Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating disease caused by degeneration of motor neurons. As with all major neurodegenerative disorders, development of disease-modifying therapies has proven challenging for multiple reasons. Nevertheless, ALS is one of the few neurodegenerative diseases for which disease-modifying therapies are approved. Significant discoveries and advances have been made in ALS preclinical models, genetics, pathology, biomarkers, imaging and clinical readouts over the last 10–15 years. At the same time, novel therapeutic paradigms are being applied in areas of high unmet medical need, including neurodegenerative disorders. These developments have evolved our knowledge base, allowing identification of targeted candidate therapies for ALS with diverse mechanisms of action. In this Review, we discuss how this advanced knowledge, aligned with new approaches, can enable effective translation of therapeutic agents from preclinical studies through to clinical benefit for patients with ALS. We anticipate that this approach in ALS will also positively impact the field of drug discovery for neurodegenerative disorders more broadly.
Key points
-
Amyotrophic lateral sclerosis (ALS), with a lifetime risk of ~1/350, represents an area of huge unmet need and is a useful model of neurodegeneration, with measurable changes in motor function over a relatively short time frame.
-
The field of ALS has advanced significantly over the last decade, with rapid progress in understanding the genetic architecture and the pathophysiological mechanisms of the disease, and in the development of robust, exploitable preclinical model systems.
-
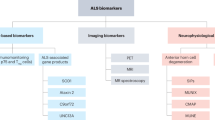
Potential biomarkers of phenotypic conversion, target engagement and therapeutic efficacy have now emerged. Plasma and cerebrospinal fluid (CSF) neurofilament protein levels look particularly promising and may improve the efficiency of future clinical trials and allow identification of responder subgroups.
-
The identification of several biological pathways with the potential to be tackled therapeutically has generated a promising pipeline of preclinical approaches and clinical trials.
-
Genetic therapy trials are now poised for successful translation. In addition, combination therapies or therapies with the potential to ameliorate several pathophysiological mechanisms contributing to motor neuron injury are now being evaluated.
-
Recent innovations in trial design are poised to enhance outcome measures, and patient selection and randomization, while minimizing the impact of disease heterogeneity and increasing statistical power.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease, is a devastating neurodegenerative disorder in which degeneration of the upper motor neurons in the motor cortex and lower motor neurons in the brainstem and spinal cord cause progressive denervation of voluntary muscles. ALS occurs globally, with an incidence of approximately 2 per 100,000 person-years, a prevalence of 6–9 per 100,000 persons (refs. 1,2) and a lifetime risk of approximately 1 in 350 (ref. 3). There is evidence that the condition is increasing in incidence1,4. This may be partially accounted for by changing population demographics, and improved clinical services supporting accurate diagnosis. A family history of ALS is present in 5–10% of affected individuals, usually with an autosomal dominant inheritance pattern. However, systematic genetic testing has revealed the presence of an identifiable genetic cause in a larger proportion of patients with ALS (see below). Advancing failure of the neuromuscular system causes progressive weakness of the muscles in the upper and lower limbs, as well as the bulbar and respiratory muscles. The rate of disease progression is variable, but the majority of patients die from neuromuscular respiratory failure within 2–3 years of symptom onset5. There is an overlap between ALS and frontotemporal dementia (FTD) — approximately 5% of patients with ALS develop overt features of FTD, but detailed neuropsychological evaluation reveals more subtle disturbance of frontal and temporal lobe function in up to 50% of patients6,7.
Pathologically the key features of ALS include loss of upper and lower motor neuron cell bodies and degeneration of the corticobulbar/corticospinal tracts and lower motor neuron axons, with denervation changes within muscles. In most patients (~97%), ALS is a TAR DNA-binding protein 43 (TDP-43) proteinopathy. As motor neurons become injured there is loss of the TDP-43 protein from the nucleus, with cytoplasmic aggregation into structures with compact or skein-like morphology8,9,10. However, there is also pathological heterogeneity: ALS caused by mutations in the SOD1 (Cu–Zn superoxide dismutase) and FUS (fused in sarcoma) genes is not a TDP-43 proteinopathy, although cytoplasmic protein aggregates of different composition are present11,12,13,14. In addition, the most common genetic subtype of ALS, caused by intronic hexanucleotide GGGGCC expansions in the C9orf72 gene, does have TDP-43 mislocalization, but has additional p62-positive protein aggregates caused by pathological dipeptide repeat proteins (DPRs)15.
The diagnosis of ALS is made by exclusion of mimic disorders16. Diagnostic investigations usually include a battery of blood tests, imaging of the brain and spine to exclude structural pathology and a neurophysiological assessment. Diagnostic criteria include the revised El Escorial criteria17, the Awaji Shima criteria18 and most recently the simplified Gold Coast criteria19.
Supplementary Table 1 highlights currently available symptomatic and disease-modifying therapies available for ALS. More than 60 compounds, with different mechanisms of action, have been evaluated in clinical trials in ALS20, but only three of these have been approved for clinical use: riluzole, edaravone and AMX0035. Riluzole was the first FDA-approved therapy for ALS21,22,23. It is considered to reduce glutamate release into the synaptic cleft by blocking voltage-gated sodium channels on presynaptic neurons, and thus to ameliorate excitotoxicity24,25. The disease-modifying effect has been regarded as modest, the initial trial results indicating a prolongation of survival by approximately 3 months on average. However, population studies comparing patients who did and did not receive riluzole treatment documented substantially larger increases in survival, ranging from 6 to 19 months26.
Edaravone, an antioxidant agent administered intravenously for 14 days per month, was evaluated in several trials in Japan27,28. Over a 6-month trial period there was evidence that edaravone slowed disability progression in selected patients early after disease onset and with rapid disease progression27. It is not yet known whether there is an effect on survival. Edaravone was approved for the treatment of ALS in Japan in 2015, and has been approved by the FDA (2017) as well as regulatory authorities in Canada (2018) and other Asian countries including China. To date, edaravone has not been approved by the EMA. An oral formulation of edaravone has recently been approved in the USA and will probably rapidly replace the intravenous formulation in the treatment of patients with ALS. The FDA recently approved AMX0035, developed by Amylyx, for the treatment of ALS after convening an expert panel on two occasions to consider the complex phase II trial data. AMX0035 is a fixed-dose combination of taurursodiol and sodium phenylbutyrate that is considered to mitigate mitochondrial dysfunction and endoplasmic reticulum (ER) stress.
Symptomatic treatment of ALS includes both pharmacological and non-pharmacological interventions as shown in Supplementary Table 1. Most patients with ALS die from neuromuscular respiratory failure. Intervening with non-invasive ventilation when significant weakness of the respiratory muscles develops has a significant impact in improving both life expectancy and quality of life29,30.
In this Review, we describe advances in the molecular subclassification of ALS based on genetics. We review new insights into the pathophysiology of ALS which show promise for drug discovery. We describe advances in preclinical disease modelling which improve the prospects for identification of effective neuroprotective therapies. We describe the current landscape of clinical trials for ALS and summarize the preclinical pipeline of potential therapeutic agents. Finally, we discuss reasons for the relatively high failure rate of ALS clinical trials to date, and suggest strategies to address the huge unmet need for new ALS therapies.
ALS pathophysiology
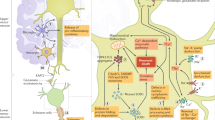
Motor neuron injury in ALS is considered to result from multiple interacting pathophysiological mechanisms that culminate in widespread network disruption. The heterogeneity of ALS suggests that different mechanisms may play more or less prominent roles in individual patients. Advances in preclinical disease modelling (Box 1) are needed to capture this complexity. To develop neuroprotective therapies, an advantageous approach could include upstream targeting of the initiating factor (for example, by gene silencing or gene replacement), and one or more drugs that ameliorate multiple facets of the disease pathophysiology. This section reviews recently emerging insights into contributory pathophysiological mechanisms (Fig. 1) and highlights novel therapeutic strategies.
Advances in large-scale genomic analysis have uncovered a variety of causative genes and risk factors for amyotrophic lateral sclerosis (ALS). These gene variants map onto key pathogenic mechanisms relevant to all motor neuron cellular compartments as well as neighbouring cells such as glia and interneurons. In this way, these mechanisms are genetically validated, enabling a greater confidence in their targeting for therapeutic benefit. Some of these mechanisms have emerged only in recent years due to new genetic information, including gene changes highlighting dysregulation of RNA processing and metabolism. There is significant overlap of some genes with those found in closely related disorders such as frontotemporal dementia (for example, C9orf72, CHCHD10, SQSTM1, TBK1, CCNF, FUS, TARDBP, OPTN, UBQLN2, TUBA4A, ATAXN2, VCP and CHMP2B). This suggests a closer relationship to broader neurodegenerative disorders, and indeed many of the pathways depicted are relevant in, for example, Alzheimer disease. ER, endoplasmic reticulum. For a complete list of the ALS loci, genes and associated proteins, see Supplementary Table 2.
Genetic architecture
Since the discovery of SOD1 mutations as a cause of familial ALS (fALS) in 1993 (ref. 31), our understanding of the genetic architecture of ALS has increased substantially. ALS is an archetypal complex disease which has a monogenic cause in ~10–21% of patients, but in the majority of affected individuals is determined by an interaction of multiple genetic and environmental risk factors.
Of patients with ALS, 5–10% have familial disease, usually with a Mendelian autosomal dominant pattern of inheritance. The genetic cause in 60–70% of individuals with fALS has now been identified32. In 90–95% of patients with ALS, the disease is currently classified as sporadic ALS (sALS), but genetic factors are considered important even in the absence of a family history. The heritability of sALS has been estimated at approximately 50%3,33. Analyses of large genome-wide association studies (GWAS) of patients apparently with sALS have indicated that the genetic architecture of ALS is based predominantly on rare variants34. Thus, the traditional subclassification of ALS into fALS and sALS is now recognized as overly simplistic and the field is moving towards a more accurate molecular subclassification based on the identification of risk genes.
More than 30 genes have been identified (Table 1; Supplementary Table 2) which are causative in or confer an increased risk of the development of ALS. Four genes account for the disease in up to 70% of patients with fALS, at least in European populations: C9orf72, SOD1, TARDBP and FUS. A recent extensive GWAS involving a meta-analysis of 29,612 patients with ALS and 122,656 controls identified 15 new genetic risk loci33. Interestingly, the risk of developing ALS and the factors controlling severity of disease (age at onset and speed of progression) appear to be genetically independent. The identified risk genes converge on several key biological pathways including: oxidative stress; dysregulation of mitochondrial function, protein homeostasis, RNA processing, axonal transport and nucleocytoplasmic transport (NCT); neuroinflammation; excitotoxicity; and DNA damage (Fig. 1). These pathways should be intensively interrogated for new therapeutic targets.
Recent studies have shown the value of systematic genetic screening of all patients with ALS as opposed to focusing on those with familial and early-onset disease. A prospective study which undertook targeted sequencing of ALS-relevant genes showed that 21% of patients had a clinically reportable pathogenic variant and a further 21% had variants of unconfirmed significance35. Among the 13% who carried more than one variant, there was a significantly earlier age at disease onset.
Key insights emerging from recent genetic studies are highlighted in Box 2.
It is likely that genetic profiling will become standard practice and will replace the traditional classification into fALS and sALS. In this effort statistical power can be improved by reducing the search space for causative genes to areas of the genome which are functional within motor neurons36. Successful complete genetic subclassification of ALS will have a major effect on therapy development, both by targeted genetic therapy approaches and by facilitating the development of more relevant ALS models for drug screening.
Many of the genetic mutations associated with ALS are present for >50 years before disease onset. It is noteworthy that the age at disease onset is variable even within pedigrees harbouring the same mutation. The late age at onset suggests a multistep process in which genetic factors are penetrant only when combined with lifestyle or environmental factors37. In addition, incomplete heritability of known pathogenic mutations implicates an interaction with environmental factors. To date, the only confirmed epidemiological risk factors associated with the development of ALS are age and male gender1. Recent Mendelian randomization studies38 have produced relatively robust evidence for a causal link between strenuous physical exercise39 and hyperlipidaemia40, and the risk of ALS. Other potential environmental risk factors proposed include smoking, military service and specific sporting activities, including soccer and American football41,42,43,44.
Oxidative stress
Oxidative stress may result from excess levels of reactive oxygen species (ROS), reactive nitrogen species or impaired functioning of antioxidant defence systems. ROS contribute significantly to neuronal injury and central nervous system (CNS) ageing by changing the structure and function of biomolecules including proteins, lipids, DNA and RNA. There is abundant evidence indicating a key role for oxidative damage in the pathophysiology of ALS45,46,47. Altered oxidative stress biomarker profiles have been reported in models of ALS and in human ALS biosamples48. There is also evidence for impaired oxidative stress defence systems in ALS, including dysregulation of glutathione homeostasis49 and the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant response element (ARE) cytoprotective system50,51.
Oxidative stress can contribute to and exacerbate multiple other pathophysiological processes that are involved in motor neuron injury45. Biochemical and cell-based assays have identified oxidative stress as a signalling cue which promotes acetylated TDP-43 aggregates, while TDP-43 acetylation impairs RNA binding and promotes accumulation of insoluble, hyperphosphorylated TDP-43 species that resemble the pathological inclusions found in the CNS of individuals with ALS52. Mislocalization of both TDP-43 and FUS occur in cellular models of ALS exposed to oxidative stress, underpinning alterations in RNA processing53. Chronic oxidative stress promotes GADD34-mediated phosphorylation of TDP-43, a hallmark of TDP-43 proteinopathy54. Cytoplasmic aggregation of TDP-43 sequesters specific microRNAs and proteins, including nuclear genome-encoded mitochondrial proteins, leading to dysregulation of mitochondrial function that further augments oxidative stress55. Thus, an accelerating cycle of oxidative stress, protein aggregation and mitochondrial dysfunction is established.
Oxidative stress is also involved in the crosstalk between motor neurons and neighbouring astrocytes and microglia56,57. For example, there is evidence that astrocytes release glutamate via upregulation of the cysteine–glutamate antiporter in response to increased oxidative stress and this in turn may augment excitotoxic injury to motor neurons56.
Effective alleviation of oxidative stress could potentially ameliorate multiple facets of the pathobiology of motor neuron degeneration. A retrospective analysis of drugs evaluated in mSOD1 mice indicated that drugs that specifically target oxidative stress could be the most promising therapeutic candidates for the prevention of motor neuron degeneration58. Alleviation of oxidative stress is likely to be the primary mechanism of action of the free radical scavenging drug edaravone.
