Abstract
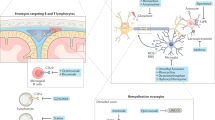
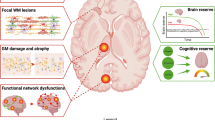
Cognitive impairment is increasingly recognized to be a core feature of multiple sclerosis (MS), with important implications for the everyday life of individuals with MS and for disease management. Unfortunately, the exact mechanisms that underlie this cognitive impairment are poorly understood and there are no effective therapeutic options for this aspect of the disease. During MS, focal brain inflammatory lesions, together with pathological changes of both CNS grey matter and normal-appearing white matter, can interfere with cognitive functions. Moreover, inflammation may alter the crosstalk between the immune and the nervous systems, modulating the induction of synaptic plasticity and neurotransmission. In this Review, we examine the CNS structures and cognitive domains that are affected by the disease, with a specific focus on hippocampal involvement in MS and experimental autoimmune encephalomyelitis, an experimental model of MS. We also discuss the hypothesis that, during MS, immune-mediated alterations of synapses’ ability to express long-term plastic changes may contribute to the pathogenesis of cognitive impairment by interfering with the dynamics of neuronal networks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ontaneda, D., Thompson, A. J., Fox, R. J. & Cohen, J. A. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet 389, 1357–1366 (2017).
Compston, A. & Coles, A. Multiple sclerosis. Lancet 359, 1221–1231 (2002).
Comi, G., Radaelli, M. & Soelberg Sørensen, P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389, 1347–1356 (2017).
Filippi, M. et al. Attendees of the correlation between pathological MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 11, 349–360 (2012).
Calabrese, M. et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 16, 147–158 (2015). This review focuses on the pathogenesis of grey matter damage during MS.
Charcot, J. M. Lectures on diseases of the nervous system (London: New Sydenham Society, 1877).
Chiaravalloti, N. D. & DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 7, 1139–1151 (2008).
Langdon, D. W. Cognition in multiple sclerosis. Curr. Opin. Neurol. 24, 244–249 (2011).
DeLuca, G. C., Yates, R. L., Beale, H. & Morrow, S. A. Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathol. 25, 79–98 (2015).
Di Filippo, M., Sarchielli, P., Picconi, B. & Calabresi, P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 29, 402–412 (2008).
Rocca, M. A. et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 14, 302–317 (2015). This paper presents an elegant review on both clinical and neuroimaging aspects of cognitive impairment in MS.
Amato, M. P. et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology 78, 309–314 (2012).
Ruano, L. et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler. 23, 1258–1267 (2017).
Goldschmidt, T., Antel, J., König, F. B., Brück, W. & Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72, 1914–1921 (2009).
Achiron, A. et al. Cognitive patterns and progression in multiple sclerosis: construction and validation of percentile curves. J. Neurol. Neurosurg. Psychiatry 76, 744–749 (2005).
Deloire, M., Ruet, A., Hamel, D., Bonnet, M. & Brochet, B. Early cognitive impairment in multiple sclerosis predicts disability outcome several years later. Mult. Scler. 16, 581–587 (2010).
Moccia, M. et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. 22, 659–667 (2016).
Zipoli, V. et al. Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult. Scler. 16, 62–67 (2010).
Morrow, S. A. et al. On-road assessment of fitness-to-drive in persons with MS with cognitive impairment: a prospective study. Mult. Scler. https://doi.org/10.1177/1352458517723991 (2017).
Schultheis, M. T. et al. Examining the relationship between cognition and driving performance in multiple sclerosis. Arch. Phys. Med. Rehabil. 91, 465–473 (2010).
Morrow, S. A. et al. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin. Neuropsychol. 24, 1131–1145 (2010).
Rao, S. M., Leo, G. J., Bernardin, L. & Unverzagt, F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41, 685–691 (1991).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013).
Cotter, J. et al. Social cognition in multiple sclerosis: a systematic review and meta-analysis. Neurology 87, 1727–1736 (2016).
Dineen, R. A. et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132, 239–249 (2009). This study investigates the possibility that cognitive dysfunction in MS is related to the disconnection of cognitively important processing regions by white matter damage.
Preziosa, P. et al. Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: a multicenter study. Hum. Brain Mapp. 37, 1627–1644 (2016).
