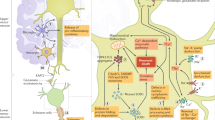
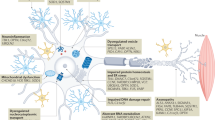
Abstract
Disease heterogeneity in amyotrophic lateral sclerosis poses a substantial challenge in drug development. Categorization based on clinical features alone can help us predict the disease course and survival, but quantitative measures are also needed that can enhance the sensitivity of the clinical categorization. In this Review, we describe the emerging landscape of diagnostic, categorical and pharmacodynamic biomarkers in amyotrophic lateral sclerosis and their place in the rapidly evolving landscape of new therapeutics. Fluid-based markers from cerebrospinal fluid, blood and urine are emerging as useful diagnostic, pharmacodynamic and predictive biomarkers. Combinations of imaging measures have the potential to provide important diagnostic and prognostic information, and neurophysiological methods, including various electromyography-based measures and quantitative EEG–magnetoencephalography-evoked responses and corticomuscular coherence, are generating useful diagnostic, categorical and prognostic markers. Although none of these biomarker technologies has been fully incorporated into clinical practice or clinical trials as a primary outcome measure, strong evidence is accumulating to support their clinical utility.
Key points
-
The heterogeneity in both the clinical presentation and underlying pathobiology of amyotrophic lateral sclerosis in humans has limited the translation from preclinical models to successful clinical trials.
-
Disease categorization might be improved using ancillary and companion measures. Such biomarkers can serve diagnostic, categorical and prognostic functions.
-
Biomarkers that are closest to use in the clinical domain include neurofilaments, which can be measured in serum and cerebrospinal fluid.
-
Promising emerging biomarkers include those derived from neurophysiological assessment such as quantitative EEG and transcranial magnetic stimulation.
-
No single biomarker modality will adequately cover all of the diagnostic, categorical prognostic and pharmacodynamic requirements to support the successful development of novel therapeutics.
-
The future landscape is likely to both integrate different biomarker modalities and use deep learning and artificial intelligence to fully address the complexity of amyotrophic lateral sclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 3, 17071 (2017).
Zarei, S. et al. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 6, 171 (2015).
Elamin, M. et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 76, 1263–1269 (2011).
Rusina, R., Vandenberghe, R. & Bruffaerts, R. Cognitive and behavioral manifestations in ALS: beyond motor system involvement. Diagnostics 11, 624 (2021).
Ling, S.-C., Polymenidou, M. & Cleveland, D. W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013).
Van Harten, A. C. M., Phatnani, H. & Przedborski, S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 44, 658–668 (2021).
Ghasemi, M. & Brown, R. H. Genetics of amyotrophic lateral sclerosis. Cold Spring Harb. Perspect. Med. 8, a024125 (2018).
Rooney, J., Burke, T., Vajda, A., Heverin, M. & Hardiman, O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 381–385 (2017).
Delaby, C. et al. Differential levels of neurofilament light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Sci. Rep. 10, 9161 (2020).
Heckler, I. & Venkataraman, I. Phosphorylated neurofilament heavy chain: a potential diagnostic biomarker in amyotrophic lateral sclerosis. J. Neurophysiol. 127, 737–745 (2022).
Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76, 1035–1048 (2019).
Zucchi, E. et al. A motor neuron strategy to save time and energy in neurodegeneration: adaptive protein stoichiometry. J. Neurochem. 146, 631–641 (2018).
Manouchehrinia, A. et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 7, 139–143 (2020).
Camu, W. et al. Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): a phase 2a randomised, double-blind, placebo-controlled trial. EBioMedicine 59, 102844 (2020).
Lu, C.-H. et al. Plasma neurofilament heavy chain levels and disease progression in amyotrophic lateral sclerosis: insights from a longitudinal study. J. Neurol. Neurosurg. Psychiatry 86, 565–573 (2015).
Mullard, A. NfL makes regulatory debut as neurodegenerative disease biomarker. Nat. Rev. Drug Discov. 22, 431–434 (2023).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Verde, F., Otto, M. & Silani, V. Neurofilament light chain as biomarker for amyotrophic lateral sclerosis and frontotemporal dementia. Front. Neurosci. 15, 679199 (2021).
Forgrave, L. M., Ma, M., Best, J. R. & DeMarco, M. L. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Alzheimers Dement. 11, 730–743 (2019).
Meyer, T. et al. Neurofilament light-chain response during therapy with antisense oligonucleotide tofersen in SOD1-related ALS: treatment experience in clinical practice. Muscle Nerve 67, 515–521 (2023).
Paganoni, S. et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383, 919–930 (2020).
[No authors listed]. MIROCALS Consortium Announces Top-line Results of European Trial of Low Dose Interleukin 2 in Amyotrophic Lateral Sclerosis at 33rd International Symposium on ALS/MND https://www.mndassociation.org/sites/default/files/2022-12/Final-MIROCALS-press-release-08122022.pdf (2022).