Excitotoxicity
Excitotoxicity mediated by excessive stimulation of postsynaptic glutamate receptors is considered a major pathophysiological mechanism in ALS59. A prolonged increase of synaptic glutamate causes excessive neuronal firing, increasing intracellular calcium levels with downstream neurotoxic effects60. Excitotoxicity causes prolonged pathological changes, such as ER stress and mitochondrial calcium overload56. Excessive glutamate exposure also decreases cysteine uptake by inhibiting the glutamate–cysteine antiporter, depleting the intracellular levels of the antioxidant glutathione and increasing indices of intracellular oxidative stress61.
Motor neurons susceptible to neurodegeneration in ALS have cell-specific features which increase their vulnerability to excitotoxic injury including: high expression of calcium-permeable AMPA receptors lacking the GluR2 subunit62 and low expression of the calcium-buffering proteins parvalbumin and calbindin63. Decreased expression of the major glutamate re-uptake transporter GLT1–EAAT2 has been reported in both animal models and the CNS of patients with ALS64,65,66. In human induced pluripotent stem cell (iPSC)-derived motor neurons, C9orf72 mutations increase Ca2+-permeable AMPA receptor-mediated excitotoxicity67 and also impair mitochondrial calcium-buffering capacity68.
Transcranial magnetic stimulation with threshold tracking has been deployed to demonstrate that cortical hyperexcitability is an early and intrinsic feature of sALS and fALS69. Magnetic resonance spectroscopy (MRS) has been used to measure glutamate and GABA levels in the brain of patients with ALS, with variable results across different studies.
Riluzole has complex and incompletely understood mechanisms of action. However, one component is an effect on presynaptic sodium channels that causes a reduction in the release of glutamate from presynaptic terminals, which is considered to ameliorate excitotoxic effects on motor neurons70. It is noteworthy that, in the current development pipeline, most active drug programmes targeting glutamate excitotoxicity are associated with riluzole reformulation efforts (for example, to produce stable liquid and sublingual preparations of the drug71). Metabotropic glutamate receptors are emerging as novel potential drug targets in ALS as their modulation may result in a decrease in glutamate release as well as induction of the production of neurotrophic factors (NTFs)72.
Mitochondrial dysfunction
Mitochondrial dysfunction has been proposed as a central determinant of the pathophysiology of ALS. Altered energy production, excess generation of ROS, disruption of mitochondrial axonal transport, altered mitochondrial structure and dynamics, perturbation of mitophagy and calcium buffering, and triggering of apoptosis have been extensively described in ALS model systems and patient biosamples73,74,75. Defects in the specialized domains on the ER mitochondrial-associated membranes have been reported to impair neuronal calcium homeostasis, mitochondrial dynamics, ER function and autophagy, culminating in axonal degeneration76.
Mutations in specific ALS genes have been linked to compromise of mitochondrial function through various mechanisms77 and multiple abnormal ALS disease-associated proteins have been shown to interact directly with mitochondria, impairing their function. For example, mutant SOD1 aggregates in the mitochondrial intermembrane space and reduces the activity of the electron transport chain (ETC) complexes. The DPR poly-GR produced in C9orf72-mutant ALS binds to the mitochondrial complex V component ATP5A1 and enhances its ubiquitination and degradation78. The C9ORF72 protein localizes to the inner mitochondrial membrane and regulates cellular energy homeostasis by stabilizing the translocase of inner mitochondrial domain containing 1 (TIMMDC1) protein, a crucial factor for the correct assembly of the oxidative phosphorylation complex. The function of mitochondrial complex 1 is impaired in patient-derived neurons from patients with C9orf72-mutant ALS79.
There is evidence that TDP-43 has a role in maintaining mitochondrial homeostasis by regulating the processing of mitochondrial transcripts, a function that is perturbed in the presence of mutations80. TDP-43 aggregates also sequester specific microRNAs and proteins that are nuclear genome-encoded mitochondrial proteins, and abnormal levels of expression cause dysregulation of mitochondrial function which in turn augments oxidative stress55.
Potential therapeutic strategies targeting mitochondrial biology have been attempted. Several drugs targeting mitochondrial function and/or ROS such as coenzyme Q10, dexpramipexole, olesoxime and creatine all showed promise in preclinical models, but were unsuccessful in clinical trials81,82,83,84. Tauroursodeoxycholic acid (TUDCA) modulates the mitochondrial pathway of neuronal injury by reducing ROS formation, inhibiting BAX translocation, cytochrome c release and caspase 3 activation85. A recently published phase II clinical trial involving 137 patients with ALS evaluated treatment with TUDCA combined with sodium phenylbutyrate over a 6-month period86,87 and showed evidence of slowing of the rate of decline on the ALS functional rating scale–revised (ALSFRS-R) score and increased survival. A European trial of TUDCA alone is ongoing (EudraCT 2018-002722-22). A recent preclinical study, using models harbouring SOD1 and TARDBP mutations, demonstrated that inhibition of protein phosphatase 1 prevented mitochondrial fragmentation, ETC impairment, disruption of axonal transport and cell death88 and this pathway is worth exploring further. 31P-MRS has been used to provide evidence for bioenergetic dysfunction in the brain and muscle of patients with ALS and provides a potential biomarker tool for use in clinical trials targeting bioenergetic dysfunction89.
Impaired protein homeostasis
The correct balance between protein production and degradation is controlled by a complex network involving the synthesis, folding, trafficking and degradation of proteins which respond to stress signals such as the unfolded protein responses of the ER and mitochondria and the cytosolic heat shock response. Loss of proteome fidelity, accumulation of non-native protein aggregates and oxidatively damaged protein species are key features of an ageing cell and may contribute to age-related neurodegenerative disorders90. There is extensive evidence that dysfunctional proteostasis contributes to the pathophysiology of ALS91,92. Several proteins encoded by genes causative in ALS regulate the proteostasis network directly or indirectly (Table 1) and some ALS-linked proteins (for example, SOD1 and TDP-43) are substrates for these pathways. C9ORF72, sequestosome 1/P62, optineurin and ubiquilin 2 play key roles in the initiation of autophagy; ubiquilin 2, alsin, FIG4, VCP and CHMP2B are important in the control of maturation of autophagosomes; TBK1 loss of function impairs substrate delivery to autophagosomes93,94,95,96,97,98,99.
Motor neurons may be particularly vulnerable to proteome stress based on their size and axonal arbour, the relatively low expression of ubiquitin proteasome genes in motor neurons that are vulnerable to degeneration in ALS100, and their relative inability to mount an effective heat shock response101.
Intracellular protein aggregates are a hallmark pathological feature of ALS. Studies focusing on the effects of TDP-43 mislocalization have highlighted both gain and loss of function consequences including altered regulation of splicing, hyperresponsiveness to cellular stress, increased DNA damage, widespread alterations in the transcriptome102,103 and alteration in the transport of ribosomal protein mRNAs to regulate local axonal translation104. TDP-43 has a key role as a repressor of cryptic exon inclusion during RNA splicing, and loss of TDP-43 from the nucleus leads to inclusion of a cryptic exon in UNC13A mRNA and reduced UNC13A protein expression, which may interfere with vesicle maturation during exocytosis and neurotransmitter release105. In mouse models, TDP-43 mislocalization has been shown to cause abnormal synaptic function and a hyperexcitability phenotype in the motor cortex106.
Stress granules (SGs) are dynamic membraneless compartments composed of mRNAs and RNA-binding proteins (RBPs) that assemble on a temporary basis to allow the cell to respond to stress by halting translation of the majority of mRNAs and directing the translation of cytoprotective proteins which allow the cell to mount an effective stress response. Normally SGs are highly dynamic structures, but in the presence of age-related changes or severe cellular stress, they may form solid aggregated inclusion bodies. SGs in ALS contain multiple RNAs and proteins and also proteins such as TDP-43 and FUS which possess so-called low complexity prion-like domains that are prone to aggregation107. There is a prevalent view that impaired SG disassembly facilitates the transition from a liquid to a solid phase, driving the formation of cytoplasmic TDP-43 inclusions108. Multiple ALS genes encode proteins which interfere with SG dynamics through mutations of their low-complexity domain including: TARDBP, FUS, EWSR1, TAF15, hnRNPA1, hnRNAPA2B1, ATXN2 and TIA1 (ref. 109). ANXA11 mutations have been shown to cause dysregulation of SG disassembly110 and VCP mutations impair autophagy-dependent SG degradation and also autophagy-independent disassembly111.
Restoration of proteostasis to reduce the burden of aggregated proteins is regarded as an attractive therapeutic approach for neuroprotection. Pathways targeted in experimental models have included: upregulation of chaperones; manipulation of the heat shock response; protection from ER stress; maintenance of EiF2A (eukaryotic initiation factor 2A) in the active state; induction of autophagy; and activation of the proteasome machinery90. An array of small molecules have been identified which can modulate several aspects of TDP-43 proteinopathy including the level of expression, nucleocytoplasmic localization, cleavage and phosphorylation status of TDP-43 (ref. 112). The heat-shock inducer arimoclomol showed promising results preclinically113, but recently failed to show efficacy in clinical trials114. The therapeutic potential of autophagy induction has been explored in experimental models91. Some success was reported with rapamycin and trehalose, and fluphenazine, methotrimeprazine and berberine have emerged as hits from screening studies115,116,117.
Studies to identify small molecules that modulate the recruitment of TDP-43 and other RBPs into SGs have highlighted compounds with planar aromatic moieties in cellular models of ALS118. Targeting the VCP–FAF2 axis to enhance SG disassembly has also been proposed as a potential therapeutic approach to promote the clearance of persistent SGs, although this approach has not yet reached human trials119.
Neuroinflammation and glial contribution
Neuroinflammation is a pathological hallmark evident in preclinical models and the CNS of patients with ALS both histologically and in imaging studies120.
Astrocytes maintain the integrity of the blood–brain barrier and modulate inflammatory signalling through the release of pro-inflammatory cytokines (interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)) or anti-inflammatory molecules (prostaglandin E2 and transforming growth factor (TGF)-β)121,122,123. Astrocytes derived from fibroblasts from patients with ALS are toxic to cocultured motor neurons124. The exact mechanisms of this toxicity have not yet been established although impaired bioenergetic support through lactate release, and pro-nerve growth factor–p75 receptor signalling, may contribute125.
Microglia can adopt a toxic M1 phenotype or a neuroprotective M2 phenotype126,127,128. In mutant SOD1-transgenic mice, the microglia switched from the M2 to the M1 phenotype after disease onset129. Peripheral blood monocytes from patients with ALS are more readily activated and differentiated to a pro-inflammatory M1 phenotype, and represent a potential target for immunomodulatory therapy130.
Microglial NLRP3 inflammasome activation has been reported as a key contributor to neuroinflammation in ALS. NLRP3 inhibition could potentially become a therapeutic target to ameliorate microglia-driven neuroinflammation and disease progression in ALS57. The nuclear factor-kappa β (NF-κB) protein has been reported as a master regulator of inflammation in ALS131. NF-κB signalling was activated within glia during disease progression in mutant SOD1 mice. Notably, NF-κB signalling has been proposed to regulate microglial activation in ALS, as the localization of NF-κB activity and deletion of NF-κB signalling in microglia rescued motor neurons from microglia-mediated death and extended survival in ALS mice by impairing pro-inflammatory microglial activation132.
The responses of glia to TDP-43 pathology have been studied133,134,135. TDP-43 proteinopathy has been reported to cause inflammation by triggering cytoplasmic release of mitochondrial DNA which activates the cytoplasmic DNA-sensing cyclic GMP-AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway. It is possible that inhibition of cGAS–STING could mitigate inflammatory pathology in ALS136,137.
Transcriptomic analysis of the motor cortex identified 1,573 differentially expressed inflammatory genes in sALS and indicated specific inflammatory molecular signatures for different patient subgroups, with the potential for personalized therapeutic targeting138. The C9ORF72 protein may modulate inflammation139 and there is evidence that reduced C9ORF72 levels cannot suppress inflammation mediated by the STING pathway140.
Biochemical and imaging biomarkers of glial activation have the potential to be used as pharmacodynamic indices in clinical trials. Patients with ALS exhibit higher cerebrospinal fluid (CSF) levels of chitotriosidase (Chit-1) and chitinase-3-like protein 1 (CHI3L1) than neurological disease controls and healthy controls, which correlate with the rate of disease progression121. Glial activation in vivo has been measured using translocator protein (TSPO) PET imaging and dynamic 18F-DPA714 or 11C-PBR28 PET–MRI141.
DNA damage and repair
Postmitotic cells such as neurons are highly susceptible to DNA damage and if unrepaired, cell death ensues136. DNA damage has been demonstrated in pathological studies showing elevated oxidized deoxyguanosine (OdG) in CNS tissue and biofluids from patients with ALS142,143. Recent evidence has been reported of elevated apurinic/apyrimidinic DNA sites (loss of purine/pyrimidine base from the DNA strand) and activation of the DNA damage response (DDR) in ALS144.
Several genes mutated in ALS lead to elevated DNA damage or have roles in DNA repair. C9orf72-related ALS shows elevated staining for γH2AX, a phosphorylated histone that acts as an important regulator of the DDR, in spinal motor neurons145. iPSC-derived motor neurons from patients with C9orf72-related ALS show a similar elevation in γH2AX which is partially rescued by inhibition of oxidative stress146. C9orf72 hexanucleotide expansions are prone to formation of G-quadruplex DNA structures147 which promote the formation of R-loops148. These DNA–RNA hybrid structures are substrates for DNA strand breaks, and have been detected in spinal cord tissue from patients with C9orf72-related ALS and mouse models in association with defective ataxia telangiectasia mutated (ATM)-mediated DNA repair signalling148.
Recently TDP-43 has been shown to play a role in DDR signalling via association with DDR proteins103. Loss of nuclear TDP-43 was also shown to correlate with strand breaks and DDR response in spinal cord tissue from patients with sALS103. Further in vitro assessment in cell models of DNA damage showed that TDP-43 participates in non-homologous end-joining-mediated repair. TARDBP mutations prevent this activity and lead to DNA damage, and DNA damage itself can cause TDP-43 mislocalization149. Loss of TDP-43 also increases R-loop formation and genome instability and the TARDBP mutation causes the same abnormalities150. FUS and NEK1 mutations have also been implicated in dysregulation of DNA repair151,152.
The emerging picture is of DNA damage and impaired repair being a common pathological feature. This is supported by multiple lines of evidence implicating loss of DDR or DNA repair functions of ALS-related genes. A causal link for DNA damage in motor neuron loss is not yet supported by the available evidence, but therapeutic opportunities to enhance repair or mitigate damage (for example, by amelioration of oxidative stress) are worth further investigation.