Calabrese, M. et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 66, 1144–1150 (2009).
Harrison, D. M. et al. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 72, 1004–1012 (2015).
Preziosa, P. et al. DT MRI microstructural cortical lesion damage does not explain cognitive impairment in MS. Mult. Scler. 23, 1918–1928 (2017).
Bellmann-Strobl, J. et al. Poor PASAT performance correlates with MRI contrast enhancement in multiple sclerosis. Neurology 73, 1624–1627 (2009). This study shows that the performance of individuals with MS on the PASAT is affected by the appearance of MRI contrast-enhancing lesions, surrogate markers of CNS inflammatory activity.
Pardini, M. et al. Isolated cognitive relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 85, 1035–1037 (2014).
Mori, F. et al. Early treatment with high-dose interferon beta-1a reverses cognitive and cortical plasticity deficits in multiple sclerosis. Funct. Neurol. 27, 163–168 (2012).
Heesen, C. et al. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav. Immun. 24, 1148–1155 (2010).
Bonnier, G. et al. Multicontrast MRI quantification of focal inflammation and degeneration in multiple sclerosis. Biomed. Res. Int. 2015, 569123 (2015).
Steenwijk, M. D. et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 139, 115–126 (2016).
Bergsland, N., Zivadinov, R., Dwyer, M. G., Weinstock-Guttman, B. & Benedict, R. H. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult. Scler. 22, 1327–1336 (2016).
Batista, S. et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 259, 139–146 (2012).
Planche, V. et al. Regional hippocampal vulnerability in early multiple sclerosis: dynamic pathological spreading from dentate gyrus to CA1. Hum. Brain Mapp. 39, 1814–1824 (2018).
Cocozza, S. et al. Cerebellar lobule atrophy and disability in progressive MS. J. Neurol. Neurosurg. Psychiatry 88, 1065–1072 (2017).
Granberg, T. et al. Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: results of a 17-year longitudinal study. Mult. Scler. 21, 1151–1158 (2015).
Batista, S. et al. Impairment of social cognition in multiple sclerosis: amygdala atrophy is the main predictor. Mult. Scler. 23, 1358–1366 (2017).
Batista, S. et al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology 89, 38–45 (2017).
Eftekhari, E. et al. Normal appearing white matter permeability: a marker of inflammation and information processing speed deficit among relapsing remitting multiple sclerosis patients. Neuroradiology 59, 771–780 (2017).
Filippi, M. et al. The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 75, 2121–2128 (2010).
Muhlert, N. et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry 85, 833–839 (2014).
Pellicano, C. et al. Cognitive impairment and its relation to imaging measures in multiple sclerosis: a study using a computerized battery. J. Neuroimag. 23, 445–452 (2013).
Sicotte, N. L. et al. Regional hippocampal atrophy in multiple sclerosis. Brain 131, 1134–1141 (2008). This study demonstrates that individuals with MS present with hippocampal atrophy and that hippocampal volume loss is associated with poor performance on word-list learning.
Benedict, R. H., Ramasamy, D., Munschauer, F., Weinstock-Guttman, B. & Zivadinov, R. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J. Neurol. Neurosurg. Psychiatry 80, 201–206 (2009).
Debernard, L. et al. Deep grey matter MRI abnormalities and cognitive function in relapsing-remitting multiple sclerosis. Psychiatry Res. 234, 352–361 (2015).
González Torre, J. A. et al. Hippocampal dysfunction is associated with memory impairment in multiple sclerosis: a volumetric and functional connectivity study. Mult. Scler. 23, 1854–1863 (2017).
Hulst, H. E. et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum. Brain Mapp. 33, 2268–2280 (2012).
Hulst, H. E. et al. Memory impairment in multiple sclerosis: relevance of hippocampal activation and hippocampal connectivity. Mult. Scler. 21, 1705–1712 (2015).
Koenig, K. A. et al. Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magn. Reson. Imaging 32, 354–358 (2014).
Longoni, G. et al. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct. Funct. 220, 435–444 (2015).
Planche, V. et al. Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Mult. Scler. 23, 1214–1224 (2016).