Brown, A.-L. et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 603, 131–137 (2022).
Akiyama, T., Koike, Y., Petrucelli, L. & Gitler, A. D. Cracking the cryptic code in amyotrophic lateral sclerosis and frontotemporal dementia: towards therapeutic targets and biomarkers. Clin. Transl. Med. 12, e818 (2022).
Mehta, P. R., Brown, A.-L., Ward, M. E. & Fratta, P. The era of cryptic exons: implications for ALS-FTD. Mol. Neurodegener. 18, 16 (2023).
Klim, J. R. et al. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci. 22, 167–179 (2019).
Vu, L. et al. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J. Neurol. Neurosurg. Psychiatry 91, 350–358 (2020).
Shepheard, S. R. et al. Urinary neopterin: a novel biomarker of disease progression in amyotrophic lateral sclerosis. Eur. J. Neurol. 29, 990–999 (2022).
Yazdani, S. et al. T cell responses at diagnosis of amyotrophic lateral sclerosis predict disease progression. Nat. Commun. 13, 6733 (2022).
Liu, H. et al. Systematic review and meta-analysis on microRNAs in amyotrophic lateral sclerosis. Brain Res. Bull. 194, 82–89 (2023).
Magen, I. et al. Circulating miR-181 is a prognostic biomarker for amyotrophic lateral sclerosis. Nat. Neurosci. 24, 1534–1541 (2021).
Lange, D. J. et al. Pyrimethamine significantly lowers cerebrospinal fluid Cu/Zn superoxide dismutase in amyotrophic lateral sclerosis patients with SOD1 mutations. Ann. Neurol. 81, 837–848 (2017).
Schmitz, A., Pinheiro Marques, J., Oertig, I., Maharjan, N. & Saxena, S. Emerging perspectives on dipeptide repeat proteins in C9ORF72 ALS/FTD. Front. Cell Neurosci. 15, 637548 (2021).
Shi, Y. et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 (2018).
Sellier, C. et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin‐2 to induce motor neuron dysfunction and cell death. EMBO J. 35, 1276–1297 (2016).
Fu, R.-H. et al. C9-ALS-associated proline-arginine dipeptide repeat protein induces activation of NLRP3 inflammasome of HMC3 microglia cells by binding of complement component 1 Q subcomponent-binding protein (C1QBP), and syringin prevents this effect. Cells 11, 3128 (2022).
Gendron, T. F. et al. Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann. Neurol. 82, 139–146 (2017).
Sturmey, E. & Malaspina, A. Blood biomarkers in ALS: challenges, applications and novel frontiers. Acta Neurol. Scand. 146, 375–388 (2022).
Berrone, E. et al. SOMAscan proteomics identifies novel plasma proteins in amyotrophic lateral sclerosis patients. Int. J. Mol. Sci. 24, 1899 (2023).
Ta, D. et al. Severity of in vivo corticospinal tract degeneration is associated with survival in amyotrophic lateral sclerosis: a longitudinal, multicohort study. Eur. J. Neurol. 30, 1220–1231 (2023).
Bharti, K. et al. Functional alterations in large-scale resting-state networks of amyotrophic lateral sclerosis: a multi-site study across Canada and the United States. PLoS One 17, e0269154 (2022).
Müller, H.-P. et al. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 87, 570–579 (2016).
Westeneng, H.-J. et al. Subcortical structures in amyotrophic lateral sclerosis. Neurobiol. Aging 36, 1075–1082 (2015).
Tahedl, M. et al. Cortical progression patterns in individual ALS patients across multiple timepoints: a mosaic-based approach for clinical use. J. Neurol. 268, 1913–1926 (2021).
Tahedl, M. et al. Brainstem-cortex disconnection in amyotrophic lateral sclerosis: bulbar impairment, genotype associations, asymptomatic changes and biomarker opportunities. J. Neurol. 270, 3511–3526 (2023).
Querin, G. et al. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J. Neurol. Neurosurg. Psychiatry 89, 1220–1221 (2018).
Querin, G. et al. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: a longitudinal neuroimaging study. Ann. Neurol. 86, 158–167 (2019).
El Mendili, M. M., Querin, G., Bede, P. & Pradat, P.-F. Spinal cord imaging in amyotrophic lateral sclerosis: historical concepts-novel techniques. Front. Neurol. 10, 350 (2019).
Barry, R. L. et al. Selective atrophy of the cervical enlargement in whole spinal cord MRI of amyotrophic lateral sclerosis. Neuroimage Clin. 36, 103199 (2022).
Rajagopalan, V. & Pioro, E. P. Graph theory network analysis provides brain MRI evidence of a partial continuum of neurodegeneration in patients with UMN-predominant ALS and ALS-FTD. NeuroImage Clin. 35, 103037 (2022).
