Abstract
Current approaches to classifying cognitive impairment in people living with HIV can overestimate disease burden and lead to ambiguity around disease mechanisms. The 2007 criteria for HIV-associated neurocognitive disorders (HAND), sometimes called the Frascati criteria, can falsely classify over 20% of cognitively healthy individuals as having cognitive impairment. Minimum criteria for HAND are met on the basis of performance on cognitive tests alone, which might not be appropriate for populations with diverse educational and socioeconomic backgrounds. Imprecise phenotyping of cognitive impairment can limit mechanistic research, biomarker discovery and treatment trials. Importantly, overestimation of cognitive impairment carries the risk of creating fear among people living with HIV and worsening stigma and discrimination towards these individuals. To address this issue, we established the International HIV-Cognition Working Group, which is globally representative and involves the community of people living with HIV. We reached consensus on six recommendations towards a new approach for diagnosis and classification of cognitive impairment in people living with HIV, intended to focus discussion and debate going forward. We propose the conceptual separation of HIV-associated brain injury — including active or pretreatment legacy damage — from other causes of brain injury occurring in people living with HIV. We suggest moving away from a quantitative neuropsychological approach towards an emphasis on clinical context. Our recommendations are intended to better represent the changing profile of cognitive impairment in people living with HIV in diverse global settings and to provide a clearer framework of classification for clinical management and research studies.
Similar content being viewed by others
Introduction
The most frequently used criteria for cognitive impairment in people living with HIV are the HIV-associated neurocognitive disorders (HAND) criteria, developed in 2007 by a working group convened by the US National Institute of Mental Health1. The HAND criteria (sometimes referred to as the Frascati criteria) were intended to harmonize research methodology to allow comparisons across diverse study settings. Although originally intended for use in research, HAND terminology has become widely used to refer to the clinical burden of cognitive impairment2. The HAND criteria have been successful in providing a consistent system of classification in global research studies for 15 years. The spectrum of HIV disease has, however, changed dramatically in the past two decades: the majority of people living with HIV globally are now virally supressed by effective antiretroviral therapy (ART), and life expectancy approaches that of uninfected cohorts3,4. Minimum criteria for HAND are met on the basis of cognitive test performance compared with HIV-negative populations, without the need for a clinical assessment of cognitive status. Several authors have argued that this approach overestimates disease burden and that the HAND criteria are not appropriate for the modern era5,6,7,8,9,10,11.
Criticism of the HAND criteria centres on three main points, as outlined by authors from our group in 2021 (ref. 12). First, the statistical approach applied to cognitive data has the potential for a very high false-positive rate: the current approach defines over 20% of cognitively healthy HIV-negative control participants as having cognitive impairment8,10. Second, cognitive test performance is strongly influenced by complex educational, cultural and socioeconomic factors, which can interact with HIV risk such that low cognitive test performance might not correspond to a pathological state13,14. Last, in the modern era of effective ART and an ageing population of people living with HIV, cognitive impairment in these individuals is frequently multifactorial and, hence, is not synonymous with the direct effect of HIV on the brain and not best described as ‘HIV-associated’, which implies a degree of causation15,16.
The HAND criteria typically classify 20–60% (and sometimes up to 90%) of people living with HIV as cognitively impaired2,14,17. This figure does not seem to align with clinical observations that cognitive impairment in people living with HIV presents less frequently in the modern era, usually affecting individuals who are not on effective ART or have relevant comorbidities, or occurring as a result of damage caused by CNS HIV replication before effective ART18,19,20. Lack of diagnostic precision could hamper clinical studies of cognitive impairment in people living with HIV and reduce the power to identify biomarkers relating to neuropathogenesis21.
A label of cognitive impairment can affect self-esteem and confidence and raise fears for future health in people living with HIV22. Overestimation of cognitive impairment might also worsen stigma and discrimination towards these individuals23. For example, people living with HIV in the UK were denied the opportunity to become airline pilots owing to concerns over the development of cognitive impairment. Following a campaign by a pilot living with HIV, the UK Civil Aviation Authority removed this ban in 2022 to reflect the improved HIV outcomes in the modern ART era24. Conversely, underestimation or misclassification of cognitive impairment in people living with HIV carries the risk of missing cases and preventing access to care. Cognitive impairment is an important complication of HIV with far-reaching consequences for quality of life22. Approaches to the diagnosis and classification of cognitive impairment must reflect the modern spectrum of disease so that prognostic information is accurate and affected individuals can receive the help they need.
The original HAND publication of 2007 acknowledged several of these potential methodological issues and recommended strongly that the criteria should be field tested and further refined going forward1. In response to the issues described above, we established the International HIV-Cognition Working Group. The broad aim of the group was to propose improvements to the diagnostic approach to cognitive impairment in people living with HIV so as to reflect changes in the spectrum of HIV disease in the modern ART era. In this Consensus Statement, we provide specific recommendations around key issues to focus discussion and help the field move forward.
Methods
The International HIV-Cognition Working Group was initiated by the HIV Mental Health Research Unit at the University of Cape Town, South Africa, and follows our HAND critique published in 2021 (ref. 12). The group was intended to be globally representative, and hence, preference was given to experts based in low-income and middle-income countries with high HIV prevalence. In high-income countries, members were invited in approximately equal numbers from Europe and the USA. We aimed to include people with direct clinical experience of people living with HIV, as well as leading researchers in the field. Representatives were invited from the community of people living with HIV in both high-income and low-income settings. Of 25 representatives invited, three declined and two withdrew after initially accepting, citing time commitments. Of the resulting 20 members, nine (45%) were based in low-income and middle-income countries in the Global South. Members included academics and clinicians from the neurology, psychiatry, neuropsychology and infectious disease fields, as well as three HIV community representatives.