Sumowski, J. F. et al. Mesial temporal lobe and subcortical grey matter volumes differentially predict memory across stages of multiple sclerosis. Mult. Scler. 24, 675–678 (2017).
Sacco, R. et al. Cognitive impairment and memory disorders in relapsing-remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J. Neurol. 262, 1691–1697 (2015).
Cawley, N. et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 138, 2584–2595 (2015).
Rahn, K. A. et al. Inhibition of glutamate carboxypeptidase II (GCPII) activity as a treatment for cognitive impairment in multiple sclerosis. Proc. Natl Acad. Sci. USA 109, 20101–20106 (2012).
Papadopoulos, D. et al. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 19, 238–253 (2009).
Dutta, R. et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 69, 445–454 (2011). This study demonstrates that demyelinated hippocampi in MS show marked decreases in synaptic density and in the levels of neuronal proteins known to be important for learning and memory processes, such as those involved in glutamate neurotransmission and synaptic plasticity.
Jürgens, T. et al. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 139, 39–46 (2016).
Michailidou, I. et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 77, 1007–1026 (2015).
Colasanti, A. et al. Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol. Psychiatry 80, 62–72 (2016).
Herranz, E. et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann. Neurol. 80, 776–790 (2016).
Dutra, R. C. et al. Spatial reference memory deficits precede motor dysfunction in an experimental autoimmune encephalomyelitis model: the role of kallikrein-kinin system. Brain Behav. Immun. 33, 90–101 (2013).
Assini, F. L., Duzzioni, M. & Takahashi, R. N. Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behav. Brain Res. 204, 206–211 (2009).
Acharjee, S. et al. Altered cognitive-emotional behavior in early experimental autoimmune encephalitis — cytokine and hormonal correlates. Brain Behav. Immun. 33, 164–172 (2013).
D’Intino, G. et al. Cognitive deficit associated with cholinergic and nerve growth factor down-regulation in experimental allergic encephalomyelitis in rats. Proc. Natl Acad. Sci. USA 102, 3070–3075 (2005).
Di Filippo, M. et al. Persistent activation of microglia and NADPH oxidase drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 6, 20926 (2016).
Lemon, N. & Manahan-Vaughan, D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J. Neurosci. 26, 7723–7729 (2006).
Ziehn, M. O. et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J. Neurosci. 32, 12312–12324 (2012).
Habbas, S. et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell 163, 1730–1741 (2015). This elegant study shows that inflammation results in persistent functional modification of hippocampal excitatory synapses and contextual learning and memory impairment in EAE.
Titley, H. K., Brunel, N. & Hansel, C. Toward a neurocentric view of learning. Neuron 95, 19–32 (2017). This recent work integrates the synaptic and neuronal mechanisms of learning.
Bliss, T. V. & Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the performant path. J. Physiol. 232, 331–356 (1973). This article presents the first description of LTP, now recognized as a neurobiological model of memory processes.
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Nicoll, R. A. A. Brief history of long-term potentiation. Neuron 93, 281–290 (2017).
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Malenka, R. C. & Nicoll, R. A. Long-term potentiation — a decade of progress? Science 285, 1870–1874 (1999).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213 (2011).
Kettenmann, H., Kirchhoff, F. & Verkhratsky, A. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18 (2013).
Wu, Y., Dissing-Olesen, L., MacVicar, B. A. & Stevens, B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 36, 605–613 (2015).
Brambilla, R. et al. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62, 452–467 (2014).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Lloyd, A. F., Davies, C. L. & Miron, V. E. Microglia: origins, homeostasis, and roles in myelin repair. Curr. Opin. Neurobiol. 47, 113–120 (2017).
Lloyd, A. F. & Miron, V. E. Cellular and molecular mechanisms underpinning macrophage activation during remyelination. Front. Cell Dev. Biol. 21, 60 (2016).
Miron, V. E. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J. Leukoc. Biol. 101, 1103–1108 (2017).
Redford, E. J., Kapoor, R. & Smith, K. J. Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain 120, 2149–2157 (1997).
Cibelli, M. et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 68, 360–368 (2010).
Williamson, L. L. & Bilbo, S. D. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav. Immun. 30, 186–194 (2013).
Di Filippo, M. et al. Synaptic plasticity and experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Brain Res. 1621, 205–213 (2015).