Fortanier, E. et al. Structural connectivity alterations in amyotrophic lateral sclerosis: a graph theory based imaging study. Front. Neurosci. 13, 1044 (2019).
Smallwood Shoukry, R. F., Clark, M. G. & Floeter, M. K. Resting state functional connectivity is decreased globally across the C9orf72 mutation spectrum. Front. Neurol. 11, 598474 (2020).
Behler, A. et al. Multimodal in vivo staging in amyotrophic lateral sclerosis using artificial intelligence. Ann. Clin. Transl. Neurol. 9, 1069–1079 (2022).
Kassubek, J. et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain 137, 1733–1740 (2014).
Behler, A., Müller, H.-P., Ludolph, A. C., Lulé, D. & Kassubek, J. A multivariate Bayesian classification algorithm for cerebral stage prediction by diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage Clin. 35, 103094 (2022).
Bede, P., Iyer, P. M., Finegan, E., Omer, T. & Hardiman, O. Virtual brain biopsies in amyotrophic lateral sclerosis: diagnostic classification based on in vivo pathological patterns. NeuroImage Clin. 15, 653–658 (2017).
Bede, P., Murad, A. & Hardiman, O. Pathological neural networks and artificial neural networks in ALS: diagnostic classification based on pathognomonic neuroimaging features. J. Neurol. 269, 2440–2452 (2022).
Schuster, C., Hardiman, O. & Bede, P. Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PLoS One 11, e0167331 (2016).
Tan, H. H. G. et al. MRI clustering reveals three ALS subtypes with unique neurodegeneration patterns. Ann. Neurol. 92, 1030–1045 (2022).
Bede, P., Murad, A., Lope, J., Hardiman, O. & Chang, K. M. Clusters of anatomical disease-burden patterns in ALS: a data-driven approach confirms radiological subtypes. J. Neurol. 269, 4404–4413 (2022).
De Vocht, J. et al. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol. 77, 1008–1017 (2020).
Chipika, R. H. et al. The presymptomatic phase of amyotrophic lateral sclerosis: are we merely scratching the surface? J. Neurol. 268, 4607–4629 (2021).
Lulé, D. E. et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers — a developmental disorder. J. Neurol. Neurosurg. Psychiatry 91, 1195–1200 (2020).
van Veenhuijzen, K. et al. Longitudinal effects of asymptomatic C9orf72 carriership on brain morphology. Ann. Neurol. 93, 668–680 (2023).
Bede, P. et al. Presymptomatic grey matter alterations in ALS kindreds: a computational neuroimaging study of asymptomatic C9orf72 and SOD1 mutation carriers. J. Neurol. 270, 4235–4247 (2023).
Bede, P. et al. Phenotypic categorisation of individual subjects with motor neuron disease based on radiological disease burden patterns: a machine-learning approach. J. Neurol. Sci. 432, 120079 (2022).
Finegan, E. et al. Widespread subcortical grey matter degeneration in primary lateral sclerosis: a multimodal imaging study with genetic profiling. Neuroimage Clin. 24, 102089 (2019).
Tahedl, M. et al. Not a benign motor neuron disease: longitudinal imaging captures relentless motor connectome disintegration in primary lateral sclerosis. Eur. J. Neurol. 30, 1232–1245 (2023).
Finegan, E. et al. Extra-motor cerebral changes and manifestations in primary lateral sclerosis. Brain Imaging Behav. 15, 2283–2296 (2021).
Pradat, P.-F. et al. The French national protocol for Kennedy’s disease (SBMA): consensus diagnostic and management recommendations. Orphanet J. Rare Dis. 15, 90 (2020).
Schuster, C., Hardiman, O. & Bede, P. Survival prediction in amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurol. 17, 73 (2017).
Dieckmann, N. et al. Cortical and subcortical grey matter atrophy in amyotrophic lateral sclerosis correlates with measures of disease accumulation independent of disease aggressiveness. Neuroimage Clin. 36, 103162 (2022).
Pallebage-Gamarallage, M. et al. Dissecting the pathobiology of altered MRI signal in amyotrophic lateral sclerosis: a post mortem whole brain sampling strategy for the integration of ultra-high-field MRI and quantitative neuropathology. BMC Neurosci. 19, 11 (2018).
Wang, C. et al. Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis. Neuroimage 222, 117216 (2020).
Zejlon, C. et al. Structural magnetic resonance imaging findings and histopathological correlations in motor neuron diseases — a systematic review and meta-analysis. Front. Neurol. 13, 947347 (2022).
Sennfält, S. et al. FDG-PET shows weak correlation between focal motor weakness and brain metabolic alterations in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 485–494 (2023).
Bede, P. et al. Degenerative and regenerative processes in amyotrophic lateral sclerosis: motor reserve, adaptation and putative compensatory changes. Neural Regen. Res. 16, 1208–1209 (2021).
Temp, A. G. M. et al. Cognitive reserve and regional brain volume in amyotrophic lateral sclerosis. Cortex 139, 240–248 (2021).
