 35, 4005–4014 (2015).
Uematsu, A., Tan, B. Z. & Johansen, J. P. Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn. Mem. 22, 444–451 (2015).
Rajkowski, J., Majczynski, H., Clayton, E. & Aston-Jones, G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J. Neurophysiol. 92, 361–371 (2004).
Kalwani, R. M., Joshi, S. & Gold, J. I. Phasic activation of individual neurons in the locus ceruleus/subceruleus complex of monkeys reflects rewarded decisions to go but not stop. J. Neurosci. 34, 13656–13669 (2014).
Varazzani, C., San-Galli, A., Gilardeau, S. & Bouret, S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J. Neurosci. 35, 7866–7877 (2015).
Borderies, N., Mattioni, J., Bornert, P., Gilardeau, S. & Bouret, S. Pharmacological evidence for the implication of noradrenaline in effort. Preprint at bioRxiv https://doi.org/10.1101/714923 (2020).
Shenhav, A. et al. Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci. 40, 99–124 (2017).
Berridge, C. W. & Arnsten, A. F. Psychostimulants and motivated behavior: arousal and cognition. Neurosci. Biobehav. Rev. 37, 1976–1984 (2013).
Schmidt, K. T. & Weinshenker, D. Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol. Pharmacol. 85, 640–650 (2014).
Espana, R. A., Schmeichel, B. E. & Berridge, C. W. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 1641, 207–216 (2016).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005). The authors present a computational approach to modelling the relation between mode of firing of LC neurons and adaptive behavioural performance.
Arnsten, A. F. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 7, 133–146 (2000). This paper reviews experiments mainly in primates supporting the notion that optimal concentration of NA plays an important role in the cognitive function of prefrontal cortex.
Yu, A. J. & Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005). The authors present a computational approach to support the notion that the LC-NA system responds to unexpected uncertainty in the environment.
Nassar, M. R. et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. Neurosci. 15, 1040–1046 (2012).
Preuschoff, K., T Hart, B. M. & Einhauser, W. Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Front. Neurosci. 5, 115 (2011).
Jepma, M. & Nieuwenhuis, S. Pupil diameter predicts changes in the exploration–exploitation trade-off: evidence for the adaptive gain theory. J. Cogn. Neurosci. 23, 1587–1596 (2011).
Muller, T. H., Mars, R. B., Behrens, T. E. & O’Reilly, J. X. Control of entropy in neural models of environmental state. eLife 8, e39404 (2019).
Sales, A. C., Friston, K. J., Jones, M. W., Pickering, A. E. & Moran, R. J. Locus Coeruleus tracking of prediction errors optimises cognitive flexibility: An Active Inference model. PLoS Comput. Biol. 15, e1006267 (2019).
Raizada, R. D. & Poldrack, R. A. Challenge-driven attention: interacting frontal and brainstem systems. Front. Hum. Neurosci. 1, 3 (2008).
Giller, F., Muckschel, M., Ziemssen, T. & Beste, C. A possible role of the norepinephrine system during sequential cognitive flexibility — evidence from EEG and pupil diameter data. Cortex 128, 22–34 (2020).
Wolff, N., Muckschel, M., Ziemssen, T. & Beste, C. The role of phasic norepinephrine modulations during task switching: evidence for specific effects in parietal areas. Brain Struct. Funct. 223, 925–940 (2018).
Alvarez, V. A., Chow, C. C., Van Bockstaele, E. J. & Williams, J. T. Frequency-dependent synchrony in locus ceruleus: role of electrotonic coupling. Proc. Natl Acad. Sci. USA 99, 4032–4036 (2002).
Ennis, M., Shipley, M. T., Aston-Jones, G. & Williams, J. T. Afferent control of nucleus locus ceruleus: differential regulation by ‘shell’ and ‘core’ inputs. Adv. Pharmacol. 42, 767–771 (1998).
Cerpa, J. C., Marchand, A. R. & Coutureau, E. Distinct regional patterns in noradrenergic innervation of the rat prefrontal cortex. J. Chem. Neuroanat. 96, 102–109 (2019).
Guedj, C. et al. Boosting norepinephrine transmission triggers flexible reconfiguration of brain networks at rest. Cereb. Cortex 27, 4691–4700 (2017).
Dahlstroem, A. & Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 62 (Suppl. 232), 1–55 (1964). This seminal paper reports the discovery of nuclei of noradrenergic neurons in the brain.
Dahl, M. J. et al. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav. 3, 1203–1214 (2019).