Working group meetings were held virtually. The framework laid out in the 2021 HAND critique was used as a starting point for discussion12, with the specific aim of outlining a diagnostic approach for cognitive impairment in people living with HIV that is applicable in the clinic as well as in research, is appropriate for diverse populations of people living with HIV globally, is applicable in both low-resource and high-resource settings, and reduces the risk of fear, stigma and discrimination for people living with HIV. Members participated in videoconference discussions and engaged further via the group e-mail chain. On the basis of this communication, a manuscript draft was prepared by S.N. and distributed to the group for comment and further input. Multiple iterations of the manuscript were reviewed, revised and redistributed. Additional smaller meetings were held virtually or in person at international conferences, and the outcomes of these meetings were subsequently shared for discussion with the wider group. This iterative process continued until a broad consensus was reached on all points by all working group members.
This process led to the six recommendations outlined below and summarized in Box 1. These recommendations should be interpreted as representing the consensus opinion of a diverse group of experts rather than being a definitive new set of criteria. Further validation and a broader consensus within the field will be required to define and implement definitive new criteria for cognitive impairment in people living with HIV.
Recommendations
Classifying cause of brain injury in people living with HIV
Recommendation 1: HIV-associated brain injury (HABI) should be considered as one cause of cognitive impairment alongside other potential causes of brain injury occurring in people living with HIV.
In the modern era, cognitive impairment is frequently multifactorial, with a direct effect of HIV on the brain representing only one cause15,19. To distinguish direct effects of HIV from other causes of brain injury in people living with HIV, we recommend that the term HABI should be used to refer to damage caused directly by HIV. Other causes of brain injury include various comorbidities and medication effects19,25, as summarized in Box 2. We recommend that HABI is conceptually separated from other causes of brain injury, although we accept that this can be difficult in practice, given that several causes can coexist and clinical manifestations can lead to overlapping symptoms and signs. Nevertheless, we feel that separating the concept of HABI from all-cause cognitive impairment in people living with HIV reduces ambiguity in terminology and facilitates examination of brain injury mechanisms.
HAND is defined as being caused by HIV, at least in part1. The HAND criteria do acknowledge, however, that people living with HIV are potentially vulnerable to the cognitive effects of other conditions. Where present, such comorbid conditions are termed ‘contributing’, as they contribute to cognitive impairment alongside the effect of HIV. If an individual has an alternative cause of cognitive impairment, this is considered a ‘confounding’ condition and a classification of HAND is not made. As such, a label of HAND means that cognitive impairment is caused by a direct effect of HIV on the brain, with or without additional contributions from comorbidities. This terminology might no longer be appropriate owing to dramatic improvements in HIV clinical care, which have reduced the frequency of brain injury caused by HIV. We recommend that a classification of cognitive impairment in people living with HIV should encompass all potential causes of brain injury, regardless of whether HABI is the cause or even a contributing factor in any given case. Moving to a classification that considers multiple causes of cognitive impairment will more accurately represent changes to the clinical burden of disease and facilitate the study of more representative samples in research, compared with the current classification.
Parallels can be drawn between milder forms of cognitive impairment in people living with HIV and mild cognitive impairment (MCI) in relation to Alzheimer disease (AD). The underlying pathology associated with MCI is heterogeneous and the majority of individuals with MCI do not go on to develop AD26. Biomarkers have been identified that reliably differentiate MCI with underlying AD pathology from other causes of MCI, and can be used to predict progression to AD27. The comparable situation in people living with HIV is to distinguish between HABI and other underlying causes of cognitive impairment. This distinction is more difficult with HABI than with AD pathology as, in contrast to AD, HABI does not generally progress to a marked dementia syndrome in individuals who receive suppressive ART28.
Numerous potential pathological mechanisms could underlie HABI, including persistent immune activation, blood–brain barrier dysfunction and more direct virus-induced neurotoxicity. Neuronal damage can be mediated by both immune-active molecules and HIV products, and could involve several mechanisms including oxidative stress, metabolic changes, glutamate dysregulation and NMDA excitotoxity29,30,31. Of note, HABI differs slightly from the existing terms HIV encephalopathy and HIV encephalitis. HIV encephalopathy refers to a predominantly subcortical cognitive–motor syndrome, also known as HIV-associated dementia, which is an AIDS-defining condition. HIV encephalitis refers to the histopathological finding of multinucleated giant cells and microglial nodules32. Although these complications continue to occur, particularly in people with untreated or advanced HIV disease, they no longer represent the prominent neuropathology of HABI in the modern ART era. For example, in one study, of 20 people diagnosed with HIV-associated dementia, only one had histopathological evidence of HIV encephalitis post mortem33. Our proposed term HABI is intended to encompass any mechanism of brain injury caused directly by HIV, including those previously described by the terms HIV encephalopathy and HIV encephalitis.
Differentiating legacy and active HABI
Recommendation 2: HABI should be differentiated on the basis of HIV RNA suppression and the activity of pathology.