Dysregulated RNA metabolism and nucleocytoplasmic transport (NCT)
Disrupted RNA metabolism appears to play a key role in the pathophysiology of ALS. Both TDP-43 and FUS are RNA-binding proteins (RBPs) with major roles described in multiple aspects of RNA biology including splicing, transcription, stability, export and microRNA biogenesis153. Both proteins have binding sites for over 5,000 genes and regulate expression levels of hundreds of mRNAs. The most profound mutation-associated changes in expression occur in genes with very long introns and roles in synaptic function154,155. C9orf72 mutations lead to the accumulation of RNA foci which sequester a range of RBPs including TDP-43 and RNA export factors155,156. This sequestration has inevitable downstream effects on RNA metabolism149 including dysregulated splicing. Targeting the repeat expansion in C9orf72 with antisense oligonucleotides (ASOs) was shown to prevent the formation of RNA foci and downstream effects157,158 and is under clinical investigation (Table 2). The sequestration of RBPs to C9orf72 RNA foci also licenses them for nuclear export, allowing the pathological transcripts to escape to the cytoplasmic ribosomal machinery, resulting in the production of toxic DPRs159. This has become a potential target for therapeutic intervention by inhibition of the nuclear export adaptor SRSF1 (ref. 159).
Deficiencies in NCT have been identified in multiple ALS model systems160,161,162,163. DPRs resulting from repeat-associated non-AUG (RAN) translation of C9orf72 repeats promote SG formation107, and an emerging model is one in which aberrant recruitment of NCT factors to SGs disrupts NCT, leading to neurodegeneration164. Inhibitors of the integrated stress response (ISR) such as ISRIB, a stabilizer of eIF2B function165 were able to protect against these effects164 and related compounds are in clinical studies in ALS (Table 2).
The mislocalization of TDP-43 itself is thought to play a role in disturbed NCT via sequestration of NCT machinery components166. A putative target to reduce TDP-43 nuclear export is exportin 1 which binds the nuclear export sequence in proteins to mediate their export from the nucleus. Inhibitors of exportin-1 (KPT-276 and KPT-335) have been shown to reduce cell death in cortical neurons expressing a TDP-43 carboxy-terminal fragment166. However, whether TDP-43 nuclear export is mediated by exportin 1 is still not established167. An ASO approach to limit exportin-1 expression (BIIB100) was discontinued after phase I trials.
Unidirectional transport of many cargoes is dictated by a cytoplasmic to nuclear gradient of RAN GTPase, which exists predominantly in a GTP-bound state in the nucleus. The loss of this gradient is predicted to be a key factor in the disruption of NCT in ALS. A pathway which maintains this gradient and restored normal NCT in C9orf72-related ALS neurons has recently been identified168. Overall, the pathways related to NCT may be fertile ground for future therapeutic discovery in ALS.
Impaired axonal transport and integrity
Due to their extreme polarization, motor neurons are particularly reliant on retrograde and anterograde transport of cargos to maintain axonal integrity. Defects in axonal transport have been described in multiple ALS models and in the human disease, as evidenced by pathological accumulations of organelles and phosphorylated neurofilaments in motor neuron axons, as well as the identification of ALS-associated mutations in cytoskeletal and axonal transport-related genes169. Mutations in KIF5A, which encodes a microtubule motor protein, and in ANXA11, which disrupt axonal RNA transport, were recently identified as causative in ALS170,171.
Targets which may be amenable to therapeutic intervention have been identified. Small-molecule screens identified P38 MAPK inhibitors that rescued axonal transport defects in SOD1-mutant mice172 and IGF1R inhibitors173 as selective potentiators of the axonal transport of signalling endosomes. Retrograde transport of neurotrophin-containing signalling endosomes is required for axonal integrity, and inhibition of P38 MAPK or IGF1R increased their rate of retrograde axonal transport172,173.
A well-described activator of axonal transport is via inhibition of HDAC6 and consequent reduction in tubulin acetylation. Knockdown or pharmacological inhibition of HDAC6 restored axonal transport defects in human FUS-associated ALS cellular models174. In vivo models have pointed to a prominent role of mutant TDP-43 in mediating axonal transport defects175. Compounds targeting HDAC6 are currently in the preclinical discovery phase for the treatment of ALS.
Wallerian degeneration is a process of controlled axonal degeneration involving enzymes which detect and trigger programmed axonal degeneration following injury176. Sterile-α and Toll/IL-1 receptor (TIR) motif-containing protein 1 (SARM1) is a NAD-degrading enzyme with a major role in facilitating fast Wallerian degeneration. Sarm1 deletion in a transgenic TARDBPQ331K mouse model reduced motor axon degeneration and denervation of neuromuscular junctions177. SARM1 inhibitors are currently being explored for several therapeutic indications, including ALS. Overall, the mechanisms underlying disrupted axonal transport and integrity are a promising avenue for future therapeutic discovery.
ALS drug pipeline
Pathophysiological mechanisms currently being targeted in preclinical development and clinical trials for ALS are highlighted in Fig. 2.
We have identified over 40 active clinical programmes (Table 2) and over 50 late-stage preclinical programmes (Table 3) and show here how these efforts map onto the pathophysiological landscape of amyotrophic lateral sclerosis (ALS). Among clinical programmes, some trends emerge. Neuroinflammation is the best represented therapeutic target covered by a variety of approaches, with the complement system representing a particular target of interest. Genetic therapies using antisense oligonucleotide (ASO) approaches also dominate the clinical space targeting specific ALS genes, although Ataxin2 modifiers may be more broadly applicable in relation to TDP-43 proteinopathy. Genetic therapies are also prominent in preclinical efforts. Other notable areas include proteostasis and cell therapies. Considering the most up to date understanding of pathomechanisms and genetics, several areas are poised for further exploration in sporadic ALS more broadly, including RNA metabolism and axonopathy. AAV, adeno‐associated virus; mAb, monoclonal antibody; MSC, mesenchymal stem cell; TUDCA, tauroursodeoxycholic acid.
We identified 91 development programmes for drugs approved or in clinical development for the treatment of ALS, using the AdisInsight Springer database (August 2022). A total of 53 drug development programmes had at least one significant ALS trial update within the last 12 months and were not discontinued programmes (Table 2). Of the 53 drug development programmes, 39 focused on novel therapeutic agents, while the other 14 were related to marketed drugs, drug repurposing or drug reformulation (including six drugs that are reformulations of edaravone or riluzole, not listed in Table 2). Six drugs were either marketed or in the pre-registration stage, while 11 additional drugs were under evaluation in pivotal trials.
The selected 53 drugs with recent updates were manually categorized according to the mechanism of action to identify trends and gaps, and several key themes emerged. The most represented target category was neuroinflammation, with 16 therapeutic agents in trials using approaches ranging from upregulation and activation of endogenous regulatory T cells (aldesleukin) to therapeutic antibodies targeting CD4+ T cells and B cells (anti-CD40L). Several approaches targeting the complement cascade, including ANX-005 (C1q inhibitor) and pegcetacoplan (C5 convertase inhibitor) were identified, although recent trials in ALS of the anti-C5 antibody ravulizumab and the C5 inhibitor zilucoplan were terminated following interim analyses, due to lack of efficacy.
Another well-represented group of compounds in trials was for genetic therapies using ASO technology to knockdown gene expression. Three of these target specific mutations in genetic subpopulations (C9orf72, FUS and SOD1) and one targets Ataxin2, a potential modifier of TDP-43 proteinopathy, which could be relevant for almost all ALS subtypes. Depletion of ataxin 2 levels limits TDP-43-mediated toxicity in multiple model systems178. The ASO ION-363 is currently being evaluated in a phase III trial (NCT04768972) targeting patients with ALS and mutations in the FUS gene. ION-363 was initially developed as an experimental treatment specifically for a patient with FUS-associated ALS and then provided to several patients in a compassionate use programme. The controlled phase III trial was established based on this initial programme179.
Other well-represented approaches include glutamate excitotoxicity, although the four different approaches identified represent generic bioequivalence trials for new formulations of riluzole. Cell therapy continues to be an area of interest, although the current focus is on cell therapy that modulates the toxic microenvironment, as opposed to cell replacement. NurOwn is one of the larger recent cell therapy trials, although the pivotal phase III trial (NCT03280056) did not meet statistical significance in the primary efficacy end point180. Additionally, troponin activators that increase muscle force for a given level of motor neuron innervation represent a symptomatic approach to improve muscle strength, rather than aiming for neuroprotection of motor neurons181.
Approaches that are under-represented, based on current knowledge of the pathophysiology of ALS, include those targeting mitochondrial dysfunction and proteostasis. AMX0035 potentially targets both of these pathways and was the subject of a recent new drug application (NDA) and marketing authorization application filings based on data from phase II trials86,87. AMX0035 was approved in 2022 for use in the USA and Canada for the treatment of ALS. The phase III PHOENIX trial of this combination therapy is ongoing.
Clinical case studies
Analysis of clinical stage ALS drug development programmes has highlighted several major target mechanisms of action. To demonstrate the current trends in ALS drug development, one representative drug programme from each target mechanism of action was selected for further discussion in the following case studies.
NurOwn
Mesenchymal stem cell (MSC)-NTF cell therapy (NurOwn), developed by BrainStorm Cell Therapeutics182, is the first ALS cell therapy programme evaluated by a randomized, placebo-controlled pivotal trial. Adult stem cells were collected from the patient’s own bone marrow, expanded and manipulated to secrete NTFs ex vivo. Subsequently, the cells were transplanted back into the patient via intrathecal injection. It was demonstrated that adult bone marrow MSCs may be differentiated into astrocyte-resembling cells that express astrocytic markers and produce significant levels of NTFs183,184. These MSC-NTF cells secrete NTFs, such as glial cell line-derived NTF (GDNF), brain-derived NTF (BDNF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) with the potential to protect motor neurons. MSC-NTF cells are also reported to stimulate new motor neuron growth and to promote neuromuscular junction reinnervation182.
A pivotal phase III trial (NCT03280056) was conducted to evaluate the safety and efficacy of repeated administration of NurOwn in patients with ALS. The randomized, double-blind trial completed enrolment of 196 patients. The trial did not meet statistical significance in the primary efficacy end point, which evaluated the percentage of trial participants who experienced a 1.25 points per month improvement in the post-treatment ALSFRS-R slope. Response rates of 33% and 28% were observed for the NurOwn and placebo groups, respectively (P = 0.45). However, positive elements did emerge in the phase III trial. For example, significant improvements in CSF biomarkers of neuroinflammation, neurodegeneration and NTF support were observed in patients treated with NurOwn, and the treatment was well tolerated87,180. Meanwhile, another MSC cell therapy programme, lenzumestrocel (Neuronata-R), is currently under evaluation in a phase III clinical trial (NCT04745299) to confirm efficacy and safety. Neuronata-R therapy was approved for the treatment of ALS by the Ministry of Food and Drug Safety in South Korea in 2014.
Tofersen
Tofersen is an ASO developed by Ionis Pharmaceuticals and licensed to Biogen, and is in development for the treatment of patients with ALS and a confirmed SOD1 mutation. Tofersen binds to SOD1 mRNA and is predicted to reduce the synthesis of the SOD1 protein and therefore ameliorate the toxic gain of function of mutant SOD1. Tofersen is administered by intrathecal injection at monthly intervals.
Tofersen was evaluated in SOD1-transgenic rodent models. The drug extended survival by more than 50 and 40 days in SOD1G93A rats and SOD1G93A mice, respectively185. The initial loss of compound muscle action potential in SOD1G93A mice was also reversed after a single dose of tofersen. Furthermore, increases in serum phosphorylated neurofilament heavy, a promising biomarker for ALS, were ameliorated by the ASO. Confidence in the approach for SOD1 gene silencing also arose from gene therapy trials in SOD1-transgenic mice using intrathecal administration of adeno-associated viral vectors to deliver short hairpin RNAs targeting SOD1 (ref. 186).
In the reported phase I/II trial (NCT02623699) of tofersen, involving 50 participants with SOD1-associated ALS, the mean level of CSF SOD1 was lowered by 33% on day 85 in the group receiving the highest dose (100 mg) compared with the group receiving placebo. The concentrations of phosphorylated neurofilament heavy and neurofilament light (NFL) in plasma and CSF were decreased during the intervention period among the ten participants who received 100 mg of tofersen, providing the first indication in an ALS trial of a potential biomarker of therapeutic efficacy. Additional exploratory measures suggested that tofersen may slow the functional decline of patients, as assessed by ALSFRS-R, pulmonary function testing and evaluation of muscle strength by hand-held dynamometry187.
Based on the above positive outcome, an additional cohort was recruited to evaluate the 100 mg dose in the tofersen phase III VALOR trial, which enrolled 108 patients internationally and completed in 2021. The VALOR trial did not achieve statistical significance on its primary end point of change in ALSFRS-R score at 6 months. However, consistent positive effects were observed across key secondary and exploratory clinical outcome measures, and these effects became more apparent with longer-term follow-up in the open-label extension study after the 6-month experimental period. Tofersen administration resulted in a slowing of decline in participants with faster progressing disease, as well as an apparent clinical stabilization in participants with slower progressing disease188,189. Biogen has filed an NDA submission with the FDA, which accepted the application in July 2022 under the accelerated pathway, and granted priority review. In October 2022, Biogen announced that the FDA had extended the review period of the NDA for tofersen by 3 months. Therefore, the updated Prescription Drug User Fee Act goal date is 25 April 2023. Biogen has also initiated a phase III ATLAS study to evaluate the ability of tofersen to delay clinical onset of ALS in pre-symptomatic individuals with a SOD1 mutation and biomarker evidence of ALS disease activity measured by a threshold level of NFL in plasma (NCT04856982). This will allow the effects of early intervention to be evaluated.
A similar approach, using intrathecally delivered ASOs, has been employed for the treatment of C9orf72-associated ALS (BIIB078 (terminated in 2022 due to lack of efficacy) and WVE004). During these trials, the measurement of DPRs in CSF could provide a useful biomarker of target engagement190. The BIIB078 ASO led to robust dose-dependent reduction of DPRs in the CSF. However, despite this, the treatment group receiving the highest dose showed a trend towards greater decline than the group receiving placebo across clinical outcome measures as well as an increase in NFL levels, suggesting increased neuronal injury191. The reasons for this unexpected outcome are uncertain but could include: the fact that the ASO targeted only the sense transcripts produced by the C9orf72 expansion, leaving antisense transcripts untouched; failure to address or possible exacerbation of C9 haploinsufficiency; or failure to adequately address downstream mechanisms of disease propagation (for example, TDP-43 proteinopathy).