Abidi, M. et al. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur. J. Neurol. 27, 121–128 (2020).
Feron, M. et al. Extrapyramidal deficits in ALS: a combined biomechanical and neuroimaging study. J. Neurol. 265, 2125–2136 (2018).
Chipika, R. H. et al. “Switchboard” malfunction in motor neuron diseases: selective pathology of thalamic nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. NeuroImage Clin. 27, 102300 (2020).
Chipika, R. H. et al. Amygdala pathology in amyotrophic lateral sclerosis and primary lateral sclerosis. J. Neurol. Sci. 417, 117039 (2020).
Finegan, E., Chipika, R. H., Li Hi Shing, S., Hardiman, O. & Bede, P. Pathological crying and laughing in motor neuron disease: pathobiology, screening, intervention. Front. Neurol. 10, 260 (2019).
Trojsi, F. et al. Resting state fMRI analysis of pseudobulbar affect in amyotrophic lateral sclerosis (ALS): motor dysfunction of emotional expression. Brain Imaging Behav. 17, 77–89 (2023).
Tahedl, M. et al. Radiological correlates of pseudobulbar affect: corticobulbar and cerebellar components in primary lateral sclerosis. J. Neurol. Sci. 451, 120726 (2023).
Bede, P. et al. Genotype-associated cerebellar profiles in ALS: focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J. Neurol. Neurosurg. Psychiatry 92, 1197–1205 (2021).
Chipika, R. H. et al. Alterations in somatosensory, visual and auditory pathways in amyotrophic lateral sclerosis: an under-recognised facet of ALS. J. Integr. Neurosci. 21, 88 (2022).
Christidi, F. et al. Neurometabolic alterations in motor neuron disease: insights from magnetic resonance spectroscopy. J. Integr. Neurosci. 21, 87 (2022).
Stagg, C. J. et al. Whole-brain magnetic resonance spectroscopic imaging measures are related to disability in ALS. Neurology 80, 610–615 (2013).
Govind, V. et al. Comprehensive evaluation of corticospinal tract metabolites in amyotrophic lateral sclerosis using whole-brain 1H MR spectroscopy. PLoS One 7, e35607 (2012).
Christidi, F. et al. Hippocampal metabolic alterations in amyotrophic lateral sclerosis: a magnetic resonance spectroscopy study. Life 13, 571 (2023).
Grapperon, A.-M. et al. Quantitative brain sodium MRI depicts corticospinal impairment in amyotrophic lateral sclerosis. Radiology 292, 422–428 (2019).
Mendili, M. M. E. et al. Alterations of microstructure and sodium homeostasis in fast amyotrophic lateral sclerosis progressors: a brain DTI and sodium MRI study. Am. J. Neuroradiol. 43, 984–990 (2022).
Müller, H.-P. et al. Relaxation-weighted 23Na magnetic resonance imaging maps regional patterns of abnormal sodium concentrations in amyotrophic lateral sclerosis. Ther. Adv. Chronic Dis. 13, 20406223221109480 (2022).
Proudfoot, M., Bede, P. & Turner, M. R. Imaging cerebral activity in amyotrophic lateral sclerosis. Front. Neurol. 9, 1148 (2019).
Abidi, M. et al. Motor imagery in amyotrophic lateral sclerosis: an fMRI study of postural control. Neuroimage Clin. 35, 103051 (2022).
Abidi, M. et al. Neural correlates of motor imagery of gait in amyotrophic lateral sclerosis. J. Magn. Reson. Imaging 53, 223–233 (2021).
Münch, M., Müller, H.-P., Behler, A., Ludolph, A. C. & Kassubek, J. Segmental alterations of the corpus callosum in motor neuron disease: a DTI and texture analysis in 575 patients. Neuroimage Clin. 35, 103061 (2022).
Broad, R. J. et al. Neurite orientation and dispersion density imaging (NODDI) detects cortical and corticospinal tract degeneration in ALS. J. Neurol. Neurosurg. Psychiatry 90, 404–411 (2019).
Wen, J. et al. Neurite density is reduced in the presymptomatic phase of C9orf72 disease. J. Neurol. Neurosurg. Psychiatry 90, 387–394 (2019).
Van Weehaeghe, D. et al. Combined brain and spinal FDG PET allows differentiation between ALS and ALS mimics. Eur. J. Nucl. Med. Mol. Imaging 47, 2681–2690 (2020).
Harada, R. et al. Imaging of reactive astrogliosis by positron emission tomography. Front. Neurosci. 16, 807435 (2022).
Raval, N. R., Wetherill, R. R., Wiers, C. E., Dubroff, J. G. & Hillmer, A. T. Positron emission tomography of neuroimmune responses in humans: insights and intricacies. Semin. Nucl. Med. 53, 213–229 (2023).