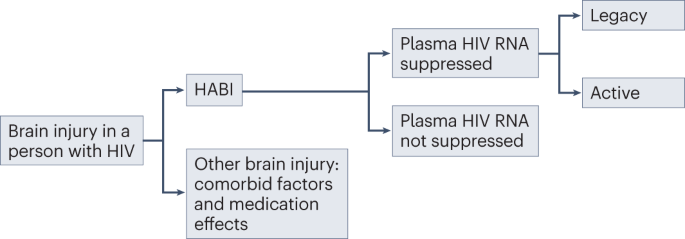
In the modern era, the majority of people living with HIV globally are virally supressed on effective ART and, as a result, are largely protected from progressive HIV disease3. Therefore, defining the risk of progressive disease caused by HABI in people with HIV RNA suppression is particularly important. Current evidence is conflicting, with regard to both how commonly HIV causes cognitive impairment in this group2,28 and whether the brain injury is the result of a progressive or static process34,35,36,37,38. To reduce ambiguity in this area, we recommend that HABI is subdivided into legacy and active HABI on the basis of progression (Fig. 1). This differentiation is important as it has implications for treatment and prognosis25,39. Of note, legacy and active HABI can coexist, as active HABI can occur on a background of legacy HABI.
HIV-associated brain injury (HABI) should be differentiated from other causes of brain injury, such as comorbid factors and medication effects. In individuals with plasma HIV RNA suppression, HABI is characterized as either legacy (inactive brain injury from pretreatment damage) or active (ongoing brain injury leading to clinical and/or radiological progression). Whether active HABI occurs in people with sustained HIV RNA suppression in plasma and cerebrospinal fluid has not been definitively shown.
HABI can also occur in people living with HIV with untreated, or incompletely treated, HIV infection40,41. In such patients, the focus should be on systemic HIV viral control25,39,42,43.
Legacy HABI
Irreversible or only partially reversible CNS damage resulting from HABI can be sustained during periods of untreated HIV infection, particularly in the context of advanced immunosuppression20. This sustained damage has been referred to as the legacy effect and represents brain injury that occurs before an individual initiates ART. In adults with vertically acquired HIV (transmitted from mother to child in utero, intrapartum or postnatally through breast feeding), legacy HABI can include sequelae from the effects of HIV infection on the developing brain44. Legacy effects are inactive and permanent and, hence, not amenable to treatment. It is possible that subclinical legacy HABI could lower cognitive reserve, thereby increasing vulnerability to cognitive impairment from other causes, such as ageing45,46,47.
Active HABI
Active HABI refers to evidence of sustained clinical or radiological progression of CNS damage over time, beyond that expected from normal ageing or variability in cognitive performance testing, with careful exclusion of alternative causes. Progression of CNS damage in the context of HIV RNA suppression in plasma should prompt examination for cerebrospinal fluid (CSF) HIV RNA escape39,43. Definitions of CSF HIV RNA escape vary, but the consensus definition is the presence of HIV RNA in the CSF combined with absence or lower levels in the plasma48. CSF RNA escape can indicate compartmentalized HIV replication in the CNS resulting from low treatment potency in the intrathecal compartment, owing to ART resistance, less effective or older ART regimens, or low adherence to treatment. CSF HIV RNA escape can lead to varying presentations, including rapidly progressive neurological disease and diffuse white matter signal abnormality on MRI49,50, although it can also be transient and asymptomatic49. Evidence suggests that CSF RNA escape is becoming less common with modern ART51. Low levels of HIV RNA in the CSF might not necessarily be the cause of active neuropathology, and its presence should not be taken as definitive evidence of CNS-compartmentalized HIV. However, in the presence of clinically active disease, CSF HIV RNA should be investigated and, if present, ideally treated in the first instance with ART directed to CSF resistance profiles52.
Another cause of active HABI is CD8 encephalitis, a severe inflammatory disorder characterized by T cell infiltration into the brain, leading to swelling and raised intracranial pressure that can be fatal31. CD8 encephalitis typically occurs in people on ART and can be associated with a number of triggers, including CSF HIV RNA escape and immune reconstitution inflammatory syndrome (IRIS), suggesting some overlap among these conditions31,53. CD8 encephalitis is responsive to corticosteroids54.
IRIS can occur in the weeks to months following the initiation of ART and can affect the brain in the absence of opportunistic pathogens31,55. This condition is thought to be a result of an immune response directed at HIV viral reservoirs in the brain and has been associated with CSF HIV RNA escape55,56. Similar to CD8 encephalitis, IRIS can lead to a severe, potentially fatal T cell encephalitis with brain oedema, which can respond to immune modulation with corticosteroids57. IRIS directed at viral, fungal, bacterial or parasitic opportunistic pathogens in the brain is considered to be a secondary effect and not part of HABI.
Beyond the uncommon cases of CD8 encephalitis and CNS IRIS described above, HIV has not been definitively shown to cause a progressive cognitive syndrome in the context of sustained HIV RNA suppression in both plasma and CSF. Nevertheless, several mechanisms have been proposed for progressive cognitive decline in controlled HIV. These mechanisms include HIV protein-associated encephalopathy; ongoing CNS HIV replication below the threshold of detection; and a neuroinflammatory process established during legacy damage that persists after effective HIV control with ART29,30,31. The 2013 consensus report from the MIND Exchange program stated that “it is not possible from existing data to conclude whether patients with successful treatment (ie, plasma HIV RNA <50 copies/mL) are at risk of progression [of cognitive impairment]”43. Since this consensus report was published, several studies have examined this question, with some suggesting that active HABI can occur in the context of controlled HIV34,58, particularly in ageing cohorts37,59,60. By contrast, others have provided longitudinal cognitive outcome data with low rates of progression20,35,38, falling prevalence rates of cognitive impairment over time28,61, cognitive deterioration associated with comorbidities rather than HIV factors19, and ageing trajectories similar to those in lifestyle-matched control individuals36. Whether controlled HIV can cause active HABI remains an important question to answer so that people living with HIV can obtain accurate prognostic information, and those at risk of cognitive impairment can be identified for treatment and management.