Theofilas, P. et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 13, 236–246 (2017).
Mann, D. M. & Yates, P. O. Lipoprotein pigments — their relationship to ageing in the human nervous system. II. The melanin content of pigmented nerve cells. Brain 97, 489–498 (1974).
Betts, M. J. et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571 (2019).
Liu, K. Y. et al. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat. Commun. 11, 1712 (2020).
Twarkowski, H. & Manahan-Vaughan, D. Loss of catecholaminergic neuromodulation of persistent forms of hippocampal synaptic plasticity with increasing age. Front. Synaptic Neurosci. 8, 30 (2016).
Weinshenker, D. Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci. 41, 211–223 (2018).
Braak, H. & Del Tredici, K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 121, 589–595 (2011).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Ehrenberg, A. J. et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 66, 115–126 (2018).
Vermeiren, Y. & De Deyn, P. P. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem. Int. 102, 22–32 (2017).
Butkovich, L. M., Houser, M. C. & Tansey, M. G. α-Synuclein and noradrenergic modulation of immune cells in Parkinson’s disease pathogenesis. Front. Neurosci. 12, 626 (2018).
Ghosh, A. et al. An experimental model of Braak’s pretangle proposal for the origin of Alzheimer’s disease: the role of locus coeruleus in early symptom development. Alzheimers Res. Ther. 11, 59 (2019).
Henrich, M. T. et al. A53T-α-synuclein overexpression in murine locus coeruleus induces Parkinson’s disease-like pathology in neurons and glia. Acta Neuropathol. Commun. 6, 39 (2018).
Koob, G. F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 46, 1167–1180 (1999).
Valentino, R. J. & Van Bockstaele, E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 583, 194–203 (2008).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Tjoumakaris, S. I., Rudoy, C., Peoples, J., Valentino, R. J. & Van Bockstaele, E. J. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J. Comp. Neurol. 466, 445–456 (2003).
Valentino, R. J. & Wehby, R. G. Morphine effects on locus ceruleus neurons are dependent on the state of arousal and availability of external stimuli: studies in anesthetized and unanesthetized rats. J. Pharmacol. Exp. Ther. 244, 1178–1186 (1988).
Curtis, A. L., Leiser, S. C., Snyder, K. & Valentino, R. J. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 62, 1737–1745 (2012).
Reyes, B. A., Zitnik, G., Foster, C., Van Bockstaele, E. J. & Valentino, R. J. Social stress engages neurochemically-distinct afferents to the rat locus coeruleus depending on coping strategy. eNeuro 2, ENEURO.0042-15.2015 (2015).
Curtis, A. L., Bethea, T. & Valentino, R. J. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544–554 (2006). This study is an important example of how gender impacts the function of LC-NA system.
Bangasser, D. A. et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15, 896–904 (2010).
Guajardo, H. M., Snyder, K., Ho, A. & Valentino, R. J. Sex differences in µ-opioid receptor regulation of the rat locus coeruleus and their cognitive consequences. Neuropsychopharmacology 42, 1295–1304 (2017).
Helena, C. et al. Effects of estrogen receptor α and β gene deletion on estrogenic induction of progesterone receptors in the locus coeruleus in female mice. Endocrine 36, 169–177 (2009).
Brady, K. T. & Randall, C. L. Gender differences in substance use disorders. Psychiatr. Clin. North. Am. 22, 241–252 (1999).
Clemow, D. B. & Bushe, C. J. Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J. Psychopharmacol. 29, 1221–1230 (2015).
Sepede, G., Corbo, M., Fiori, F. & Martinotti, G. Reboxetine in clinical practice: a review. Clin. Ter. 163, e255–e262 (2012).
Fukada, K. et al. l-threo-3,4-dihydroxyphenylserine (L-DOPS) co-administered with entacapone improves freezing of gait in Parkinson’s disease. Med. Hypotheses 80, 209–212 (2013).
Doughty, B., Morgenson, D. & Brooks, T. Lofexidine: a newly FDA-approved, nonopioid treatment for opioid withdrawal. Ann. Pharmacother. 53, 746–753 (2019).
Bowrey, H. E., James, M. H. & Aston-Jones, G. New directions for the treatment of depression: targeting the photic regulation of arousal and mood (PRAM) pathway. Depress. Anxiety 34, 588–595 (2017).
Conway, C. R. & Xiong, W. The mechanism of action of vagus nerve stimulation in treatment-resistant depression: current conceptualizations. Psychiatr. Clin. North. Am. 41, 395–407 (2018).