Chew, S. & Atassi, N. Positron emission tomography molecular imaging biomarkers for amyotrophic lateral sclerosis. Front. Neurol. 10, 135 (2019).
Canosa, A. et al. Brain metabolic differences between pure bulbar and pure spinal ALS: a 2-[18F]FDG-PET study. J. Neurol. 270, 953–959 (2023).
De Vocht, J. et al. Differences in cerebral glucose metabolism in ALS patients with and without C9orf72 and SOD1 mutations. Cells 12, 933 (2023).
Cistaro, A. et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur. J. Nucl. Med. Mol. Imaging 41, 844–852 (2014).
Marini, C. et al. A PET/CT approach to spinal cord metabolism in amyotrophic lateral sclerosis. Eur. J. Nucl. Med. Mol. Imaging 43, 2061–2071 (2016).
Juengling, F. D. et al. Simultaneous PET/MRI: the future gold standard for characterizing motor neuron disease — a clinico-radiological and neuroscientific perspective. Front. Neurol. 13, 890425 (2022).
Zanovello, M. et al. Brain stem glucose hypermetabolism in amyotrophic lateral sclerosis/frontotemporal dementia and shortened survival: an 18F-FDG PET/MRI study. J. Nucl. Med. 63, 777–784 (2022).
Costagli, M. et al. Distribution indices of magnetic susceptibility values in the primary motor cortex enable to classify patients with amyotrophic lateral sclerosis. Brain Sci. 12, 942 (2022).
Dean, K. E. et al. A specific biomarker for amyotrophic lateral sclerosis: quantitative susceptibility mapping. Clin. Imaging 75, 125–130 (2021).
Toh, C. et al. Analysis of brain and spinal MRI measures in a common domain to investigate directional neurodegeneration in motor neuron disease. J. Neurol. 270, 1682–1690 (2023).
Kriss, A. & Jenkins, T. Muscle MRI in motor neuron diseases: a systematic review. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 161–175 (2022).
Ma, J. et al. Fasciculation score: a sensitive biomarker in amyotrophic lateral sclerosis. Neurol. Sci. 42, 4657–4666 (2021).
Rajula, R. R. et al. Muscle ultrasonography in detecting fasciculations: a noninvasive diagnostic tool for amyotrophic lateral sclerosis. J. Clin. Ultrasound 50, 286–291 (2022).
Tahedl, M., Murad, A., Lope, J., Hardiman, O. & Bede, P. Evaluation and categorisation of individual patients based on white matter profiles: single-patient diffusion data interpretation in neurodegeneration. J. Neurol. Sci. 428, 117584 (2021).
Dey, A. et al. Motor cortex functional connectivity is associated with underlying neurochemistry in ALS. J. Neurol. Neurosurg. Psychiatry 94, 193–200 (2023).
Verstraete, E., Turner, M. R., Grosskreutz, J., Filippi, M. & Benatar, M. Mind the gap: the mismatch between clinical and imaging metrics in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 524–529 (2015).
Prell, T. & Grosskreutz, J. The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 507–515 (2013).
Segovia, F., Górriz, J. M., Ramírez, J., Martínez-Murcia, F. J. & Salas-Gonzalez, D. Preprocessing of 18F-DMFP-PET data based on hidden Markov random fields and the Gaussian distribution. Front. Aging Neurosci. 9, 326 (2017).
Rajagopalan, V., Chaitanya, K. G. & Pioro, E. P. Quantitative brain MRI metrics distinguish four different ALS phenotypes: a machine learning based study. Diagnostics 13, 1521 (2023).
Mazón, M., Vázquez Costa, J. F., Ten-Esteve, A. & Martí-Bonmatí, L. Imaging biomarkers for the diagnosis and prognosis of neurodegenerative diseases. the example of amyotrophic lateral sclerosis. Front. Neurosci. 12, 784 (2018).
Rizzo, G. et al. Diagnostic and prognostic value of conventional brain MRI in the clinical work-up of patients with amyotrophic lateral sclerosis. J. Clin. Med. 9, 2538 (2020).
Grollemund, V. et al. Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front. Neurosci. 13, 135 (2019).
McMackin, R., Bede, P., Pender, N., Hardiman, O. & Nasseroleslami, B. Neurophysiological markers of network dysfunction in neurodegenerative diseases. NeuroImage Clin. 22, 101706 (2019).
McMackin, R. et al. Measuring network disruption in neurodegenerative diseases: new approaches using signal analysis. J. Neurol. Neurosurg. Psychiatry 90, 1011–1020 (2019).
Wang, F.-C. & Delwaide, P. J. Number and relative size of thenar motor units estimated by an adapted multiple point stimulation method. Muscle Nerve 18, 969–979 (1995).
McComas, A. J., Fawcett, P. R., Campbell, M. J. & Sica, R. E. Electrophysiological estimation of the number of motor units within a human muscle. J. Neurol. Neurosurg. Psychiatry 34, 121–131 (1971).