Biomarker changes without brain injury
Some CSF, plasma and imaging biomarkers that are used in research have indicated an active and, hence, potentially injurious process in the brain despite viral suppression and stable cognition34,58,62,63,64,65. Examples include diffusion tensor imaging and functional MRI, and various CSF biomarkers of immune activation and neuronal damage66. Although such markers sometimes indicate ongoing inflammation in the CNS of people living with HIV, the extent to which this inflammation has a clinical correlate or represents an injurious process, as opposed to subclinical effects or a healing process in response to pre-existing damage, is not clear25. Furthermore, some biomarkers change with age and can be altered in HIV-negative control individuals matched with people living with HIV for comorbidities and lifestyle factors67. Therefore, we recommend that abnormalities indicated by such markers should not be considered definitive evidence of an active injurious process, unless they are demonstrated to correspond to clinical or radiological progression as described earlier in this section. This distinction is intended not to undermine the potential importance of biomarker abnormalities but to acknowledge the difference between a research finding of concern and definitive evidence of a clinical effect.
A biomarker that consistently corresponds to clinical or radiological progression of brain injury could be used as evidence of active HABI, similar to the use of CSF amyloid and tau proteins to predict progression of AD27. One biomarker that might be useful in this context is CSF neurofilament light chain (NfL), a robust and sensitive marker of neuronal injury68. Research is needed to investigate the use of NfL as a biomarker for active HABI in people with sustained HIV RNA suppression. It should be noted that NfL is not specific for HABI versus other causes of brain injury, so other causes should be carefully excluded.
Neuroinflammation, as indicated by raised biomarker levels, could cause an active process of neuronal dysfunction without progressive injury, which could lower cognition in a stable or fluctuant way without sustained progression, akin to a metabolic encephalopathy. Supporting this theory, some CSF and imaging biomarkers were reported to correlate with clinical outcomes in people living with HIV; however, the associations were inconsistent and generally weak66,69. Trials of anti-inflammatory and neuroprotective compounds aimed at improving cognition in this patient population have not shown clinical effects70,71,72,73. Further research is needed to determine whether a non-progressive yet active process of neuronal dysfunction that is potentially amenable to treatment can occur in people with sustained suppression of HIV RNA. If shown to occur, this condition would warrant a third HABI subtype in addition to legacy and active HABI.
Interpretation of cognitive tests
Recommendation 3: Low performance on cognitive tests should not be labelled as cognitive impairment without clinical context.
Cognitive testing is an important element of assessment in people living with HIV who are suspected of having cognitive impairment. Despite the appeal of cognitive scores as an objective measure of neuronal function, the results vary widely depending on non-biological factors such as educational background and socioeconomic status13,14. Indeed, this issue was stressed in the original HAND criteria publication1. For example, the average score on the Montreal Cognitive Assessment (MoCA) in a study of healthy HIV-negative individuals without cognitive impairment in a low-income area of Cape Town was 21.7 out of 30 (ref. 74), which falls below what is considered a normal score in North America (26–30), where the MoCA was developed. These differences do not imply impaired cognition, but rather that performance on these tests can be culture-bound and vary substantially between groups with different educational and sociodemographic backgrounds.
In research studies, cognitive scores are typically compared with those in normative control populations. These comparisons can be improved by the collection of normative data from populations with similar demographics to the measured population of people living with HIV, or by controlling for demographic factors in established normative datasets with regression-based techniques75. However, these approaches have several limitations. First, studies have demonstrated wide variation in normative data among and within countries76, and developing extensive normative data for each setting in which people living with HIV reside would be impractical. Second, matching for all lifestyle and comorbid factors associated with HIV status is difficult owing to the broad diversity of HIV demographics. Last, in some regions, HIV acquisition is associated with poverty and low education levels77,78, increasing the likelihood that people living with HIV will return lower test scores than the population average. In clinical practice, these factors are taken into account by neuropsychologists and clinicians with experience in cognitive testing, who use cut-offs appropriate for the population or interpret an individual’s performance subjectively on the basis of educational background and estimates of premorbid functioning. In research studies, these approaches are often impractical and might be considered too subjective, and hence normative control scores are used to make comparisons.
The HAND criteria define statistical methodology to determine cut-offs for cognitive performance in people living with HIV compared with normative controls1. Cognitive performance within a particular domain must fall more than 1 s.d. below the normative average for that domain to be considered impaired. This threshold must be crossed in two or more cognitive domains for a classification of HAND. Several other statistical approaches to define cognitive impairment have also been used as alternatives to the HAND criteria, including the Global Deficit Score, Multivariate Normative Comparison, Novel Multivariate Method and the Gisslén criteria8,25. Some of these methods can be applied in several different ways (discussed under Recommendation 4 below), which creates the potential for large variation in the statistical methodology used to define cognitive impairment in people living with HIV. The percentage of individuals classified as having cognitive impairment can vary widely with different methods. For example, when 20 different methods were applied to a clinical cohort in South Africa, the rate of cognitive impairment ranged from 20% to 97%79. Therefore, the same individual can be classified as having cognitive impairment by one statistical method but not by another.