Oliveira, T., Francisco, A. N., Demartini, Z. J. & Stebel, S. L. The role of vagus nerve stimulation in refractory epilepsy. Arq. Neuropsiquiatr. 75, 657–666 (2017).
Vonck, K. et al. Vagus nerve stimulation 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev. 45, 63–71 (2014).
Swift, K. M. et al. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol. 28, 3599–3609.e3594 (2018).
Ribeiro, S. et al. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 22, 10914–10923 (2002).
Ribeiro, S., Goyal, V., Mello, C. V. & Pavlides, C. Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem. 6, 500–508 (1999).
Sara, S. J. Sleep to remember. J. Neurosci. 37, 457–463 (2017).
Poe, G. R. Sleep is for forgetting. J. Neurosci. 37, 464–473 (2017).
Booth, V. & Poe, G. R. Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: relevance to REM sleep reactivation and memory consolidation. Hippocampus 16, 161–173 (2006).
Poe, G. R., Nitz, D. A., McNaughton, B. L. & Barnes, C. A. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000).
Novitskaya, Y., Sara, S. J., Logothetis, N. K. & Eschenko, O. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem. 23, 238–248 (2016).
Vanderheyden, W. M., Poe, G. R. & Liberzon, I. Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. Exp. Brain Res. 232, 1575–1584 (2014).
Wassing, R. et al. Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol. 29, 2351–2358.e4 (2019).
Cabrera, Y., Holloway, J. & Poe, G. R. Sleep changes across the female hormonal cycle affecting memory: implications for resilient adaptation to traumatic experiences. J. Womens Health 29, 446–451 (2020).
Acknowledgements
Funding for the 3-day workshop that generated this Perspective was provided by a grant from the Albert and Elaine Borchard Foundation Center on International Education to G.R.P. and S.J.S.Research funding to D.M.-V.: German Research Foundation project no.: 316803389, SFB 1280/A04.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests
Additional information
Peer review information
Nature Reviews Neuroscience thanks M. Mather and the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chemogenetics
-
Viral introduction of chemically engineered neurotransmitter receptors into neuronal membranes. These can be subsequently activated by pharmacological ligands that are specific to the receptor.
- Co-transmitters
-
Neuromodulators released from a neuron along with a primary neurotransmitter.
- Fast-scan voltammetry
-
Voltammetry examines fluctuations in current that are driven by variations in voltage/potential. In cyclic voltammetry, after the desired potential is reached, the potential is ramped in the opposite direction to return to the initial potential (time-locked voltage oscillations), causing the substance of interest to be oxidized and reduced in predetermined cycles. The concentration of the substance can be calculated by generating a calibration curve of current against concentration, allowing the relative concentration to be calculated within milliseconds, and thus the real-time detection of neurotransmitter concentration.
- Fear extinction
-
Learning that a context or cue that was associated with an aversive event no longer predicts that event, and thus the fear response to that context or cue is no longer expressed.
- Frequency tuning
-
In the auditory cortex, individual neurons exhibit a specific response pattern based on the sound frequency applied. Delivery of a set of different sound frequencies determines the frequency tuning of the neuron.
- Optogenetics
-
Analysis via the viral introduction of light-sensitive channels or ion pumps into neuronal membranes, which subsequently can be driven by the external application of a specific light wavelength.
- RNAi
-
RNA interference, which comprises the inhibition of gene expression or translation by silencing the target mRNA.
- Terminal fields
-
Neural areas targeted by axonal projections.
Rights and permissions
About this article
Cite this article
Poe, G.R., Foote, S., Eschenko, O. et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21, 644–659 (2020). https://doi.org/10.1038/s41583-020-0360-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-020-0360-9
This article is cited by
-
The crucial role of locus coeruleus noradrenergic neurons in the interaction between acute sleep disturbance and headache
The Journal of Headache and Pain (2024)
-
Evidence of an active role of dreaming in emotional memory processing shows that we dream to forget
Scientific Reports (2024)
-
Social activity mediates locus coeruleus tangle-related cognition in older adults
Molecular Psychiatry (2024)
-
Premotor projections from the locus coeruleus and periaqueductal grey are altered in two rat models with inborn differences in emotional behavior
Experimental Brain Research (2024)
-
Neurogenic orthostatic hypotension in Parkinson’s disease: is there a role for locus coeruleus magnetic resonance imaging?
Journal of Neural Transmission (2024)