Di Filippo, M. et al. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol. Dis. 52, 229–236 (2013).
Kim, D. Y. et al. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLOS ONE 7, e35476 (2012).
Mosayebi, G., Soleyman, M. R., Khalili, M., Mosleh, M. & Palizvan, M. R. Changes in synaptic transmission & long-term potentiation induction as a possible mechanism for learning disability in an animal model of multiple sclerosis. Int. Neurourol. J. 20, 26–32 (2016).
Prochnow, N., Gold, R. & Haghikia, A. An electrophysiologic approach to quantify impaired synaptic transmission and plasticity in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 264, 48–53 (2013).
Nisticò, R. et al. Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLOS ONE 8, e54666 (2013).
Novkovic, T., Shchyglo, O., Gold, R. & Manahan-Vaughan, D. Hippocampal function is compromised in an animal model of multiple sclerosis. Neuroscience 309, 100–112 (2015).
Planche, V. et al. Selective dentate gyrus disruption causes memory impairment at the early stage of experimental multiple sclerosis. Brain Behav. Immun. 60, 240–254 (2017).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Gardoni, F. et al. Decreased NR2B subunit synaptic levels cause impaired long-term potentiation but not long-term depression. J. Neurosci. 29, 669–677 (2009).
Kamsler, A. & Segal, M. Hydrogen peroxide modulation of synaptic plasticity. J. Neurosci. 23, 269–276 (2003).
Minagar, A. et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 80, 210–219 (2013). This comprehensive manuscript discusses the role of thalamic damage in MS.
Kipp, M. et al. Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell. Mol. Life Sci. 72, 1127–1147 (2015).
Parmar, K. et al. The role of the cerebellum in multiple sclerosis-150 years after Charcot. Neurosci. Biobehav Rev. 89, 85–98 (2018). This article presents a review on cerebellar involvement in MS, including its potential role in MS-related cognitive impairment.
Benedict, R. H. et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult. Scler. 19, 1478–1484 (2013).
Bisecco, A. et al. Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum. Brain Mapp. 36, 2809–2825 (2015).
Bisecco, A. et al. Attention and processing speed performance in multiple sclerosis is mostly related to thalamic volume. Brain Imag. Behav. 12, 20–28 (2017).
Ruet, A. et al. Information processing speed impairment and cerebellar dysfunction in relapsing-remitting multiple sclerosis. J. Neurol. Sci. 347, 246–250 (2014).
Allen, G., Buxton, R. B., Wong, E. C. & Courchesne, E. Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943 (1997).
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C. & Yeo, B. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
D’Ambrosio, A. et al. Cerebellar contribution to motor and cognitive performance in multiple sclerosis: an MRI sub-regional volumetric analysis. Mult. Scler. 23, 1194–1203 (2017).
Moroso, A. et al. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 146–151 (2017).
Houtchens, M. K. et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 69, 1213–1223 (2007).
Schoonheim, M. M. et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84, 776–783 (2015).
DeLuca, J., Chelune, G. J., Tulsky, D. S., Lengenfelder, J. & Chiaravalloti, N. D. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J. Clin. Exp. Neuropsychol. 26, 550–562 (2004).
Costa, S. L., Genova, H. M., DeLuca, J. & Chiaravalloti, N. D. Information processing speed in multiple sclerosis: past, present, and future. Mult. Scler. 23, 772–789 (2017).
Kern, K. C. et al. Thalamic-hippocampal-prefrontal disruption in relapsing-remitting multiple sclerosis. Neuroimage Clin. 8, 440–447 (2014).
Foong, J. et al. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain. 120, 15–26 (1997).
Foong, J. et al. Correlates of executive function in multiple sclerosis: the use of magnetic resonance spectroscopy as an index of focal pathology. J. Neuropsychiatry Clin. Neurosci. 11, 45–50 (1999).
Muhlert, N. et al. Diffusion MRI-based cortical complexity alterations associated with executive function in multiple sclerosis. J. Magn. Reson. Imaging 38, 54–63 (2013).
Muhlert, N. et al. The grey matter correlates of impaired decision-making in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 86, 530–536 (2015).