AMX0035
AMX0035 (Relyvrio) is the first combination potential disease-modifying therapy trialled in ALS, although another combination therapy of dextromethorphan hydrobromide and quinidine sulfate was approved for the symptomatic treatment of pseudobulbar affect in ALS (NCT00573443, NCT01799941; Nuedexta Prescribing Information). AMX0035 is an oral proprietary, fixed-dose combination of sodium phenylbutyrate and taurursodiol (also known as TUDCA) developed by Amylyx Pharmaceuticals as a therapeutic approach targeting multiple pathophysiological mechanisms11 in ALS and Alzheimer disease. Sodium phenylbutyrate is a histone deacetylase inhibitor that upregulates chaperones192, while taurursodiol inhibits mitochondrial associated apoptosis193.
In the phase II CENTAUR trial (NCT03127514), 137 participants were randomly assigned 2:1 to receive AMX0035 or placebo for 6 months. Participants completing the 6-month randomized phase were eligible to receive phenylbutyrate and taurursodiol in an open-label extension. In a modified intention-to-treat analysis, AMX0035 was associated with a statistically significantly lower mean rate of change in the ALSFRS-R score at −1.24 points per month, compared to −1.66 points per month in those receiving placebo. Secondary outcomes did not differ significantly between the two groups87. Median overall survival was 25.0 months in participants originally randomized to AMX0035 and 18.5 months in those originally randomized to placebo. Initiation of active treatment at baseline resulted in a 6.5-month longer median survival compared to placebo. These trial results suggest that AMX0035 may have functional and survival benefits in ALS86 although several aspects of the trial have been criticized194.
Amylyx has sought market authorization from multiple regulatory authorities and has also initiated recruitment into a phase III PHOENIX trial evaluating the safety and efficacy of AMX0035 in approximately 600 patients with ALS (NCT05021536). AMX0035 has received market authorization in the USA and Canada for the treatment of ALS (2022). The drug approval by Health Canada was under its notice of compliance with conditions policy, which enables therapies showing the potential to fill an unmet medical need in severe, life-threatening diseases to reach the market sooner, provided that certain conditions are met. Those conditions included the provision of data from the phase III Phoenix trial (NCT05021536), as well as additional planned or ongoing studies. Several post-marketing studies were also required by the FDA for the approval of AMX0035, including carcinogenicity studies, additional drug interaction studies, and clinical pharmacokinetic studies in subjects with hepatic and renal impairment195.
One of the active ingredients of AMX0035, taurursodiol, is currently being evaluated in a randomized phase III TUDCA-ALS trial as an add-on treatment to riluzole in patients with ALS, sponsored by Humanitas Mirasole SpA (NCT03800524).
Aldesleukin
Aldesleukin is being investigated in a phase II/III ALS clinical trial (MIROCALS), with an embedded experimental medicine approach. Aldesleukin, recombinant human IL-2 with essentially similar pharmacodynamic properties to endogenous IL-2, is being developed by Clinigen for the treatment of ALS. It has been approved by the FDA for the treatment of metastatic melanoma and renal cell carcinoma under the brand name Proleukin. In vitro studies using human cell lines demonstrated the immunoregulatory properties of aldesleukin, including activation and proliferation of cytotoxic T lymphocytes and natural killer and lymphokine-activated killer cells (Proleukin Prescribing Information). It was also noted that low-dose IL-2 enhanced regulatory T (Treg) cell function in autoinflammatory conditions, while neuroinflammation represents a key pathogenic feature of ALS196,197.
The phase II IMODALS trial (NCT02059759) was conducted to evaluate low-dose aldesleukin in 36 patients with ALS. The trial met its primary end point, with both doses of aldesleukin resulting in a significant, dose-dependent, increase in Treg cell numbers, compared with placebo. This increase was accompanied by enhancement of Treg cell suppressive function, suggesting an improved ability to control the inflammatory mechanisms that contribute to neuronal injury in ALS198. There was also a significant, dose-dependent drop in CCL2 (MCP-1) levels, an inflammatory biomarker which is frequently elevated in patients with ALS197. The peripheral blood mononuclear cell (PBMC) transcriptome was also analysed at different time points198. Participants were classified into low, moderate and high responders based on the magnitude of the upregulation in the Treg cell count. Substantial baseline differences were observed between the PBMC transcriptomes of participants, with the least responsive patients showing a more inflammatory-prone phenotype at baseline. Low-dose IL-2 caused a reduction in the pro-inflammatory transcriptome signature in both high-responder and low-responder groups. It was also observed that pretreatment expression levels of two genes correlated with the magnitude of drug responsiveness, allowing the development of a two-biomarker-based regression model to predict the Treg cell response to low-dose IL-2. These findings and the application of this embedded experimental medicine approach could be particularly relevant for future application of precision medicine approaches in ALS clinical trial design198.
The IMODALS trial data supported the evaluation of aldesleukin in the randomized phase II/III MIROCALS trial (NCT03039673) to further evaluate the safety and effectiveness of the most favourable dose (2 million IU/day subcutaneous injection for five consecutive days every 4 weeks) in a larger trial of 220 participants over a longer period of 18 months. The outcome of this trial is currently under analysis.
Ravulizumab
Several approaches targeting the complement cascade are currently in clinical trials for the treatment of ALS, and it may be informative to consider the recent failure of ravulizumab in the CHAMPION-ALS trial. Complement is an essential effector component of both innate and adaptive humoral immune responses activated by the alternative, lectin and classic pathways. It is well known that complement can be activated in response to CNS inflammation, enhancing tissue injury, and complement deposition has been identified in CNS tissue from patients with ALS199. A potential role for complement in both central motor neuron loss and peripheral neuromuscular junction injury200 has stimulated interest in targeting the complement cascade in ALS clinical trials.
Ravulizumab is a humanized anti-C5 antibody developed by Alexion and AstraZeneca for the treatment of ALS and other rare diseases. It is a terminal complement inhibitor that specifically binds to the C5 complement protein with high affinity, thereby inhibiting its cleavage to C5a and C5b, and preventing the generation of the terminal membrane attack complex C5b9 and production of the pro-inflammatory C5a fragment. Ravulizumab inhibits terminal complement-mediated intravascular haemolysis and has been approved in the USA for the treatment of paroxysmal nocturnal haemoglobinuria.
The phase III CHAMPION-ALS trial (NCT04248465) was conducted to evaluate the efficacy and safety of ravulizumab for the treatment of ALS. AstraZeneca discontinued the CHAMPION-ALS trial in August 2021 due to lack of efficacy, following a prespecified interim analysis. This outcome suggests that peripheral targeting of the complement terminal pathway is not beneficial in ALS. More recently, a phase II/III trial of another C5 inhibitor zilucoplan (NCT04297683, NCT04436497) was also discontinued for futility. However, further approaches target C3 convertases and utilize smaller peptidic inhibitors, which may have better access to the CNS and the potential to target an earlier phase in the complement activation pathway.
Verdiperstat
Verdiperstat is an orally administered small molecule inhibitor of myeloperoxidase (MPO) developed by Biohaven Pharmaceuticals. MPO increases oxidative stress and inflammation in the brain, which play roles in multiple neurodegenerative diseases. Inhibition of MPO could potentially alleviate the pathological mechanisms associated with neuroinflammation. A placebo-controlled phase II/III trial (NCT04297683, NCT04436510) under the HEALEY ALS Platform Trial was conducted in 167 patients to evaluate the safety and efficacy of verdiperstat. In September 2022, Biohaven reported the failure of this trial. Specifically, the drug did not achieve statistically significant differences on the prespecified primary outcome, disease progression, as measured by ALSFRS-R, survival and other key secondary measures. The safety profile of verdiperstat appeared to be similar to that found in previous clinical trials. More details of the study results are expected to be provided in the near future. The failure of verdiperstat highlights the complexity of patholophysiological mechanisms involved in ALS. It is possible that therapies targeting multiple pathways contributing to neuronal injury may have a higher probability of success.
Drugs in preclinical development
A search using the AdisInsight Springer database (August 2022) identified 91 preclinical ALS drug development programmes. Of these, 22 defined a lead therapeutic candidate, listed ALS as the lead target indication, had had at least one significant update within the previous 12 months, and were not discontinued programmes (Table 3). Moreover, all drug development programmes except one (a riluzole microencapsulated formulation) were focused on the development of new therapies. Of the preclinical development programmes, 11 were for genetic therapies, which represent an exciting new therapeutic approach for subgroups of patients with ALS.
Preclinical drug development programmes are highlighted in Table 3 and themes emerge which indicate the potential future direction of ALS clinical trials. The best represented category is genetic therapy approaches targeting specific misfolded proteins (for example, TDP-43, FUS and SOD1). Neuroinflammation is less well represented preclinically, with three approaches documented (Alpha-5, COYA-201 and LP143). Several novel mechanisms are represented for the first time. Cortical hyperexcitability is an emerging mechanism, and QRA-244, which targets voltage-gated potassium channel Kv7 family members, represents a potential approach to address this pathophysiological mechanism. Preclinical and clinical studies highlighting the potential of inhibiting retrovirally-derived transposable elements from causing genomic instability has led to the strategy of repurposing existing CNS-penetrant reverse transcriptase inhibitors such as censavudine and Triumeq. HDAC-6 inhibitors for correction of axonal transport defects are noteworthy, as this mechanism contributes in multiple neurodegenerative diseases, although a lead compound has not yet been identified. Three representative preclinical drug development programmes are highlighted in the following case studies.
QRA-244
QRA-244 is a Kv7 ion channel opener developed by the QurAlis Corporation for the treatment of motor neuron hyperexcitability-induced disease progression in ALS. QurAlis utilized a live-cell screening strategy to investigate abnormal electrophysiological properties and reveal targets that modulate the intrinsic hyperexcitability of ALS motor neurons reprogrammed from fibroblasts201. This unbiased screen identified Kv7 as a strongly upregulated drug target. Kv7 as a therapeutic target was further supported by a recent clinical study of a Kv7 channel opener that decreased motor neuron excitability202. QurAlis conducted a precision medicine programme to develop QRA-244, a selective Kv7 ion channel opener, for the treatment of ALS. Preclinical development of QRA-244 is underway in the USA.
M102
M102 is an orally bioavailable small molecule under development by Aclipse Therapeutics in collaboration with the University of Sheffield, UK. M102 is the S-enantiomer of the marketed R-enantiomer Apokyn (R-apomorphine), a dopamine agonist used in the management of advanced Parkinson disease. The S-enantiomer is a very weak dopamine antagonist203,204, and thus M102 does not show the adverse effects associated with dopamine agonism that occur following exposure to R-apomorphine.
M102 was identified as a lead therapeutic candidate by the University of Sheffield in an effort to identify CNS-penetrant activators of the Nrf2 pathway205,206. M102 is unique as a pro-electrophilic drug which only activates the Nrf2 pathway in cells under oxidative stress, thus reducing potential off-target effects207,208. Importantly, M102 at similar doses is also capable of activating the heat shock factor 1 (HSF1) signalling pathway that upregulates molecular chaperones to improve proteotoxic stress209.
Preclinical pharmacological data demonstrated that M102 significantly slows disease progression in SOD1G93A mice205 and reversed disease indices in the TDP-43Q331K mouse model210. Unlike currently available ALS drugs, Nrf2 and HSF1 activation target multiple pathophysiological mechanisms contributing to motor neuron injury. M102 has received orphan drug designation from the FDA and EMA and is expected to advance to phase I/II clinical trials in the near future.
COYA-201
COYA-201 is a Treg cell-derived exosome preparation under development by Coya Therapeutics for the treatment of ALS. Neuroinflammation contributes to neurodegeneration, which is driven by an imbalance of pro-inflammatory effector T cells and Treg cells. COYA-201 was developed using the immunosuppressive cell exosome (iscEXO) platform, derived from Treg cells and M2 macrophages, both of which are prominent anti-inflammatory and neuroprotective cells211.
Exosomes are membrane-derived small vesicles, rich in proteins and RNAs and which can avoid potential cell-based issues such as immune rejection212 and polarization to a pro-inflammatory cell type213. Compared to MSC-derived exosomes, Treg cell-derived exosomes have much higher suppressive capacity and anti-inflammatory function213. The first-in-human trial of COYA-201 was expected in 2022.
Strategies to improve ALS R&D
Clinical trials in ALS have historically seen high failure rates for various reasons (Box 3). We highlight below mitigation strategies for these factors which may lead to significant enhancement of successful therapy development.
Improving preclinical studies
Target identification
Major recent advances have taken place that underpin a strong pipeline of candidates for neuroprotective therapy development. Our understanding of disease pathophysiology has increased, with greater knowledge of the genetic architecture of ALS and of the multiple overlapping mechanisms that contribute to motor neuron injury in the presence of specific genetic mutations.
This wealth of additional genetic and pathophysiological information means that there is no shortage of potential novel therapeutic targets. To date successful neuroprotective approaches for ALS are exclusively CNS-penetrant small molecules, which presents challenges for identifying druggable targets. Approaches that draw on the plethora of existing publicly available biological data could help to rapidly identify small molecule targets, a recent example being TargetDB214. Machine learning-based approaches could also be deployed to assist with target identification; for example, the use of knowledge graphs which link disparate types of data with relational inferences derived from natural language processing215. Such strategies have been used to identify COVID-19 therapies216 and are being deployed in ALS to identify new targets, validated using patient-derived cell models (BenevolentAI).
Additional therapeutic modalities are expanding the list of tractable targets, and the successful use of gene therapy (onasemnogene abeparvovec) and ASO (nusinersen) approaches for amelioration of motor neuron injury in spinal muscular atrophy provide realistic prospects for therapeutic advances in defined genetic subtypes of ALS. The limited options for large-molecule (antibody) therapeutics in neurology may be abrogated by new technologies that enable CNS transport of antibody therapeutic agents. This was recently demonstrated in mice and primates using a transferrin-targeting moiety engineered into the Fc portion of a BACE antibody217. This strategy is being applied in several neurodegenerative conditions including FTD (DNL53, Denali Therapeutics; NCT05262023).
Multiple mechanisms, as described above, can contribute to the ultimate demise of motor neurons. This raises the prospect that multiple targets may need to be addressed simultaneously to achieve significant slowing of disease progression. This can be achieved with polypharmacology (for example, AMX0035) and indeed the combination of riluzole and edaravone has become the standard of care for ALS in some countries. A second approach may be to identify single upstream targets that can in turn address multiple downstream mechanisms (for example, M102 which targets the transcription factors Nrf2 and HSF1).