Fluctuation of cognitive scores in an individual over time presents another challenge80. Minor variation around domain cut-offs can have large effects on binary classifications81, so an individual on the margin of impairment can be classified as having impairment at one time point but not at another. This issue is evident in longitudinal studies, where fluctuations in cognitive function are frequently observed1,82.
For the reasons above, we suggest that the use of cross-sectional quantitative neuropsychological approaches alone is limited as a method of determining impairment in diverse populations. No tool provides a perfect indication of neuronal function, and any statistical method of dichotomization on the basis of cognitive performance will be to some extent arbitrary. Perfectly matching a normative population to factors associated with HIV acquisition in all settings is extremely difficult. Although defining a group at the lower end of the cognitive spectrum can be useful, we recommend that they be classified as having low cognitive performance rather than being diagnosed with cognitive impairment, unless supporting information exists in other areas of assessment (see Recommendation 5 below).
Comparisons can be made with the diagnosis of MCI in relation to AD. Statistical cut-offs for low cognitive performance in MCI vary from 1 to 2 s.d., resulting in wide variation in MCI prevalence depending on the method used83. Less stringent definitions using 1 s.d. are generally not favoured as they have a high false-positive rate84, fail to show an association with medial temporal atrophy and apolipoprotein E genotype (an important genetic determinant of AD risk)85, and result in considerable diagnostic instability — that is, fluctuation between a classification of MCI and cognitively normal — over time83. The potential for false classification of MCI is mitigated by the requirement for symptomatology, in contrast to HAND, for which symptoms are not a requirement (see Recommendation 6).
Individuals with isolated low cognitive performance, though not meeting our proposed criteria for cognitive impairment, might represent an important group for future research. Subclinical impairment and/or reduced cognitive reserve in those with low cognitive performance could increase vulnerability to other brain injuries, which is particularly important as the population of people living with HIV ages45,46,47. It should be noted that individuals at the lower end of the spectrum of cognitive performance might be more likely to have lower education levels and socioeconomic status, as well as different comorbid and lifestyle factors, compared with those at the upper end86,87. Consequently, those with low cognitive performance might have worse general health-related outcomes, owing to the interaction with social determinants of health88.
False classifications in cognitive data
Recommendation 4: When interpreting cognitive data, the false-classification rate should be considered.
As stated in the previous section, HAND criteria define cognitive impairment as performance of at least 1 s.d. below the normative average in at least two cognitive domains1. If population performance is normally distributed, then approximately 16% of scores on each test will fall more than 1 s.d. below the mean, so a sizeable proportion of individuals without cognitive impairment will be falsely classified as impaired. This false-classification rate depends on the number of domains measured, the number of tests used per domain, and the relationships among different tests, but is typically in excess of 20% and can rise to over 70% in some cases9,10,80.
As mentioned in the previous section, several other statistical approaches can be used to determine cut-offs for cognitive performance in people living with HIV compared with normative controls8,25. Some methods are more stringent than others, with improved false-positive rates generally at the expense of decreased sensitivity. Some methods can be applied in several different ways; for example, when more than one test is used per cognitive domain to improve accuracy, that domain can be defined as impaired by one test being positive, both tests being positive, or by the average domain T-score. These variations can alter the false-classification rate quite markedly80,81.
The false-classification rate is an important consideration when interpreting study findings: a study reporting low performance on cognitive tests in 30% of a population has a different interpretation when the false-classification rate is known to be 25% compared with 2.5%, for example. Currently, the false-classification rate is rarely acknowledged or reported in studies reporting HAND prevalence11. The range of tools to help estimate the false-classification rate for different statistical methodologies should be expanded8.
To help avoid false classification of cognitive impairment, alternative approaches can be used to handle cognitive data in research studies. One approach is to longitudinally assess the trajectory of cognitive performance rather than applying dichotomization cross-sectionally. For longitudinal assessment, fluctuation in cognitive performance and practice effects must be taken into account80,89. Another approach is to use cognitive performance as a continuous variable rather than as a binary measure with a statistically determined cut-off. The use of continuous variables captures the full spectrum of cognition and provides greater statistical power than the comparison of proportions below a cut-off81.
Defining cognitive impairment in people living with HIV
Recommendation 5: A research classification of cognitive impairment in people with HIV should consider a combination of cognitive symptoms, low performance on cognitive testing and abnormality on neurological investigations.
Assessment for cognitive impairment falls broadly into three areas: clinical history, performance on cognitive testing and the results of neurological investigations. Each area has strengths and weaknesses if used alone to determine cognitive impairment (Table 1). The presence of cognitive symptoms, defined here as a change in cognition that has been noticed by the individual, is clinically important but is a subjective measure, and reporting of symptoms varies among settings (see Recommendation 6). The results of cognitive testing can be more objective than clinical history but are strongly influenced by complex educational and socioeconomic factors and must be interpreted in the context of the individual or population background (as discussed in Recommendation 3). Evidence of brain injury on neurological investigations, such as neuroimaging or CSF examination, is the most objective measure of pathology, but abnormalities can represent subclinical damage, and these tests are not universally available or accessible in low-resource settings. In addition, neurological investigations can be insensitive for some causes of brain injury, including HABI, and the absence of evident abnormalities on routine investigations does not exclude the presence of brain injury50.