Weygandt, M. et al. Brain activity, regional gray matter loss, and decision-making in multiple sclerosis. Mult. Scler. 24, 1163–1173 (2017).
Koini, M. et al. Correlates of executive functions in multiple sclerosis based on structural and functional MR imaging: insights from a multicenter study. Radiology 280, 869–879 (2016).
Leavitt, V. M., Lengenfelder, J., Moore, N. B., Chiaravalloti, N. D. & DeLuca, J. The relative contributions of processing speed and cognitive load to working memory accuracy in multiple sclerosis. J. Clin. Exp. Neuropsychol. 33, 580–586 (2011).
Lengenfelder, J. et al. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch. Clin. Neuropsychol. 21, 229–238 (2006).
Macniven, J. A. et al. Stroop performance in multiple sclerosis: information processing, selective attention, or executive functioning? J. Int. Neuropsychol. Soc. 14, 805–814 (2008).
Sachdev, P. S. et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat. Rev. Neurol. 10, 634–642 (2014).
Henry, J. D., von Hippel, W., Molenberghs, P., Lee, T. & Sachdev, P. S. Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 12, 28–39 (2016).
Chalah, M. A. et al. Theory of mind in multiple sclerosis: a neuropsychological and MRI study. Neurosci. Lett. 658, 108–113 (2017).
Mesulam, M. M. (ed.). Principles of Behavioral and Cognitive Neurology 2nd edn (Oxford Univ. Press, 2000).
Lezak, M. D., Howieson, D. B., Loring, D. W. & Fischer, J. S. Neuropsychological Assessment 4th edn (Oxford Univ. Press, 2004).
Sumowski, J. F. et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology 90, 278–288 (2018).
Benedict, R. H. et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 23, 721–733 (2017).
Benedict, R. H. et al. Validity of the minimal assessment of cognitive function in multiple sclerosis. (MACFIMS). J. Int. Neuropsychol. Soc. 12, 549–558 (2006).
Rocca, M. A. et al. Hippocampal-DMN disconnectivity in MS is related to WM lesions and depression. Hum. Brain Mapp. 36, 5051–5063 (2015).
Rossi, F. et al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLOS ONE 7, e44826 (2012).
Mandolesi, G. et al. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 11, 711–724 (2015).
Stampanoni Bassi, M. et al. Neurophysiology of synaptic functioning in multiple sclerosis. Clin. Neurophysiol. 128, 1148–1157 (2017).
Mori, F. et al. Cognitive and cortical plasticity deficits correlate with altered amyloid-β CSF levels in multiple sclerosis. Neuropsychopharmacology 36, 559–568 (2011).
Mancini, A. et al. Hippocampal neuroplasticity and inflammation: relevance for multiple sclerosis. Mult. Scler. Dem. Dis. 2, 2 (2017).
Giovannoni, G. et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult. Scler. Relat. Disord. 12, 70–78 (2017).
Correale, J., Gaitán, M. I., Ysrraelit, M. C. & Fiol, M. P. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain 140, 527–546 (2017).
Hemmer, B., Kerschensteiner, M. & Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 14, 406–419 (2015).
Lassmann, H. & Bradl, M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 133, 223–244 (2017).
Baxter, A. G. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 7, 904–912 (2007).
Baker, D., Gerritsen, W., Rundle, J. & Amor, S. Critical appraisal of animal models of multiple sclerosis. Mult. Scler. 17, 647–657 (2011).
Sriram, S. & Steiner, I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann. Neurol. 58, 939–945 (2005).
Lisman, J., Yasuda, R. & Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012).
Felten, D. L. & Shetty, A. N. Atlante di Neuroscienze di Netter [Italian] 2nd edn (eds Gulisano, M., Falcieri, E. & Cappello, F.) 298 (Elsevier, 2010).
Acknowledgements
M.D.F. and P.C. received funding from Fondazione Italiana Sclerosi Multipla (FISM; project codes 2010/R/10, 2011/R/10 and 2013/R/12). M.D.F. also received support from the Ministero della Salute — Ricerca Finalizzata — Bando Giovani Ricercatori (project code GR-2010-2312924).