Drug repurposing is often proposed as a potential shortcut to identifying novel therapies and has shown some success for neurological disorders, including dimethyl fumarate for multiple sclerosis218. However, greater consideration of the drawbacks and potential limitations of repurposing is needed218. The most fruitful approach may well be ‘on-target’ repurposing where the original target of the therapy is the intended target for the new indication. However, the need for CNS penetration and whether the risk–benefit and dosing level is appropriate in the new indication are often limiting issues.
Target validation
The use of patient-derived cell models has added an extra dimension to enable early validation of therapeutic hypotheses and complements traditional preclinical in vitro and in vivo models. Fibroblasts donated by patients with ALS can now be reprogrammed into the CNS cells relevant for ALS (for example, motor neurons, astrocytes and microglia). iPSC-derived neurons and glia, where cells are reverted to an embryonic stage of development, have the potential to show early pathophysiological abnormalities. However, direct reprogramming via induced neural progenitor cells has the advantages of generating non-clonal cell lines which retain the features of ageing, of crucial importance in the study of age-related neurodegenerative disorders124,219. These human CNS-relevant cell models are being used to define new therapeutic targets and for the screening of libraries to identify novel neuroprotective therapies220.
Non-mammalian model systems are also emerging as powerful tools to enable target validation. Drosophila melanogaster (fruitfly) and Danio rerio (zebrafish) are increasingly used as models to probe disease mechanisms and have the potential to expand the number of available genetic models and to rapidly validate mechanisms in ALS using genetic or pharmacological approaches221. For example, drosophila models carrying mutations in a range of ALS-related genes have demonstrated a role for dysregulation of RNA metabolism which has been subsequently validated in human biosamples222. Zebrafish carrying C9orf72 hexanucleotide repeats were shown to replicate key features of ALS/FTD223 and to complement mammalian models.
Preclinical screening cascades
It is now possible to develop preclinical screening cascades which take us far beyond unreliable readouts of survival in SOD1-transgenic mice. A combined approached can be used, with target-specific screening in vitro validated early in orthogonal patient-derived cell assays for selected compounds. SOD1-transgenic rodents can be used for early mechanistic pharmacokinetic–pharmacodynamic work, followed by studies focused on evaluating more global effects on motor system degeneration. Finally, validation in alternative model systems such as patient-derived in vitro models and more physiologically relevant mouse models should enhance confidence in clinical translatability. Such an approach should enable a step-change in our ability to predict therapeutic efficacy in the clinic (Fig. 3).
In terms of efficacy assessment and increasing confidence in translation from the preclinical to the clinical arena, an enhanced approach would take advantage of the latest developments in preclinical disease modelling in rodents and non-rodent in vivo systems, as well as patient-derived cellular models. A standard in vitro cascade is shown for a target-based approach, which is subsequently confirmed at an early stage in a relevant patient-derived phenotypic model. Such models can be used as orthogonal screens through a development programme to ensure that efficacy against a relevant disease phenotype is maintained. At later stages in vivo mammalian models can be used as purely mechanistic pharmacokinetic/pharmacodynamic systems to ensure that therapeutic approaches have the required drug metabolism and pharmacokinetics, potency and specificity in vivo. Subsequently, confidence in clinical translation can be obtained in either more extensive screening in patient-derived model systems or complex disease models. Ideally several approaches should be pursued. These complex models with behavioural readouts of motor function would incorporate ‘translational’ biomarkers that have the potential to predict likely clinical benefit, such as measurements of compound muscle action potential (CMAP) and neurofilament light. ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; iPSC, induced pluripotent stem cell; MN, motor neuron; PD, pharmacodynamic; PK, pharmacokinetic.
Advances in ALS clinical trials
A consensus has emerged that optimizing the use of resources, widening eligibility criteria for trials and minimizing exposure of participants facing a life-threatening disease to ineffective treatments and to placebo are of crucial importance. There has been a recent concerted effort by the international ALS community to address the limitations now recognized in traditional ALS clinical trials methodology. The revised Airlie House consensus guidelines were developed in 2019 to accelerate translational impact for ALS and addressed nine areas of need within ALS research ranging from preclinical research through to trial design and statistical methods, as well as advocating the incorporation of biomarkers and the development of home-based outcome measures for clinical trials224,225. There have been recent promising innovations in trial design, patient selection and randomization, and biomarker development.
Patient stratification
Patient stratification can be based on clinical parameters, genetics, disease stage and the rate of disease progression. Advances in the understanding of the genetic landscape of ALS and overlapping disease pathophysiological mechanisms have accelerated in recent years, as outlined above. Recent exciting progress in precision medicine, exemplified by genetically targeted therapies, is poised to change the natural history of ALS for some subgroups of patients. It is apparent that a precision medicine approach is required to optimize the rapid development of effective neuroprotective therapies for patients with ALS.
A case is emerging for routine genetic subclassification of ALS, regardless of whether a family history is present35,226. We now have improved systems for stratifying patients by disease progression rate224,227 ensuring that patients with fast and slow disease progression can be evenly balanced in trial groups, and simple disease-staging systems228,229 may also add value in future clinical trials. Using data from across 14 European cohorts, an eight-factor validated multivariate model was developed for predicting survival in individual patients with ALS230,231. The use of such a model is predicted to allow up to 80% of patients with ALS to take part in clinical trials, while minimizing the impact of variable rates of disease progression. Thus, prognostic heterogeneity in ALS can now be quantified232. Risk profiles can be used to improve randomization, explore risk-based outcomes and increase statistical power as a covariate in the final analysis of trial data. Stratifying predicted survival into tertiles may be the optimal approach233. In addition, stratifying patients with ALS based on baseline NFL levels is emerging as a useful approach which was recently deployed in the phase III tofersen trial189.
Innovations in trial design and outcome measures
The traditional approach has been the randomized controlled trial in which the effects of the drug candidate are compared to that of placebo, and with survival or ALSFRS-R score as the commonly used primary outcome measures. This approach is costly, time-consuming and inefficient. Many preclinical and early phase I/II trials in ALS have yielded promising results that were not replicated in phase III trials. Substantial consideration has recently been devoted to the refinement of clinical trials in ALS. The ALSFRS-R has been used extensively as an outcome measure, but violates Rasch model expectations and is limited by the multidimensionality that arises from the summation of multiple subscales, which prevents direct comparison of patients with identical total scores233. A new self-reported ALS disability scale has been developed, with improved responsiveness. It is a 28-item Rasch-built overall ALS Disability Scale, with each item scored as 0, 1 or 2, and which demonstrates excellent test–retest reliability227. Other novel outcome measures are in development including modifications to the ALSFRS-R.
Multi-arm, multistage platform trials (MAMS) that incorporate biomarkers of target engagement/therapeutic efficacy are now poised to accelerate drug discovery and increase trial participation. In a MAMS trial with a sequential adaptive design, the sample size is not fixed in advance and the emerging trial data are sequentially analysed with in-built, pre-determined futility and superiority analyses. This enables ineffective treatment arms to be discontinued and studies with a positive signal to seamlessly graduate to the next trial phase. Platform trial designs (for example, Healey ALS Platform Trial and MND-SMART) have emerged to allow more rapid assessments and inclusivity for patients, and more efficient deployment of a pooled placebo arm233,234.
A further European platform trial, TRICALS, will start imminently as several of the initial portfolio of trials are recruiting patients in multiple European centres.
A master protocol allowing the simultaneous evaluation of multiple compounds and efficient use of a common placebo group has been successfully used in other disease areas, most notably oncology235. However, it is noteworthy that in these conditions robust biomarkers of disease burden are available. Such biomarkers have not yet been established in ALS, although promising candidates are now emerging; for example, neurofilaments and specific protein biomarkers in genetic therapy trials.
These recent proposals to modify the traditional design of ALS clinical trials have the potential to reduce the sample size required and the placebo exposure time for trial participants, as well as the cost, burden for patients and the trial duration232. Event-driven trial designs may ameliorate the consequences of inaccurate baseline assumptions for trial design and the resulting unknown trial duration can be mitigated by deployment of planned interim analyses.
Improved patient-reported outcome measures, including home assessments, are predicted to improve the reliability and sensitivity of trial end points. Evidence is emerging that home assessments may be a viable strategy for measuring the disease trajectory in ALS clinical trials232,236,237. This strategy has been accelerated by the shift towards telemedicine for the provision of clinical care to patients with ALS during the COVID-19 pandemic.
Dialogue with regulatory bodies will be essential in bringing to fruition these proposed innovations for ALS clinical trials. It is noteworthy that the FDA has already published guidance for adaptive platform master protocol trial designs238 and the EMA has established a plan to carefully explore this issue.
Biomarkers
Robust biomarkers are needed in ALS to measure disease progression and provide prognostic information, to identify potential therapeutic targets, to stratify patients prior to clinical trial entry, and as measures of target engagement and therapeutic efficacy in clinical trials. A large volume of biomarker research has been undertaken, but only a few robust, validated biomarkers have so far emerged (Box 4). Recent reviews have comprehensively described the current state-of-the-art for clinical, biochemical, imaging and electrophysiological biomarkers225,239.
Outlook
Significant recent advances have been made in understanding the genetic architecture and pathophysiology of ALS. A strategy has emerged where more accurate subclassification of patients can be made based on identification of risk genes beyond the traditional boundaries of sALS and fALS. This expanded genetic understanding has also enabled the validation of several pathophysiological biological pathways for therapeutic targeting and, alongside the expansion of therapeutic modalities, there is now no shortage of therapeutic hypotheses to explore preclinically.
In the preclinical space, the number, variety and clinical relevance of available model systems to enable more rigorous preclinical screening have expanded. This enables validation of therapeutic hypotheses and targets across models and reduces the risk of failure due to lack of efficacy.
In the clinical space, recent innovations in trial design will enhance outcome measures, patient selection and randomization, minimize the impact of clinical variability and increase statistical power. Platform trials and patient-reported outcomes have the potential to improve the pace of validation of therapeutic agents. This is particularly the case if combined with surrogate biomarkers of disease burden, which are now emerging.
The development of biomarkers of target engagement and efficacy to bridge the gap from preclinical to clinical testing is a key future need, as is the application of sound pharmacological principles in therapy development. Several biomarkers (biochemical, physiological, imaging) that can be applied across the translational pathway show great promise.
Of added importance is the continued impact of patient advocacy groups encouraging and financially supporting researchers and clinicians. This strong and visible advocacy has been a factor in elevating the priority of regulatory agencies to address the high unmet need of individuals facing ALS. For example, regulatory agencies appear to be more receptive to considering adequate and well-controlled phase II data as part of a streamlined approval process, as was recently shown with AMX0035. Having the attention and guidance of regulatory agencies continues to be an important factor in promoting rapid access to new therapies for patients with ALS.
In summary, there is great potential for developing improved neuroprotective treatments for ALS in the near future. We advocate for academia–industry partnerships which accelerate the pace of rigorous testing of therapeutic hypotheses, incorporating the latest advances in preclinical screening, biomarker discovery and trial design. The unmet need of patients with ALS is clear and a range of tools are now poised for effective translation.
References
Longinetti, E. & Fang, F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr. Opin. Neurol. 32, 771–776 (2019).
Brown, C. A., Lally, C., Kupelian, V. & Flanders, W. D. Estimated prevalence and incidence of amyotrophic lateral sclerosis and SOD1 and C9orf72 genetic variants. Neuroepidemiology 55, 342–353 (2021).
Ryan, M., Heverin, M., McLaughlin, R. L. & Hardiman, O. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 76, 1367–1374 (2019).
GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 1083–1097 (2018).
Masrori, P. & Van Damme, P. Amyotrophic lateral sclerosis: a clinical review. Eur. J. Neurol. 27, 1918–1929 (2020).
Elamin, M. et al. Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology 80, 1590–1597 (2013).
Phukan, J. et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J. Neurol. Neurosurg. Psychiatry 83, 102–108 (2012).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Ling, S. C., Polymenidou, M. & Cleveland, D. W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013).
Tan, R. H., Ke, Y. D., Ittner, L. M. & Halliday, G. M. ALS/FTLD: experimental models and reality. Acta Neuropathol. 133, 177–196 (2017).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 3, 17085 (2017).
Mackenzie, I. R. et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427–434 (2007). This paper highlights the important molecular features of SOD1-ALS, which is not a TDP-43 proteinopathy and may partially explain why treatments evaluated in SOD1-transgenic mouse models have not generally translated well to the clinic.
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
Kato, S. et al. Copper chaperone for superoxide dismutase co-aggregates with superoxide dismutase 1 (SOD1) in neuronal Lewy body-like hyaline inclusions: an immunohistochemical study on familial amyotrophic lateral sclerosis with SOD1 gene mutation. Acta Neuropathol. 102, 233–238 (2001).
Ramos-Campoy, O. et al. Systematic screening of ubiquitin/p62 aggregates in cerebellar cortex expands the neuropathological phenotype of the C9orf72 expansion mutation. J. Neuropathol. Exp. Neurol. 77, 703–709 (2018).
Turner, M. R. & Talbot, K. Mimics and chameleons in motor neurone disease. Pract. Neurol. 13, 153–164 (2013).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1, 293–299 (2000).
de Carvalho, M. et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 119, 497–503 (2008).
Shefner, J. M. et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 131, 1975–1978 (2020).
Petrov, D., Mansfield, C., Moussy, A. & Hermine, O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 9, 68 (2017).
Lacomblez, L. et al. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology 47, S242–S250 (1996).
Lacomblez, L., Bensimon, G., Leigh, P. N., Guillet, P. & Meininger, V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347, 1425–1431 (1996).
Bensimon, G., Lacomblez, L. & Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 330, 585–591 (1994).
Kretschmer, B. D., Kratzer, U. & Schmidt, W. J. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn Schmiedebergs Arch. Pharmacol. 358, 181–190 (1998).
Wang, S. J., Wang, K. Y. & Wang, W. C. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes). Neuroscience 125, 191–201 (2004).
Andrews, J. A. et al. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 509–518 (2020).
The Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert. Opin. Pharmacother. 18, 735–738 (2017).
Bourke, S. C. et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 5, 140–147 (2006).
Lechtzin, N. et al. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph. Lateral Scler. 8, 185–188 (2007).
Rosen, D. R. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59-62 (1993).
Ranganathan, R. et al. Multifaceted genes in amyotrophic lateral sclerosis-frontotemporal dementia. Front. Neurosci. 14, 684 (2020).
van Rheenen, W. et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 53, 1636–1648 (2021). This paper demonstrates that the genetic architecture of ALS is largely based on rare variants and that powerful new genetic insights can arise through the analysis of large numbers of cases available through the Project MinE consortium.
van Rheenen, W. et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048 (2016).