Kadrie, H. A., Yates, S. K., Milner-Brown, H. S. & Brown, W. F. Multiple point electrical stimulation of ulnar and median nerves. J. Neurol. Neurosurg. Psychiatry 39, 973–985 (1976).
Jagtap, S. A. et al. Multipoint incremental motor unit number estimation versus amyotrophic lateral sclerosis functional rating scale and the medical research council sum score as an outcome measure in amyotrophic lateral sclerosis. Ann. Indian Acad. Neurol. 17, 336–339 (2014).
Shefner, J. M. Motor unit number estimation in human neurological diseases and animal models. Clin. Neurophysiol. 112, 955–964 (2001).
de Carvalho, M., Barkhaus, P. E., Nandedkar, S. D. & Swash, M. Motor unit number estimation (MUNE): where are we now? Clin. Neurophysiol. 129, 1507–1516 (2018).
Nandedkar, S. D., Nandedkar, D. S., Barkhaus, P. E. & Stalberg, E. V. Motor unit number index (MUNIX). IEEE Trans. Biomed. Eng. 51, 2209–2211 (2004).
Fathi, D., Nafissi, S., Attarian, S., Neuwirth, C. & Fatehi, F. An overview of motor unit number index reproducibility in amyotrophic lateral sclerosis. Iran. J. Neurol. 18, 119–126 (2019).
Wirth, A. M. et al. Combinatory biomarker use of cortical thickness, MUNIX, and ALSFRS-R at baseline and in longitudinal courses of individual patients with amyotrophic lateral sclerosis. Front. Neurol. 9, 614 (2018).
Grimaldi, S. et al. Global motor unit number index sum score for assessing the loss of lower motor neurons in amyotrophic lateral sclerosis. Muscle Nerve 56, 202–206 (2017).
Kaya, R. D., Hoffman, R. L. & Clark, B. C. Reliability of a modified motor unit number index (MUNIX) technique. J. Electromyogr. Kinesiol. 24, 18–24 (2014).
Ebersbach, T. et al. Motor unit number index (MUNIX) in the D50 disease progression model reflects disease accumulation independently of disease aggressiveness in ALS. Sci. Rep. 12, 15997 (2022).
Neuwirth, C. & Weber, M. Motor Unit Number Index (MUNIX) Instructions & Qualification Process ENCALS https://www.encals.eu/wp-content/uploads/2017/12/MUNIX-Protocol_v1.0_Dec2017.pdf (2017).
Bostock, H., Jacobsen, A. B. & Tankisi, H. Motor unit number index and compound muscle action potential amplitude. Clin. Neurophysiol. 130, 1734–1740 (2019).
Zhang, S. et al. Application value of the motor unit number index in patients with Kennedy disease. Front. Neurol. 12, 705816 (2021).
Jacobsen, A. B. et al. Reproducibility, and sensitivity to motor unit loss in amyotrophic lateral sclerosis, of a novel MUNE method: MScanFit MUNE. Clin. Neurophysiol. 128, 1380–1388 (2017).
Jacobsen, A. B., Bostock, H. & Tankisi, H. Following disease progression in motor neuron disorders with 3 motor unit number estimation methods. Muscle Nerve 59, 82–87 (2019).
Kanai, K. et al. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain 129, 953–962 (2006).
Iwai, Y. et al. Axonal dysfunction precedes motor neuronal death in amyotrophic lateral sclerosis. PLoS One 11, e0158596 (2016).
Lugg, A., Schindle, M., Sivak, A., Tankisi, H. & Jones, K. E. Nerve excitability as a biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. Preprint at medRxiv https://doi.org/10.1101/2022.02.11.22270866 (2022).
Stikvoort García, D. J. L., Sleutjes, B. T. H. M., van Schelven, L. J., Goedee, H. S. & van den Berg, L. H. Diagnostic accuracy of nerve excitability and compound muscle action potential scan derived biomarkers in amyotrophic lateral sclerosis. Eur. J. Neurol. 30, 3068–3078 (2023).
Kanai, K. et al. Motor axonal excitability properties are strong predictors for survival in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 83, 734–738 (2012).
Wainger, B. J. et al. Effect of ezogabine on cortical and spinal motor neuron excitability in amyotrophic lateral sclerosis. JAMA Neurol. 78, 186–196 (2021).
Cao, B. et al. Neurophysiological index is associated with the survival of patients with amyotrophic lateral sclerosis. Clin. Neurophysiol. 130, 1730–1733 (2019).
Swash, M. & de Carvalho, M. The neurophysiological index in ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 5, 108–110 (2004).
Alix, J. J. P. et al. Multi-dimensional electrical impedance myography of the tongue as a potential biomarker for amyotrophic lateral sclerosis. Clin. Neurophysiol. 131, 799–808 (2020).
Schooling, C. N. et al. Tensor electrical impedance myography identifies bulbar disease progression in amyotrophic lateral sclerosis. Clin. Neurophysiol. 139, 69–75 (2022).