Given the limitations discussed above, we propose that in research settings, a classification of cognitive impairment can be made when an individual shows abnormalities in at least two of the three assessment areas. Using this pragmatic approach, someone with low cognitive performance would be considered to have cognitive impairment if supporting evidence of cognitive symptoms and/or brain injury was available. Similarly, someone with cognitive symptoms and evidence of brain injury would be considered to have cognitive impairment, even if cognitive performance did not fall below a threshold and, hence, would not currently be classified as HAND — for example, individuals with high pre-morbid function and/or cognitive reserve.
The importance of an isolated abnormality in any one assessment area should not be undermined by altering the criteria for cognitive impairment as described here. Individuals in whom the criteria for cognitive impairment are not met could still represent a group with clinical and research importance, and our recommendation is simply to alter the terminology used to describe these groups. Individuals previously defined as having asymptomatic neurocognitive impairment (ANI) — a form of HAND and therefore considered a neurocognitive disorder — would instead be referred to as having ‘low performance on cognitive tests’. These individuals would not be considered to have cognitive impairment in the absence of supporting evidence of abnormality from another assessment area.
Defining cognitive symptoms
Recommendation 6: Cognitive symptoms should refer to any change in cognition that has been noticed by the individual or an observer, whether or not this change impacts daily functioning.
When assessing someone at risk of cognitive impairment, obtaining a history of cognitive symptoms is important. We recommend a subtly different use of the term ‘symptom’ from that applied in the HAND criteria. HAND criteria define symptomatic cognitive impairment as a change in activities of daily living (ADLs) resulting from cognitive issues1. As treatment for HIV has improved, cognitive symptoms in people living with HIV have generally become milder28. Symptoms such as forgetfulness or difficulty concentrating can considerably affect an individual’s quality of life and ability to work but might not be severe enough to limit ADLs22. This level of symptoms is similar to that required for the diagnosis of MCI in relation to AD, for which cognitive change should be noticeable but not yet have a substantial effect on ADLs90.
Cognitive symptoms are inherently subjective: some cultures might be reluctant to acknowledge cognitive issues and some languages have limited vocabulary for cognitive complaints91,92. Furthermore, cognitive dysfunction can impair insight, reducing the probability of an individual reporting their difficulties93. Where available, an observer account — for example, a collateral history from a partner, family member or carer — can improve accuracy of symptom reporting and already forms part of the criteria for MCI94. In these cases, gaining consent from the individual with HIV is important, as the observer account can include sensitive information. Difficulties with ADLs reported on functional scales should be confirmed as related to cognitive issues rather than physical disability, intercurrent illness, psychological factors or fatigue.
Cognitive symptoms can be transient and reactive to psychological stressors or life events95,96. Symptoms can be more common in people with depression, which could be related to the effects of mood on cognitive processes or to shared biological mechanisms of neuroinflammation97,98,99. Where uncertainty arises, repeated assessments over longer periods of time are useful. Rapidly evolving symptoms should trigger urgent investigation for CNS opportunistic infection, CD8 encephalitis, fulminant presentations of symptomatic CSF HIV RNA escape, or neurological disorders unrelated to HIV infection49,55.
Discussion and potential limitations
Our recommendations differ from the HAND criteria in two main ways: first, by distinguishing HABI as a separate entity from all-cause cognitive impairment, and second, by recommending a clinical assessment for a label of cognitive impairment to be applied. Although perhaps not as appealingly simple as the HAND classification, our approach reflects the complexity of assessing cognitive impairment in people living with HIV in the modern era. Application of this approach in clinical settings would require no additional measures beyond the current recommended standard of care. Assessment of a person suspected of having cognitive issues should, at a minimum, involve a clinical history (ideally with an observer account), backed up with a cognitive measure. Assessment for brain injury depends on locally available resources, and detailed neurological investigations are not essential for this classification.
Historically, not all research studies have collected the information necessary to diagnose cognitive impairment in this way. We feel that collecting a clinical history and objective markers of brain injury is important to conduct research with relevance to the outcomes and concerns of people living with HIV. Studies that consider individual assessment areas in isolation without a clinical history (for example, a study of cross-sectional cognitive performance or neuroimaging), could provide useful mechanistic information. However, we suggest that such studies avoid reporting rates of cognitive impairment and making assumptions about the cause of impairment. Even in advanced HIV disease, diagnostic measures interpreted without clinical context have been shown to have poor inter-rater agreement for assigning the aetiology of cognitive impairment, owing to a myriad of comorbidities100.
Although we highlight issues with the HAND criteria and their potential to overestimate prevalence, our approach should not be interpreted as implying that cognitive impairment is no longer an issue in people living with HIV. Cognitive impairment remains a vitally important complication of HIV with multiple causes and can have profound effects on many aspects of an individual’s functioning and quality of life22. Furthermore, cognitive issues could become even more important as the population of people living with HIV ages. A robust set of criteria is essential to focus research and ensure that those at risk are identified and receive the help they need.