Shepheard, S. R. et al. Value of systematic genetic screening of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 92, 510–518 (2021). This prospective study demonstrates the importance of offering genetic screening to all patients with ALS irrespective of a family history of the disease. Clinically reportable genetic changes can frequently be found in patients with apparently sporadic disease.
Zhang, S. et al. Genome-wide identification of the genetic basis of amyotrophic lateral sclerosis. Neuron 110, 992–1008.e11 (2022). This paper describes the use of machine learning to identify ALS risk genes by integrating GWAS and epigenetic data, which led to a fivefold increase in recovered heritability.
Al-Chalabi, A. et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 13, 1108–1113 (2014). This paper highlights the importance of gene–environment interactions in the multistep process required for ALS to manifest clinically.
Julian, T. H. et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain 145, 832–842 (2021).
Julian, T. H. et al. Physical exercise is a risk factor for amyotrophic lateral sclerosis: convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine 68, 103397 (2021). This study provides evidence that Mendelian randomization can overcome some of the confounding factors that have been identified in previous studies attempting to identify environmental risk factors for ALS.
Bandres-Ciga, S. et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann. Neurol. 85, 470–481 (2019).
McKay, K. A. et al. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol. Scand. 143, 39–50 (2021).
Ingre, C., Roos, P. M., Piehl, F., Kamel, F. & Fang, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 7, 181–193 (2015).
Lacorte, E. et al. Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: a systematic review. Neurosci. Biobehav. Rev. 66, 61–79 (2016).
Armon, C. Smoking may be considered an established risk factor for sporadic ALS. Neurology 73, 1693–1698 (2009).
Barber, S. C. & Shaw, P. J. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free. Radic. Biol. Med. 48, 629–641 (2010).
Ferraiuolo, L., Kirby, J., Grierson, A. J., Sendtner, M. & Shaw, P. J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 616–630 (2011).
D’Amico, E., Factor-Litvak, P., Santella, R. M. & Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 65, 509–527 (2013).
Mitsumoto, H. et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph. Lateral Scler. 9, 177–183 (2008).
Kim, K. Glutathione in the nervous system as a potential therapeutic target to control the development and progression of amyotrophic lateral sclerosis. Antioxidants 10, 1011 (2021).
Cuadrado, A. et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18, 295–317 (2019).
Jimenez-Villegas, J. et al. NRF2 as a therapeutic opportunity to impact in the molecular roadmap of ALS. Free Radic. Biol. Med. 173, 125–141 (2021).
Cohen, T. J. et al. An acetylation switch controls TDP-43 function and aggregation propensity. Nat. Commun. 6, 5845 (2015).
Colombrita, C. et al. TDP-43 is recruited to stress granules in conditions of oxidative insult. J. Neurochem. 111, 1051–1061 (2009).
Goh, C. W. et al. Chronic oxidative stress promotes GADD34-mediated phosphorylation of the TAR DNA-binding protein TDP-43, a modification linked to neurodegeneration. J. Biol. Chem. 293, 163–176 (2018).
Zuo, X. et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 28, 132–142 (2021).
Kazama, M. et al. Astrocytes release glutamate via cystine/glutamate antiporter upregulated in response to increased oxidative stress related to sporadic amyotrophic lateral sclerosis. Neuropathology 40, 587–598 (2020).
Deora, V. et al. The microglial NLRP3 inflammasome is activated by amyotrophic lateral sclerosis proteins. Glia 68, 407–421 (2020).
Benatar, M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 26, 1–13 (2007).
King, A. E., Woodhouse, A., Kirkcaldie, M. T. & Vickers, J. C. Excitotoxicity in ALS: overstimulation, or overreaction? Exp. Neurol. 275, 162–171 (2016).
Van Damme, P., Dewil, M., Robberecht, W. & Van Den Bosch, L. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener. Dis. 2, 147–159 (2005).
Lewerenz, J. et al. The cystine/glutamate antiporter system xc− in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 18, 522–555 (2013).
Williams, T. L., Day, N. C., Ince, P. G., Kamboj, R. K. & Shaw, P. J. Calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 42, 200–207 (1997).
Ince, P. et al. Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropathol. Appl. Neurobiol. 19, 291–299 (1993).
Bruijn, L. I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Lauriat, T. L. & McInnes, L. A. EAAT2 regulation and splicing: relevance to psychiatric and neurological disorders. Mol. Psychiatry 12, 1065–1078 (2007).
Pajarillo, E., Rizor, A., Lee, J., Aschner, M. & Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology 161, 107559 (2019).
Selvaraj, B. T. et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca2+-permeable AMPA receptor-mediated excitotoxicity. Nat. Commun. 9, 347 (2018).
Dafinca, R. et al. Impairment of mitochondrial calcium buffering links mutations in C9ORF72 and TARDBP in iPS-derived motor neurons from patients with ALS/FTD. Stem Cell Rep. 14, 892–908 (2020).
Vucic, S., Pavey, N., Haidar, M., Turner, B. J. & Kiernan, M. C. Cortical hyperexcitability: diagnostic and pathogenic biomarker of ALS. Neurosci. Lett. 759, 136039 (2021).
Lamanauskas, N. & Nistri, A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 27, 2501–2514 (2008).
Dyer, A. M. & Smith, A. Riluzole 5 mg/mL oral suspension: for optimized drug delivery in amyotrophic lateral sclerosis. Drug. Des. Devel Ther. 11, 59–64 (2017).
Battaglia, G. & Bruno, V. Metabotropic glutamate receptor involvement in the pathophysiology of amyotrophic lateral sclerosis: new potential drug targets for therapeutic applications. Curr. Opin. Pharmacol. 38, 65–71 (2018).
Cabral-Costa, J. V. & Kowaltowski, A. J. Neurological disorders and mitochondria. Mol. Asp. Med. 71, 100826 (2020).
Jhanji, R., Behl, T., Sehgal, A. & Bungau, S. Mitochondrial dysfunction and traffic jams in amyotrophic lateral sclerosis. Mitochondrion 58, 102–110 (2021).
Debska-Vielhaber, G. et al. Impairment of mitochondrial oxidative phosphorylation in skin fibroblasts of SALS and FALS patients is rescued by in vitro treatment with ROS scavengers. Exp. Neurol. 339, 113620 (2021).
Bernard-Marissal, N., Chrast, R. & Schneider, B. L. Endoplasmic reticulum and mitochondria in diseases of motor and sensory neurons: a broken relationship? Cell Death Dis. 9, 333 (2018).
Jankovic, M. et al. Current concepts on genetic aspects of mitochondrial dysfunction in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 22, 9832 (2021).
Choi, S. Y. et al. C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat. Neurosci. 22, 851–862 (2019).
Wang, T. et al. C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab. 33, 531–546.e9 (2021).
Izumikawa, K. et al. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci. Rep. 7, 7709 (2017).
Cudkowicz, M. E. et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 12, 1059–1067 (2013).
Pastula, D. M., Moore, D. H. & Bedlack, R. S. Creatine for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 12, CD005225 (2012).
Kaufmann, P. et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 66, 235–244 (2009).
Lenglet, T. et al. A phase II-III trial of olesoxime in subjects with amyotrophic lateral sclerosis. Eur. J. Neurol. 21, 529–536 (2014).
Elia, A. E. et al. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 23, 45–52 (2016).
Paganoni, S. et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve 63, 31–39 (2021).
Paganoni, S. et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383, 919–930 (2020). This phase II clinical trial provided some evidence of benefit on decline in the ALSFRS-R and used a combination of agents to tackle several pathophysiological disease mechanisms. This drug combination has been licensed for use in the USA and Canada.
Choi, S. Y. et al. Prevention of mitochondrial impairment by inhibition of protein phosphatase 1 activity in amyotrophic lateral sclerosis. Cell Death Dis. 11, 888 (2020).
Sassani, M. et al. Magnetic resonance spectroscopy reveals mitochondrial dysfunction in amyotrophic lateral sclerosis. Brain 143, 3603–3618 (2020).
Webster, C. P., Smith, E. F., Shaw, P. J. & De Vos, K. J. Protein homeostasis in amyotrophic lateral sclerosis: therapeutic opportunities? Front. Mol. Neurosci. 10, 123 (2017).
Ramesh, N. & Pandey, U. B. Autophagy dysregulation in ALS: when protein aggregates get out of hand. Front. Mol. Neurosci. 10, 263 (2017).
Montibeller, L., Tan, L. Y., Kim, J. K., Paul, P. & de Belleroche, J. Tissue-selective regulation of protein homeostasis and unfolded protein response signalling in sporadic ALS. J. Cell Mol. Med. 24, 6055–6069 (2020).
Oakes, J. A., Davies, M. C. & Collins, M. O. TBK1: a new player in ALS linking autophagy and neuroinflammation. Mol. Brain 10, 5 (2017).
Webster, C. P. et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 35, 1656–1676 (2016).
Foster, A. D. & Rea, S. L. The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Neural Regen. Res. 15, 2186–2194 (2020).
Ying, H. & Yue, B. Y. Optineurin: the autophagy connection. Exp. Eye Res. 144, 73–80 (2016).
Rothenberg, C. et al. Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy. Hum. Mol. Genet. 19, 3219–3232 (2010).
Wu, J. J. et al. ALS/FTD mutations in UBQLN2 impede autophagy by reducing autophagosome acidification through loss of function. Proc. Natl Acad. Sci. USA 117, 15230–15241 (2020).
Nguyen, D. K. H., Thombre, R. & Wang, J. Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci. Lett. 697, 34–48 (2019).
Brockington, A. et al. Unravelling the enigma of selective vulnerability in neurodegeneration: motor neurons resistant to degeneration in ALS show distinct gene expression characteristics and decreased susceptibility to excitotoxicity. Acta Neuropathol. 125, 95–109 (2013).
Yerbury, J. J., Farrawell, N. E. & McAlary, L. Proteome homeostasis dysfunction: a unifying principle in ALS pathogenesis. Trends Neurosci. 43, 274–284 (2020).
Suk, T. R. & Rousseaux, M. W. C. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol. Neurodegener. 15, 45 (2020).
Mitra, J. et al. Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc. Natl Acad. Sci. USA 116, 4696–4705 (2019).
Nagano, S. et al. TDP-43 transports ribosomal protein mRNA to regulate axonal local translation in neuronal axons. Acta Neuropathol. 140, 695–713 (2020).
Brown, A. L. e. a. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 603, 131–137 (2022). This paper provides new insights into the consequences of loss of the normal nuclear function of TDP-43 that occurs as a result of nuclear depletion during the process of motor neuron injury and TDP-43 proteinopathy.
Dyer, M. S. et al. Mislocalisation of TDP-43 to the cytoplasm causes cortical hyperexcitability and reduced excitatory neurotransmission in the motor cortex. J. Neurochem. 157, 1300–1315 (2021).
Boeynaems, S. et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 65, 1044–1055.e5 (2017).
Ratti, A. et al. Chronic stress induces formation of stress granules and pathological TDP-43 aggregates in human ALS fibroblasts and iPSC-motoneurons. Neurobiol. Dis. 145, 105051 (2020).
Baradaran-Heravi, Y., Van Broeckhoven, C. & van der Zee, J. Stress granule mediated protein aggregation and underlying gene defects in the FTD-ALS spectrum. Neurobiol. Dis. 134, 104639 (2020).
Nahm, M. et al. ANXA11 mutations in ALS cause dysregulation of calcium homeostasis and stress granule dynamics. Sci. Transl. Med. 12, eaax3993 (2020).
Gwon, Y. et al. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 372, eabf6548 (2021).
Buratti, E. Targeting TDP-43 proteinopathy with drugs and drug-like small molecules. Br. J. Pharmacol. 178, 1298–1315 (2021).
Kalmar, B. & Greensmith, L. Cellular chaperones as therapeutic targets in ALS to restore protein homeostasis and improve cellular function. Front. Mol. Neurosci. 10, 251 (2017).
GlobalData Healthcare. Orphazyme’s arimoclomol fails to show efficacy in a specific ALS population. Clinical Trials Arena https://www.clinicaltrialsarena.com/comment/orphazyme-arimoclomol-als/ (2021).
Wang, I. F., Tsai, K. J. & Shen, C. K. Autophagy activation ameliorates neuronal pathogenesis of FTLD-U mice: a new light for treatment of TARDBP/TDP-43 proteinopathies. Autophagy 9, 239–240 (2013).
Li, Y. et al. Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience 298, 12–25 (2015).
Barmada, S. J. et al. Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nat. Chem. Biol. 10, 677–685 (2014).
Fang, M. Y. et al. Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron 103, 802–819.e11 (2019).
Dubinski, A. & Vande Velde, C. Altered stress granule disassembly: links to neurodegenerative disease? Trends Neurosci. 44, 765–766 (2021).
Appel, S. H., Beers, D. R. & Zhao, W. Amyotrophic lateral sclerosis is a systemic disease: peripheral contributions to inflammation-mediated neurodegeneration. Curr. Opin. Neurol. 34, 765–772 (2021).
Vu, L. et al. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J. Neurol. Neurosurg. Psychiatry 91, 350–358 (2020).
Westergard, T. & Rothstein, J. D. Astrocyte diversity: current insights and future directions. Neurochem. Res. 45, 1298–1305 (2020).
Yamanaka, K. & Komine, O. The multi-dimensional roles of astrocytes in ALS. Neurosci. Res. 126, 31–38 (2018).
Meyer, K. et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl Acad. Sci. USA 111, 829–832 (2014). This paper demonstrates the advantages of direct reprogramming of ALS patient fibroblasts, via induced neural progenitor cells, into astrocytes, which are toxic to motor neurons in coculture and are non-clonal and retain the epigenetic features of ageing.
Ferraiuolo, L. et al. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 134, 2627–2641 (2011).
Butovsky, O. & Weiner, H. L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 (2018).
Cassina, P., Miquel, E., Martinez-Palma, L. & Cassina, A. Glial metabolic reprogramming in amyotrophic lateral sclerosis. Neuroimmunomodulation 28, 204–212 (2021).
Haukedal, H. & Freude, K. Implications of microglia in amyotrophic lateral sclerosis and frontotemporal dementia. J. Mol. Biol. 431, 1818–1829 (2019).
Liao, B., Zhao, W., Beers, D. R., Henkel, J. S. & Appel, S. H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 237, 147–152 (2012).