Dukic, S. et al. Patterned functional network disruption in amyotrophic lateral sclerosis. Hum. Brain Mapp. 40, 4827–4842 (2019).
Fraschini, M. et al. Functional brain connectivity analysis in amyotrophic lateral sclerosis: an EEG source-space study. Biomed. Phys. Eng. Express 4, 037004 (2017).
Nasseroleslami, B. et al. Characteristic increases in EEG connectivity correlate with changes of structural MRI in amyotrophic lateral sclerosis. Cereb. Cortex 29, 27–41 (2019).
Vinding, M. C. et al. Attenuated beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci. Rep. 9, 2604 (2019).
Peter, J. et al. Movement-related beta ERD and ERS abnormalities in neuropsychiatric disorders. Front. Neurosci. 16, 1045715 (2022).
McMackin, R. et al. Sustained attention to response task-related beta oscillations relate to performance and provide a functional biomarker in ALS. J. Neural Eng. 18, 026006 (2021).
Proudfoot, M. et al. Altered cortical beta‐band oscillations reflect motor system degeneration in amyotrophic lateral sclerosis. Hum. Brain Mapp. 38, 237–254 (2017).
Corp, D. T. et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin. Neurophysiol. 132, 2639–2653 (2021).
Tankisi, H. et al. Early diagnosis of amyotrophic lateral sclerosis by threshold tracking and conventional transcranial magnetic stimulation. Eur. J. Neurol. 28, 3030–3039 (2021).
Tankisi, H. et al. Three different short-interval intracortical inhibition methods in early diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 139–147 (2023).
Menon, P. et al. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: a prospective study. Lancet Neurol. 14, 478–484 (2015).
Proudfoot, M. et al. Increased cerebral functional connectivity in ALS: a resting-state magnetoencephalography study. Neurology 90, e1418–e1424 (2018).
McMackin, R. et al. Cognitive network hyperactivation and motor cortex decline correlate with ALS prognosis. Neurobiol. Aging 104, 57–70 (2021).
Nguyen, C., Caga, J., Mahoney, C. J., Kiernan, M. C. & Huynh, W. Behavioural changes predict poorer survival in amyotrophic lateral sclerosis. Brain Cognition 150, 105710 (2021).
Dang, J. S., Figueroa, I. J. & Helton, W. S. You are measuring the decision to be fast, not inattention: the sustained attention to response task does not measure sustained attention. Exp. Brain Res. 236, 2255–2262 (2018).
Koenig, T., Smailovic, U. & Jelic, V. Past, present and future EEG in the clinical workup of dementias. Psychiatry Res. Neuroimaging 306, 111182 (2020).
McMackin, R. et al. Localization of brain networks engaged by the sustained attention to response task provides quantitative markers of executive impairment in amyotrophic lateral sclerosis. Cereb. Cortex 30, 4834–4846 (2020).
Seer, C. et al. Executive dysfunctions and event-related brain potentials in patients with amyotrophic lateral sclerosis. Front. Aging Neurosci. 7, 225 (2015).
Lange, F. et al. Neural correlates of cognitive set shifting in amyotrophic lateral sclerosis. Clin. Neurophysiol. 127, 3537–3545 (2016).
Iyer, P. M. et al. Mismatch negativity as an indicator of cognitive sub-domain dysfunction in amyotrophic lateral sclerosis. Front. Neurol. 8, 395 (2017).
Tao, L. et al. Eye tracking metrics to screen and assess cognitive impairment in patients with neurological disorders. Neurol. Sci. 41, 1697–1704 (2020).
Girardi, A., MacPherson, S. E. & Abrahams, S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology 25, 53–65 (2011).
Proudfoot, M. et al. Eye-tracking in amyotrophic lateral sclerosis: a longitudinal study of saccadic and cognitive tasks. Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 101–111 (2016).
Poletti, B. et al. An eye-tracker controlled cognitive battery: overcoming verbal-motor limitations in ALS. J. Neurol. 264, 1136–1145 (2017).
Gorges, M. et al. Eye movement deficits are consistent with a staging model of pTDP-43 pathology in amyotrophic lateral sclerosis. PLoS One 10, e0142546 (2015).
Dukic, S. et al. Resting-state EEG reveals four subphenotypes of amyotrophic lateral sclerosis. Brain 145, 621–631 (2022).
Vucic, S. et al. Study protocol of RESCUE-ALS: a phase 2, randomised, double-blind, placebo-controlled study in early symptomatic amyotrophic lateral sclerosis patients to assess bioenergetic catalysis with CNM-Au8 as a mechanism to slow disease progression. BMJ Open 11, e041479 (2021).
Meininger, V. et al. Safety, pharmacokinetic, and functional effects of the Nogo-A monoclonal antibody in amyotrophic lateral sclerosis: a randomized, first-in-human clinical trial. PLoS One 9, e97803 (2014).