Our proposed approach has several potential limitations. The recommendation to include a clinical assessment to determine clinically meaningful cognitive impairment can be difficult to transfer to the research environment. In many low-resource settings, standardized cognitive measures are applied by local-language-speaking research assistants who do not have sufficient medical or neuropsychology training to obtain a detailed history17. Despite this challenge, clinical assessments often form part of the inclusion criteria for studies of other diseases, including studies that involve people living with HIV in low-resource settings101. The 1991 American Academy of Neurology criteria for HIV-associated dementia and minor cognitive motor disorder required a clinical history. The criteria stated that mild cognitive deficits should be verified by a reliable history, if possible from an informant, to ensure that the timing and nature of impairment are consistent with HIV as the cause102. The 2007 HAND criteria moved away from this requirement by creating the category of ANI1. ANI was intended to represent a preclinical stage of impairment that might be amenable to treatment. However, as ANI is based on cognitive performance alone, without clinical correlates or other evidence of brain injury, the ability to reliably identify a pathological phenotype could be limited. To facilitate the collection of clinical assessments and observer accounts in research, tools are needed that can distinguish cognitive limitations from physical or mental health issues. Such tools exist for other diseases; for example, the Community Screening Instrument for Dementia includes a cognitive assessment, functional measures and an observer account, and has been used across diverse settings globally103.
Sensitivity to detect cognitive impairment on the basis of overlap of at least two areas of assessment (Recommendation 5) would differ depending on the method. For example, the percentage of individuals identified as having brain injury would vary depending on whether neuroimaging was available and, if so, whether MRI or CT was used. This limitation is not uncommon in clinical situations, where sensitivity to detect disease can be lower when access to investigative methods is limited. We propose Recommendation 5 as a pragmatic alternative to HAND that considers clinical context. As this recommendation is perhaps the most speculative of those proposed here, validation against clinical diagnosis compared with existing approaches will be important.
The term HABI has been coined to refer to a degree of brain injury as a direct result of HIV, but what constitutes sufficient injury in this context is to some extent subjective. HIV enters the brain during early stages of infection, making it difficult to exclude some level of HABI in all people living with HIV29. Furthermore, the separation between HABI and other causes of brain injury might not be completely distinct. For example, cerebrovascular disease can be exacerbated by HIV-associated endothelial dysfunction and/or ART effects104. As another example, some evidence indicates that HIV interacts with substance abuse to compound the injurious effects on the brain105. Nevertheless, we feel that distinguishing injury caused directly by HIV, classed as HABI, from indirect or combined effects, which would not be described as HABI, is useful.
Although a combination of investigations and clinical context can diagnose HABI, no single imaging or CSF biomarker has been identified for all stages and types of HABI that is robust enough for clinical use69. This lack could in part be attributable to variations in the underlying pathology in people categorized with HAND. As discussed in Recommendations 1 and 4, HAND can encompass a mixture of different pathologies and include individuals without true cognitive impairment. As a result, any genuine association between biomarkers and HABI-related cognitive impairment could be diluted by the inclusion of individuals without HABI. Conceptual separation of HABI from low cognitive performance is intended to improve clarity in this area. Notably, this issue is not unique to HABI: many other causes of brain injury have no definitive test and rely on clinical judgement of medical history, risk factors and investigative findings. Although not always straightforward, attempting to identify the relative contribution of HABI to cognitive impairment is important, particularly as treatment for HIV becomes more effective and widespread. This knowledge will allow people living with HIV to better understand the risk of developing cognitive impairment and enable treatments to be tailored to the cause of impairment.
Conclusions
In this Consensus Statement, we have outlined a series of recommendations for a new approach to cognitive impairment in people living with HIV. These recommendations reflect the consensus opinion of our diverse group of experts and are intended to drive discussion and debate towards the development of new criteria for cognitive impairment in people living with HIV. Our recommendations will require assessment, validation, refinement and a broader consensus within the field. More detail will be needed in several areas, including: which cognitive tools and functional scales to use in different settings; which statistical methods to apply to determine low cognitive performance; how best to obtain a history of cognitive symptoms; how to determine severity of cognitive impairment; and how to interpret investigations of HABI. Overall, our approach is intended to better represent the changing profile of cognitive impairment in people living with HIV in diverse global settings and to provide a clearer framework of classification for clinical management and research studies.
References
Antinori, A. et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799 (2007).
Wang, Y. et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95, e2610–e2621 (2020).
Frescura, L. et al. Achieving the 95 95 95 targets for all: a pathway to ending AIDS. PLoS ONE 17, e0272405 (2022).
van Sighem, A. I. et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24, 1527–1535 (2010).
Torti, C., Foca, E., Cesana, B. M. & Lescure, F. X. Asymptomatic neurocognitive disorders in patients infected by HIV: fact or fiction? BMC Med. 9, 138 (2011).
Ciccarelli, N. Considerations on nosology for HIV-associated neurocognitive disorders: it is time to update? Infection 48, 37–42 (2020).
Nightingale, S. et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 13, 1139–1151 (2014).
Underwood, J. et al. Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS ONE 13, e0194760 (2018).
Meyer, A. C., Boscardin, W. J., Kwasa, J. K. & Price, R. W. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology 41, 208–216 (2013).
Gisslen, M., Price, R. W. & Nilsson, S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect. Dis. 11, 356 (2011).
Nightingale, S., Joska, J., Winston, A., Gisslén, M. & Barber, T. Reader response: global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95, e2610–e2621 (2020).
Nightingale, S. et al. Moving on from HAND: why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin. Infect. Dis. 73, 1113–1118 (2021).
Guinosso, S. A., Johnson, S. B. & Riley, A. W. Multiple adverse experiences and child cognitive development. Pediatr. Res. 79, 220–226 (2016).