Reviewer information
Nature Reviews Neuroscience thanks M. Friese, D. Langdon and B. Weinstock-Guttman and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors all researched data for the article, provided substantial contributions to discussion of content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.D.F. participated on advisory boards for and received speaker or writing honoraria and funding for travelling from Bayer, Biogen Idec, Genzyme, Merck, Novartis, Roche and Teva. E.P. served on scientific advisory boards for Biogen Idec and Merck Serono, received honoraria for speaking and funding for travelling from Biogen, Genzyme, Novartis, Merck and Teva and received research support from Merck Serono. A.M. declares no competing interests. P.C. participated on advisory boards for and received funding for travelling, speaker honoraria and research support from AbbVie, Biogen Idec, Merck, Genzyme, Novartis, Prexton, Teva, UCB and Zambon.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Radiologically isolated syndrome
-
(RIS). A condition that is characterized by the incidental MRI finding of brain white matter lesions that are highly suggestive of MS and are not explained by another disease process in people without historical accounts of typical MS symptoms. RIS is not considered an MS subtype per se, but patients with RIS can show MRI signs of radiological progression and/or neurological MS symptoms during follow-up.
- Clinically isolated syndrome
-
(CIS). A first clinical episode with features suggestive of MS. It usually occurs in young adults with signs suggesting a lesion in the optic nerve, spinal cord, brainstem or cerebellum or, more rarely, a cerebral hemisphere. CIS develops acutely or subacutely, it lasts more than 24 hours, with or without recovery, and is often the first manifestation of MS.
- Expanded Disability Status Scale
-
(EDSS). A clinical scale aimed at quantifying the neurological disability of patients with MS. The disability score ranges from 0 (normal) to 10 (death due to MS) in half-point increments. The scale measures disability accrual due to MS and takes into account a wide range of neurological functions, particularly ambulation–lower limb function.
- T2-weighted images
-
Specific conventional MRI sequences widely applied to detect MS lesions. Acute and chronic MS lesions appear on T2-weighted images as areas of high signal intensity compared with the adjacent normal regions.
- Contrast-enhancing lesions
-
Intravenously administered contrast agents, such as gadolinium, accumulate in brain regions where the blood–brain barrier is damaged, an early pathological event in inflammatory MS lesions. The presence of a new inflammatory lesion or the recurrence of inflammation in a pre-existing lesion is thus visualized as areas of enhancement on specific MRI images (postcontrast T1-weighted sequences).
- Paced Auditory Serial Addition Test
-
(PASAT). A neuropsychological test developed to assess IPS. Administration of the test involves the oral presentation of a series of single-digit numbers (either every 3 or 2 seconds) in which the two most recent digits must be summed. It is now recognized that other cognitive domains can contribute to PASAT performance, including attention and working memory.
- Symbol Digit Modalities Test
-
(SDMT). A neuropsychological test designed to assess IPS and sustained attention. During the test, the individual is required to rapidly associate symbols and numbers, and the score depends on the number of correct associations performed in a limited time. Other functions (such as learning and visual performance) can influence the execution of the SDMT.
- Long-term potentiation
-
(LTP). LTP is the best-known form of synaptic plasticity, it is expressed by excitatory synapses throughout the brain and it manifests as a persistent increase in the size of the synaptic component of the evoked response following repeated synaptic activation. It represents a compelling cellular model for learning and memory.
- Long-term depression
-
(LTD). The other major form of long-lasting synaptic plasticity in the mammalian brain, characterized by a long-lasting decrease in synaptic strength. Converging evidence supports a key role of LTD in some learning and memory processes.
- Homing
-
The recruitment of circulating immune cells to a specific tissue.
Rights and permissions
About this article
Cite this article
Di Filippo, M., Portaccio, E., Mancini, A. et al. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci 19, 599–609 (2018). https://doi.org/10.1038/s41583-018-0053-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-018-0053-9
This article is cited by
-
The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology
Molecular Brain (2024)
-
Syntaxin 1A gene polymorphism in multiple sclerosis: a case–control study
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice
Molecular Neurobiology (2024)
-
The validation of the Italian version of multiple sclerosis neuropsychological screening questionnaire in Huntington’s disease
Neurological Sciences (2023)
-
A systematic review and meta-analysis exploring the efficacy of mindfulness-based interventions on quality of life in people with multiple sclerosis
Journal of Neurology (2023)