Du, Y. et al. Increased activation ability of monocytes from ALS patients. Exp. Neurol. 328, 113259 (2020).
Haidet-Phillips, A. M. et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 (2011).
Frakes, A. E. et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023 (2014).
Smethurst, P. et al. Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis. Brain 143, 430–440 (2020).
Svahn, A. J. et al. Nucleo-cytoplasmic transport of TDP-43 studied in real time: impaired microglia function leads to axonal spreading of TDP-43 in degenerating motor neurons. Acta Neuropathol. 136, 445–459 (2018).
Jara, J. H. et al. MCP1-CCR2 and neuroinflammation in the ALS motor cortex with TDP-43 pathology. J. Neuroinflammation 16, 196 (2019).
Yu, C. H. et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183, 636–649.e18 (2020).
Lee, J. D. & Woodruff, T. M. TDP-43 puts the STING in ALS. Trends Neurosci. 44, 81–82 (2021).
Morello, G., Spampinato, A. G. & Cavallaro, S. Neuroinflammation and ALS: transcriptomic insights into molecular disease mechanisms and therapeutic targets. Mediators Inflamm. 2017, 7070469 (2017).
Pang, W. & Hu, F. Cellular and physiological functions of C9ORF72 and implications for ALS/FTD. J. Neurochem. 157, 334–350 (2021).
McCauley, M. E. et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature 585, 96–101 (2020).
Werry, E. L. et al. Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. Int. J. Mol. Sci. 20, 3161 (2019).
Kok, J. R., Palminha, N. M., Dos Santos Souza, C., El-Khamisy, S. F. & Ferraiuolo, L. DNA damage as a mechanism of neurodegeneration in ALS and a contributor to astrocyte toxicity. Cell Mol. Life Sci. 78, 5707–5729 (2021). This paper highlights the importance of DNA damage as a contributory pathophysiological mechanism in ALS.
Ferrante, R. J. et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074 (1997).
Bogdanov, M. et al. Increased oxidative damage to DNA in ALS patients. Free. Radic. Biol. Med. 29, 652–658 (2000).
Kim, B. W., Jeong, Y. E., Wong, M. & Martin, L. J. DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol. Commun. 8, 7 (2020).
Farg, M. A., Konopka, A., Soo, K. Y., Ito, D. & Atkin, J. D. The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum. Mol. Genet. 26, 2882–2896 (2017).
Lopez-Gonzalez, R. et al. Poly(GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron 92, 383–391 (2016).
Walker, C. et al. C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat. Neurosci. 20, 1225–1235 (2017).
Konopka, A. et al. Impaired NHEJ repair in amyotrophic lateral sclerosis is associated with TDP-43 mutations. Mol. Neurodegener. 15, 51 (2020).
Giannini, M. et al. TDP-43 mutations link amyotrophic lateral sclerosis with R-loop homeostasis and R loop-mediated DNA damage. PLoS Genet. 16, e1009260 (2020).
Wang, H. et al. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat. Commun. 9, 3683 (2018).
Higelin, J. et al. NEK1 loss-of-function mutation induces DNA damage accumulation in ALS patient-derived motoneurons. Stem Cell Res. 30, 150–162 (2018).
Butti, Z. & Patten, S. A. RNA dysregulation in amyotrophic lateral sclerosis. Front. Genet. 9, 712 (2018).
Lagier-Tourenne, C. et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 15, 1488–1497 (2012).
Lee, Y. B. et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 5, 1178–1186 (2013).
Cooper-Knock, J. et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 137, 2040–2051 (2014).
Donnelly, C. J. et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 (2013).
Lagier-Tourenne, C. et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl Acad. Sci. USA 110, E4530–E4539 (2013).
Hautbergue, G. M. et al. SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nat. Commun. 8, 16063 (2017).
Boeynaems, S. et al. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci. Rep. 6, 20877 (2016).
Freibaum, B. D. et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129–133 (2015).
Jovicic, A. et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci. 18, 1226–1229 (2015).
Zhang, K. et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56–61 (2015).
Zhang, K. et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell 173, 958–971.e17 (2018). This paper demonstrates the importance of dysregulation of stress granule dynamics and its interaction with nucleocytoplasmic transport as a pathophysiological mechanism contributing to motor neuron injury in models of ALS.
Anand, A. A. & Walter, P. Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response. FEBS J. 287, 239–245 (2020).
Chou, C. C. et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 21, 228–239 (2018).
Ederle, H. et al. Nuclear egress of TDP-43 and FUS occurs independently of exportin-1/CRM1. Sci. Rep. 8, 7084 (2018).
Lee, J. et al. LSM12-EPAC1 defines a neuroprotective pathway that sustains the nucleocytoplasmic RAN gradient. PLoS Biol. 18, e3001002 (2020).
De Vos, K. J., Grierson, A. J., Ackerley, S. & Miller, C. C. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151–173 (2008).
Nicolas, A. et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 97, 1268–1283.e6 (2018).
Liao, Y. C. et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164.e20 (2019).
Gibbs, K. L. et al. Inhibiting p38 MAPK alpha rescues axonal retrograde transport defects in a mouse model of ALS. Cell Death Dis. 9, 596 (2018).
Fellows, A. D., Rhymes, E. R., Gibbs, K. L., Greensmith, L. & Schiavo, G. IGF1R regulates retrograde axonal transport of signalling endosomes in motor neurons. EMBO Rep. 21, e49129 (2020).
Guo, W. et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 8, 861 (2017).
Sleigh, J. N. et al. Mice carrying ALS mutant TDP-43, but not mutant FUS, display in vivo defects in axonal transport of signaling endosomes. Cell Rep. 30, 3655–3662.e2 (2020).
Coleman, M. P. & Hoke, A. Programmed axon degeneration: from mouse to mechanism to medicine. Nat. Rev. Neurosci. 21, 183–196 (2020).
White, M. A. et al. Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss. Acta Neuropathol. Commun. 7, 166 (2019).
Becker, L. A. et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 544, 367–371 (2017). The demonstration that depletion of ataxin 2 ameliorates TDP-43 proteinopathy has led to genetic therapy approaches in preclinical models and a clinical trial. It may theoretically be beneficial for patients with sporadic ALS as well as those with ALS with known genetic causes.
Ray, F. Ionis Opening Phase 3 Trial of ION363, Antisense Therapy for FUS-ALS. ALS https://alsnewstoday.com/news/ionis-opening-phase-3-trial-ion363-antisense-therapy-for-fus-als/ (2021).
Cudkowicz, M. E. et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 65, 291–302 (2022).
Shefner, J. M. et al. A phase 2, double-blind, randomized, dose-ranging trial of reldesemtiv in patients with ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 287–299 (2021).
Berry, J. D. et al. NurOwn, phase 2, randomized, clinical trial in patients with ALS: safety, clinical, and biomarker results. Neurology 93, e2294–e2305 (2019).
Gothelf, Y., Abramov, N., Harel, A. & Offen, D. Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin. Transl. Med. 3, 21 (2014).
Petrou, P. et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 73, 337–344 (2016). This was one of the first cellular therapy approaches to be taken into a randomized clinical trial with evidence of safety and tolerability.
McCampbell, A. et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Invest. 128, 3558–3567 (2018).
Iannitti, T. et al. Translating SOD1 gene silencing toward the clinic: a highly efficacious, off-target-free, and biomarker-supported strategy for fALS. Mol. Ther. Nucleic Acids 12, 75–88 (2018).
Miller, T. et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 383, 109–119 (2020). A clinical trial of the ASO tofersen in 50 patients with SOD1-ALS showed evidence of target engagement. NFL levels were also lowered suggesting motor neuron protection, and exploratory clinical end points looked favourable in the high-dose arm.
Miller, T., Cudkowicz, M., on behalf of the VALOR Working Group. Results from the phase 3 VALOR study and its open-label extension: evaluating the clinical efficacy and safety of tofersen in adults with ALS and confirmed SOD1 mutation. Biogen https://biogen.gcs-web.com/static-files/b2154d4e-f69f-49d4-9a61-e834387293ea (2021).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Sonobe, Y. et al. Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol. Dis. 116, 155–165 (2018).
Van den Berg, L. H., et al. Results from the phase 1 trial and open-label extension evaluating BIIB078 in adults with C9orf72-ALS. Presented at ENCALS Meeting (2022).
Suaud, L. et al. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J. Biol. Chem. 286, 45083–45092 (2011).
Rodrigues, C. M., Sola, S., Sharpe, J. C., Moura, J. J. & Steer, C. J. Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome c release in isolated mitochondria. Biochemistry 42, 3070–3080 (2003).
Mullard, A. Amylyx’s ALS therapy secures FDA approval, as regulatory flexibility trumps underwhelming data. Nat. Rev. Drug Discov. 21, 786 (2022).
FDA Briefing Document NDA# 216660 (Peripheral and Central Nervous System Drugs Advisory Committee, 2022); https://www.fda.gov/media/161378/download
Tang, Q. et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008).
Camu, W. et al. Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): A phase 2a randomised, double-blind, placebo-controlled trial. EBioMedicine 59, 102844 (2020). This short phase IIa IMODALS trial, as a prelude to the phase IIb/III MIROCALS trial, showed that low-dose IL-2 (aldesleukin) produces a robust upregulation of the peripheral Treg cell count and a lowering of at least one inflammatory mediator (MCP-1/CCL2).
Giovannelli, I. et al. Amyotrophic lateral sclerosis transcriptomics reveals immunological effects of low-dose interleukin-2. Brain Commun. 3, fcab141 (2021).
Dalakas, M. C., Alexopoulos, H. & Spaeth, P. J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 16, 601–617 (2020).
Bahia El Idrissi, N. et al. Complement activation at the motor end-plates in amyotrophic lateral sclerosis. J. Neuroinflammation 13, 72 (2016).
Wainger, B. J. et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1–11 (2014).
Wainger, B. J. et al. Effect of ezogabine on cortical and spinal motor neuron excitability in amyotrophic lateral sclerosis: a randomized clinical trial. JAMA Neurol. 78, 186–196 (2021).
Campbell, A., Baldessarini, R. J., Teicher, M. H. & Neumeyer, J. L. S(+)apomorphines. selective inhibition of excitatory effects of dopamine injected into the limbic system of the rat. Neuropharmacology 24, 391–399 (1985).
Schaus, J. M., Titus, R. D., Foreman, M. M., Mason, N. R. & Truex, L. L. Aporphines as antagonists of dopamine D-1 receptors. J. Med. Chem. 33, 600–607 (1990).
Mead, R. J. et al. S[+] Apomorphine is a CNS penetrating activator of the Nrf2-ARE pathway with activity in mouse and patient fibroblast models of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 61, 438–452 (2013).
Dinkova-Kostova, A. T., Kostov, R. V. & Kazantsev, A. G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 285, 3576–3590 (2018).
Satoh, T. et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J. Neurochem. 104, 1116–1131 (2008).
Satoh, T. et al. Dual neuroprotective pathways of a pro-electrophilic compound via HSF-1-activated heat-shock proteins and Nrf2-activated phase 2 antioxidant response enzymes. J. Neurochem. 119, 569–578 (2011).
Mead, R. J., Shaw, P. J., Ogoe, C., Shan, N. & Ferraiuolo, L. Treatment of neurological diseases. Patent PCT/US2019/056996 (2020).
Mead, R. J., Shaw, . J., Ogoe, C., Shan, N. & Ferraiuolo, L. Treatment of neurological diseases. WO patent WO 2020/081973 A1 (2020).
Coya_Non-Confidential_Deck_2022.05.16 (Coya Therapeutics, 2022). https://coyatherapeutics.com
Zheng, Q., Zhang, S., Guo, W. Z. & Li, X. K. The unique immunomodulatory properties of MSC-derived exosomes in organ transplantation. Front. Immunol. 12, 659621 (2021).
Thome, A. D. et al. Extracellular vesicles derived from ex vivo expanded regulatory T cells modulate in vitro and in vivo inflammation. Front. Immunol. 13, 875825 (2022).
De Cesco, S., Davis, J. B. & Brennan, P. E. TargetDB: a target information aggregation tool and tractability predictor. PLoS ONE 15, e0232644 (2020).
Myszczynska, M. A. et al. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat. Rev. Neurol. 16, 440–456 (2020).
Smith, D. P. et al. Expert-augmented computational drug repurposing identified baricitinib as a treatment for COVID-19. Front. Pharmacol. 12, 709856 (2021).
Kariolis, M. S. et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. 12, eaay1359 (2020).
Begley, C. G. et al. Drug repurposing: misconceptions, challenges, and opportunities for academic researchers. Sci. Transl. Med. 13, eabd5524 (2021).
Gatto, N. et al. Directly converted astrocytes retain the ageing features of the donor fibroblasts and elucidate the astrocytic contribution to human CNS health and disease. Aging Cell 20, e13281 (2021).
Stopford, M. J., Allen, S. P. & Ferraiuolo, L. A high-throughput and pathophysiologically relevant astrocyte-motor neuron co-culture assay for amyotrophic lateral sclerosis therapeutic discovery. Bio. Protoc. 9, e3353 (2019).
Braems, E., Tziortzouda, P. & Van Den Bosch, L. Exploring the alternative: fish, flies and worms as preclinical models for ALS. Neurosci. Lett. 759, 136041 (2021).
Zhang, K., Coyne, A. N. & Lloyd, T. E. Drosophila models of amyotrophic lateral sclerosis with defects in RNA metabolism. Brain Res. 1693, 109–120 (2018).
Shaw, M. P. et al. Stable transgenic C9orf72 zebrafish model key aspects of the ALS/FTD phenotype and reveal novel pathological features. Acta Neuropathol. Commun. 6, 125 (2018).
van den Berg, L. H. et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology 92, e1610–e1623 (2019).
Verber, N. & Shaw, P. J. Biomarkers in amyotrophic lateral sclerosis: a review of new developments. Curr. Opin. Neurol. 33, 662–668 (2020).
van Blitterswijk, M. et al. Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum. Mol. Genet. 21, 3776–3784 (2012).
Fournier, C. N. et al. Development and validation of the Rasch-built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS). JAMA Neurol. 77, 480–488 (2020).
Balendra, R., Al Khleifat, A., Fang, T. & Al-Chalabi, A. A standard operating procedure for King’s ALS clinical staging. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 159–164 (2019).
Al-Chalabi, A. et al. Clinical staging in amyotrophic lateral sclerosis: analysis of Edaravone Study 19. J. Neurol. Neurosurg. Psychiatry 92, 165–171 (2021).
Westeneng, H. J. et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 17, 423–433 (2018).