Abramova, A. A., Broutian, A. G. & Zakharova, M. N. Motor unit number index (MUNIX): a biomarker for evaluation of lower motor neuron involvement in amyotrophic lateral sclerosis. Hum. Physiol. 46, 900–911 (2020).
Neuwirth, C. et al. Motor Unit Number Index (MUNIX): a novel neurophysiological marker for neuromuscular disorders; test-retest reliability in healthy volunteers. Clin. Neurophysiol. 122, 1867–1872 (2011).
Vucic, S. RESCUE-ALS: a phase 2, randomized, double-blind, placebo-controlled study of CNM-Au8 to slow disease progression in ALS. MNDA Virtual Symposium, https://www.mdaconference.org/abstract-library/rescue-als-trial-results-a-phase-2-randomized-double-blind-placebo-controlled-study-of-cnm-au8-to-slow-disease-progression-in-als/ (2021).
Scheltens, P. et al. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J. Alzheimers Dis. 31, 225–236 (2012).
Scheltens, P. et al. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimers Res. Ther. 10, 107 (2018).
Yin, W. et al. Safety, pharmacokinetics and quantitative EEG modulation of TAK-071, a novel muscarinic M1 receptor positive allosteric modulator, in healthy subjects. Br. J. Clin. Pharmacol. 88, 600–612 (2022).
Benatar, M. et al. Design of a randomized, placebo-controlled, phase 3 trial of tofersen initiated in clinically presymptomatic SOD1 variant carriers: the ATLAS study. Neurotherapeutics 19, 1248–1258 (2022).
Bertrand, A. et al. Early cognitive, structural, and microstructural changes in presymptomatic C9orf72 carriers younger than 40 years. JAMA Neurol. 75, 236–245 (2018).
Galvin, M. et al. The path to specialist multidisciplinary care in amyotrophic lateral sclerosis: a population-based study of consultations, interventions and costs. PLoS One 12, e0179796 (2017).
Vucic, S., Nicholson, G. A. & Kiernan, M. C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131, 1540–1550 (2008).
Bensimon, G. & Leigh, P. N. Modifying immune response and outcomes in ALS (MIROCALS): design and results of a phase 2b, double-blind randomized placebo-controlled trial of low dose interleukin-2 (ld IL2) in ALS. 33rd International Symposium on ALS/MND Abstract C03, https://doi.org/10.1080/21678421.2022.2082738 (2022).
Giovannelli, I. et al. Amyotrophic lateral sclerosis transcriptomics reveals immunological effects of low-dose interleukin-2. Brain Commun. 3, fcab141 (2021).
Cudkowicz, M. E. et al. A randomized placebo‐controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 65, 291–302 (2022).
Miller, R. G. et al. Phase 2B randomized controlled trial of NP001 in amyotrophic lateral sclerosis: pre‐specified and post hoc analyses. Muscle Nerve 66, 39–49 (2022).
Acknowledgements
The authors wish to thank Juliette Foucher, PhD student in the Department of Clinical Neuroscience, Karolinska Institute, for her contribution to the figures in this Review. This work was funded in part by Science Foundation Ireland grants SP20/SP/8953, 16/RC/3948 and 13/RC/2106_P2. R. McMackin is supported by the Motor Neurone Disease Association UK (McMackin/Oct20/972–799). P.B. is supported by the Health Research Board (HRB EIA-2017–019 & JPND-Cofund-2–2019–1), the Irish Institute of Clinical Neuroscience (IICN) and the EU Joint Programme – Neurodegenerative Disease Research (JPND).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
C.I. has consulted for Cytokinetics and Pfizer and is a data monitoring committee member for Appelis Pharmaceutical. A.M. has acted as an adviser for Roche and Accure Therapeutics and has given a Lecture to Pfizer. He has been part of biomarker data licencing to Biogen. R.M. has provided consultancy to The Science Behind. O.H. has consulted for the following companies: Biogen, Novartis, Denali and Cytokinetics. She is Chair of a Data Safety Monitoring Board for Accelsiors. She is Lead PrincipaI Investigator on an academic–industry collaboration funded by Science Foundation Ireland, in partnership with Biogen, Takeda, Novartis, IQVIA and Accenture. She is Editor-in-Chief of the journal Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. P.B. declares no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks H. Tankisi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Accelerating Medicines Partnership: https://www.nih.gov/research-training/accelerating-medicines-partnership-amp
Precision ALS: https://www.precisionals.ie/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McMackin, R., Bede, P., Ingre, C. et al. Biomarkers in amyotrophic lateral sclerosis: current status and future prospects. Nat Rev Neurol 19, 754–768 (2023). https://doi.org/10.1038/s41582-023-00891-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00891-2
This article is cited by
-
Corneal nerves and amyotrophic lateral sclerosis: an in vivo corneal confocal imaging study
Journal of Neurology (2024)