Berry, J. D. et al. Improved stratification of ALS clinical trials using predicted survival. Ann. Clin. Transl. Neurol. 5, 474–485 (2018).
van Eijk, R. P. A. et al. Innovating clinical trials for amyotrophic lateral sclerosis: challenging the established order. Neurology 97, 528–536 (2021).
van Eijk, R. P. A. et al. An old friend who has overstayed their welcome: the ALSFRS-R total score as primary endpoint for ALS clinical trials. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 300–307 (2021).
Paganoni, S. et al. Adaptive platform trials to transform ALS therapy development. Ann. Neurol. 91, 165–175 (2022).
Kiernan, M. C. et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 17, 104–118 (2021). This is an excellent review of strategies with the potential to improve the prospects for clinical trials to deliver beneficial outcomes for patients with ALS.
Rutkove, S. B. et al. Improved ALS clinical trials through frequent at-home self-assessment: a proof of concept study. Ann. Clin. Transl. Neurol. 7, 1148–1157 (2020).
Govindarajan, R., Berry, J. D., Paganoni, S., Pulley, M. T. & Simmons, Z. Optimizing telemedicine to facilitate amyotrophic lateral sclerosis clinical trials. Muscle Nerve 62, 321–326 (2020).
US Department of Health and Human Services. Adaptive designs for clinical trials of drugs and biologics: guidance for industry. FDA https://www.fda.gov/media/78495/download (2019).
Verber, N. S. et al. Biomarkers in motor neuron disease: a state of the art review. Front. Neurol. 10, 291 (2019).
Vissers, M., Heuberger, J. & Groeneveld, G. J. Targeting for success: demonstrating proof-of-concept with mechanistic early phase clinical pharmacology studies for disease-modification in neurodegenerative disorders. Int. J. Mol. Sci. 22, 1615 (2021).
Rosen, D. R. et al. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 3, 981–987 (1994).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Scott, S. et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 9, 4–15 (2008).
Ludolph, A. C. et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 11, 38–45 (2010).
Turner, B. J. et al. TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy. BMC Neurosci. 9, 104 (2008).
Gurney, M. E., Fleck, T. J., Himes, C. S. & Hall, E. D. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology 50, 62–66 (1998).
Ito, H. et al. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp. Neurol. 213, 448–455 (2008).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008).
Gendron, T. F. & Petrucelli, L. Rodent models of TDP-43 proteinopathy: investigating the mechanisms of TDP-43-mediated neurodegeneration. J. Mol. Neurosci. 45, 486–499 (2011).
Xu, Z. S. Does a loss of TDP-43 function cause neurodegeneration? Mol. Neurodegener. 7, 27 (2012).
Arnold, E. S. et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc. Natl Acad. Sci. USA 110, E736–E745 (2013).
Watkins, J. A., Alix, J. J. P., Shaw, P. J. & Mead, R. J. Extensive phenotypic characterisation of a human TDP-43(Q331K) transgenic mouse model of amyotrophic lateral sclerosis (ALS). Sci. Rep. 11, 16659 (2021).
White, M. A. et al. TDP-43 gains function due to perturbed autoregulation in a Tardbp knock-in mouse model of ALS-FTD. Nat. Neurosci. 21, 552–563 (2018).
Lin, Z. et al. MRI-guided histology of TDP-43 knock-in mice implicates parvalbumin interneuron loss, impaired neurogenesis and aberrant neurodevelopment in amyotrophic lateral sclerosis-frontotemporal dementia. Brain Commun. 3, fcab114 (2021).
Devoy, A. et al. Humanized mutant FUS drives progressive motor neuron degeneration without aggregation in ‘FUSDelta14’ knockin mice. Brain 140, 2797–2805 (2017).
Peters, O. M. et al. Human C9ORF72 hexanucleotide expansion reproduces RNA foci and dipeptide repeat proteins but not neurodegeneration in BAC transgenic mice. Neuron 88, 902–909 (2015).
Jiang, J. et al. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90, 535–550 (2016).
Liu, Y. et al. C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron 90, 521–534 (2016).
Nguyen, L. et al. Survival and motor phenotypes in FVB C9-500 ALS/FTD BAC transgenic mice reproduced by multiple labs. Neuron 108, 784–796.e3 (2020).
Mordes, D. A. et al. Absence of survival and motor deficits in 500 repeat C9ORF72 BAC mice. Neuron 108, 775–783.e4 (2020).
Ferraiuolo, L. & Maragakis, N. J. Mini-review: induced pluripotent stem cells and the search for new cell-specific ALS therapeutic targets. Neurosci. Lett. 755, 135911 (2021).
Imamura, K. et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci. Transl. Med. 9, aaf3962 (2017).
Fujimori, K. et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 24, 1579–1589 (2018).
Boillee, S., Vande Velde, C. & Cleveland, D. W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006).
Lapasset, L. et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 25, 2248–2253 (2011).
Pereira, J. D. et al. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat. Commun. 12, 4744 (2021).
Morgan, S. et al. A comprehensive analysis of rare genetic variation in amyotrophic lateral sclerosis in the UK. Brain 140, 1611–1618 (2017).
Cooper-Knock, J. et al. Targeted genetic screen in amyotrophic lateral sclerosis reveals novel genetic variants with synergistic effect on clinical phenotype. Front. Mol. Neurosci. 10, 370 (2017).
Li, C., Ou, R., Wei, Q. & Shang, H. Shared genetic links between amyotrophic lateral sclerosis and obesity-related traits: a genome-wide association study. Neurobiol. Aging 102, 211.e1–211.e9 (2021).
Ling, J. P., Pletnikova, O., Troncoso, J. C. & Wong, P. C. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349, 650–655 (2015).
Prudencio, M. et al. Truncated stathmin-2 is a marker of TDP-43 pathology in frontotemporal dementia. J. Clin. Invest. 130, 6080–6092 (2020).
Elden, A. C. et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075 (2010).
Smith, B. N. et al. Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaad9157 (2017).
Cooper-Knock, J. et al. Rare variant burden analysis within enhancers identifies CAV1 as an ALS risk gene. Cell Rep. 33, 108456 (2020).
Cooper-Knock, J. et al. Mutations in the glycosyltransferase domain of GLT8D1 are associated with familial amyotrophic lateral sclerosis. Cell Rep. 26, 2298–2306.e5 (2019).
Farhan, S. M. K. et al. Exome sequencing in amyotrophic lateral sclerosis implicates a novel gene, DNAJC7, encoding a heat-shock protein. Nat. Neurosci. 22, 1966–1974 (2019).
Mohassel, P. et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat. Med. 27, 1197–1204 (2021).
Johnson, J. O. et al. Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. 78, 1236–1248 (2021).
Nakamura, R. et al. A multi-ethnic meta-analysis identifies novel genes, including ACSL5, associated with amyotrophic lateral sclerosis. Commun. Biol. 3, 526 (2020).
Iacoangeli, A. et al. Genome-wide meta-analysis finds the ACSL5-ZDHHC6 locus is associated with ALS and links weight loss to the disease genetics. Cell Rep. 33, 108323 (2020).
Freischmidt, A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 (2015).
Kenna, K. P. et al. NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 48, 1037–1042 (2016).
Chow, C. Y. et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 84, 85–88 (2009).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017). This landmark gene replacement trial for patients with SMA showed that motor neuron degenerative disorders are potentially treatable conditions.
Gendron, T. F. et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaai7866 (2017).
Benatar, M. et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 95, e59–e69 (2020). This publication provides validated evidence that neurofilament proteins can serve as biomarkers to subclassify disease severity in patients with ALS and also as markers of phenoconversion in pre-symptomatic carriers of pathogenic mutations.
Zucchi, E. et al. Neurofilaments in motor neuron disorders: towards promising diagnostic and prognostic biomarkers. Mol. Neurodegener. 15, 58 (2020).
Poesen, K. & Van Damme, P. Diagnostic and prognostic performance of neurofilaments in ALS. Front. Neurol. 9, 1167 (2018).
Lu, C. H. et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84, 2247–2257 (2015).
Lu, C. H. et al. Plasma neurofilament heavy chain levels and disease progression in amyotrophic lateral sclerosis: insights from a longitudinal study. J. Neurol. Neurosurg. Psychiatry 86, 565–573 (2015).
Mitsumoto, H., Brooks, B. R. & Silani, V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 13, 1127–1138 (2014).
Cudkowicz, M. E. et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann. Neurol. 60, 22–31 (2006).
Forgrave, L. M., Ma, M., Best, J. R. & DeMarco, M. L. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Alzheimers Dement. 11, 730–743 (2019).
Gagliardi, D. et al. Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int. J. Mol. Sci. 20, 4152 (2019).
Thompson, A. G. et al. Multicentre appraisal of amyotrophic lateral sclerosis biofluid biomarkers shows primacy of blood neurofilament light chain. Brain Commun. 4, fcac029 (2022).
Benatar, M., Wuu, J., Andersen, P. M., Lombardi, V. & Malaspina, A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 84, 130–139 (2018).
Magen, I. et al. Circulating miR-181 is a prognostic biomarker for amyotrophic lateral sclerosis. Nat. Neurosci. 24, 1534–1541 (2021).
Neuwirth, C. et al. Tracking motor neuron loss in a set of six muscles in amyotrophic lateral sclerosis using the Motor Unit Number Index (MUNIX): a 15-month longitudinal multicentre trial. J. Neurol. Neurosurg. Psychiatry 86, 1172–1179 (2015).
Pinto, S. & de Carvalho, M. Symmetry of phrenic nerve motor response in amyotrophic lateral sclerosis. Muscle Nerve 42, 822–825 (2010).
de Carvalho, M. et al. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 11, 456–460 (2010).
Ellis, C. M. et al. A proton magnetic resonance spectroscopic study in ALS: correlation with clinical findings. Neurology 51, 1104–1109 (1998).
Pagani, M. et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83, 1067–1074 (2014).
De Vocht, J. et al. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol. 77, 1008–1017 (2020).
Zurcher, N. R. et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [11C]-PBR28. Neuroimage Clin. 7, 409–414 (2015).
Ikawa, M., Okazawa, H., Nakamoto, Y. & Yoneda, M. PET imaging for oxidative stress in neurodegenerative disorders associated with mitochondrial dysfunction. Antioxidants 9, 861 (2020).
Malpetti, M. et al. Synaptic density in carriers of C9orf72 mutations: a [11C]UCB-J PET study. Ann. Clin. Transl. Neurol. 8, 1515–1523 (2021).
Acknowledgements
P.J.S. is supported as an Emeritus NIHR Senior Investigator and by the NIHR Sheffield Biomedical Research Centre (IS-BRC-1215-20017), the Motor Neurone Disease Association (AMBRoSIA MNDA 974-797) the Medical Research Council (MRC COEN 4017), the EU Horizon 2020 programme (H2020-633413) and the My Name’5 Doddie Foundation (DOD/14/43). N.S., P.J.S. and R.J.M. are funded by FightMND Australia (03_DDG_2020_Shan). The authors thank L. Evans for her skilled assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
R.J.M., N.S. and P.J.S. researched data for the article. All authors contributed substantially to discussion of the content. R.J.M., N.S. and P.J.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.J.M. is cofounder of and holds shares in Keapstone Therapeutics, collaborates and receives funding from BenevolentAI, Quell Therapeutics, Sosei Heptares and MSD, is a consultant to Aclipse Therapeutics, has shares in Aclipse One Inc and is an inventor on patents related to M102. N.S. is an employee and shareholder of Aclipse Therapeutics. H.J.R. is the chairman of the Board of Aclipse Therapeutics. F.M. is an employee and shareholder of Merck and Co. P.J.S. is an advisory board member and consultant for Biogen, Aclipse Therapeutics, Quell Therapeutics, BenevolentAI, QurAlis, Astex, GeniUS and Eli Lilly and collaborates with and receives research funding from Quell Therapeutics, Aclipse Therapeutics, Pfizer and SwanBio. She is a cofounder of and holds shares in Keapstone Therapeutics and holds shares in Aclipse One Inc. She is an inventor on patents related to low-dose IL-2, SRSF1 and M102. Support for clinical trials participation in the last five years has been received from Biogen, Alexion, Orion Pharma, WAVE, the EU Horizon 2020 programme and UK NIHR.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BenevolentAI: https://www.benevolent.com/amyotrophic-lateral-sclerosis
Supplementary information
Glossary
- Cryptic exon
-
An exon that is not normally included in the final mRNA transcript of a gene but which can be included under certain circumstances such as mutations elsewhere in the gene, changes in the state of the cell or loss of factors that normally regulate exon splicing.
- DNA damage response
-
The process by which cells maintain genomic integrity in the face of continuous mutation-causing insults. It comprises both DNA repair mechanisms and cell-cycle checkpoint regulation.
- Embedded experimental medicine approach
-
Incorporation of multiple readouts of a biological mechanism related to the therapeutic approach being tested within a clinical trial in order to understand the impact of the therapeutic agent on disease processes.
- Heat shock response
-
A rapid cellular response to stress that increases, usually via transcriptional activation, the availability of molecular chaperones to enable cytoprotection.
- Inflammasome
-
Multiprotein complex of innate immune receptors which are required for activation of inflammatory responses and specifically lead to maturation and secretion of IL-1β and IL-18.
- Proteinopathy
-
The accumulation of a specific protein, either the wild-type or a mutant variety, in excess with altered conformations that facilitate aggregation.
- Rasch model
-
A mathematical framework against which test developers can compare empirical data to assess an instrument’s capacity to emulate the properties of fundamental measurement (invariance and unidimensionality) and thus serve as a tool for quantifying unobservable human conditions.
- Repeat-associated non-AUG (RAN) translation
-
The initiation of protein translation in the absence of a classic AUG start codon in the RNA species being translated. It is often associated with GC-rich repetitive sequences and can occur in multiple reading frames.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mead, R.J., Shan, N., Reiser, H.J. et al. Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov 22, 185–212 (2023). https://doi.org/10.1038/s41573-022-00612-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-022-00612-2
This article is cited by
-
The role of CD56bright NK cells in neurodegenerative disorders
Journal of Neuroinflammation (2024)
-
TBK1, a prioritized drug repurposing target for amyotrophic lateral sclerosis: evidence from druggable genome Mendelian randomization and pharmacological verification in vitro
BMC Medicine (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
AAV-mediated upregulation of VDAC1 rescues the mitochondrial respiration and sirtuins expression in a SOD1 mouse model of inherited ALS
Cell Death Discovery (2024)
-
Disruption of MAM integrity in mutant FUS oligodendroglial progenitors from hiPSCs
Acta Neuropathologica (2024)