Wei, J. et al. The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front. Neurol. 11, 581346 (2020).
Clifford, D. B. HIV-associated neurocognitive disorder. Curr. Opin. Infect. Dis. 30, 117–122 (2017).
Heaton, R. K. et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75, 2087–2096 (2010).
Nyamayaro, P., Chibanda, D., Robbins, R. N., Hakim, J. & Gouse, H. Assessment of neurocognitive deficits in people living with HIV in Sub Saharan Africa: a systematic review. Clin. Neuropsychol. 33, 1–26 (2019).
Ferretti, F. et al. Cognitive impairment in a clinical setting. J. Acquir. Immune Defic. Syndr. 77, e10–e13 (2018).
Heaton, R. K. et al. Twelve-year neurocognitive decline in HIV is associated with comorbidities, not age: a CHARTER study. Brain 146, 1121–1131 (2023).
Qu, Y. et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS 36, 19–27 (2022).
Lu, J. et al. Effects of Glasgow Outcome Scale misclassification on traumatic brain injury clinical trials. J. Neurotrauma 25, 641–651 (2008).
Alford, K. et al. “A fog that impacts everything”: a qualitative study of health-related quality of life in people living with HIV who have cognitive impairment. Qual. Life Res. 31, 3019–3030 (2022).
Low, L. F. & Purwaningrum, F. Negative stereotypes, fear and social distance: a systematic review of depictions of dementia in popular culture in the context of stigma. BMC Geriatr. 20, 477 (2020).
UK Civil Aviation Authority. Infectious Diseases Guidance Material https://www.caa.co.uk/aeromedical-examiners/medical-standards/pilots/conditions/infectious-disease/infectious-diseases-guidance-material-gm/ (2023).
Winston, A. & Spudich, S. Cognitive disorders in people living with HIV. Lancet HIV 7, e504–e513 (2020).
Petersen, R. C. et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90, 126–135 (2018).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Mastrorosa, I. et al. Declining prevalence of HIV-associated neurocognitive disorders in more recent years and associated factors, in a large cohort of ART-treated HIV-infected individuals. Clin. Infect. Dis. 76, e629–e637 (2023).
Saylor, D. et al. HIV-associated neurocognitive disorder – pathogenesis and prospects for treatment. Nat. Rev. Neurol. 12, 309 (2016).
Irollo, E., Luchetta, J., Ho, C., Nash, B. & Meucci, O. Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders. Cell Mol. Life Sci. 78, 4283–4303 (2021).
Johnson, T. P. & Nath, A. Biotypes of HIV-associated neurocognitive disorders based on viral and immune pathogenesis. Curr. Opin. Infect. Dis. 35, 223–230 (2022).
Navia, B. A., Cho, E. S., Petito, C. K. & Price, R. W. The AIDS dementia complex: II. Neuropathology. Ann. Neurol. 19, 525–535 (1986).
Levine, A. J. et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol. 22, 431–441 (2016).
Rourke, S. B. et al. Asymptomatic neurocognitive impairment is a risk for symptomatic decline over a 3-year study period. AIDS 35, 63–72 (2021).
Cole, M. A. et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology 69, 2213–2220 (2007).
Cole, J. H. et al. No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin. Infect. Dis. 66, 1899–1909 (2018).
Goodkin, K. et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV 4, e411–e422 (2017).
Sanford, R. et al. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin. Infect. Dis. 67, 1697–1704 (2018).
[No authors listed] IN BRIEF: BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022. HIV Med. 23 (Suppl. 3), 3–14 (2022).
Heaton, R. K. et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16 (2011).
Cysique, L. A. & Brew, B. J. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol. Rev. 19, 169–185 (2009).
Liner, K. J. 2nd, Hall, C. D. & Robertson, K. R. Effects of antiretroviral therapy on cognitive impairment. Curr. HIV/AIDS Rep. 5, 64–71 (2008).
Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the Mind Exchange Program. Clin. Infect. Dis. 56, 1004–1017 (2013).
Nichols, S. L. Central nervous system impact of perinatally acquired HIV in adolescents and adults: an update. Curr. HIV/AIDS Rep. 19, 121–132 (2022).
Narsi, K., Tomita, A. & Ramlall, S. Neuropsychological functioning and cognitive reserve in newly HIV diagnosed antiretroviral-naive South African adults from peri-urban and informal settlements. PLoS ONE 16, e0260260 (2021).
Narsi, K., Tomita, A. & Ramlall, S. Cognitive reserve and its determinants in newly HIV diagnosed antiretroviral-naive adults from periurban and informal settlements: evidence from an HIV hyperendemic South African setting. J. Acquir. Immune Defic. Syndr. 85, 387–393 (2020).
Chan, P. & Valcour, V. Neurocognition and the aging brain in people with HIV: implications for screening. Top. Antivir. Med. 29, 423–429 (2022).
Winston, A. et al. Defining cerebrospinal fluid HIV RNA escape: editorial review AIDS. AIDS 33, S107–S111 (2019).
Ferretti, F., Gisslen, M., Cinque, P. & Price, R. W. Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr. HIV/AIDS Rep. 12, 280–288 (2015).
Kugathasan, R. et al. Diffuse white matter signal abnormalities on magnetic resonance imaging are associated with human immunodeficiency virus type 1 viral escape in the central nervous system among patients with neurological symptoms. Clin. Infect. Dis. 64, 1059–1065 (2017).
Perez-Valero, I. et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33, 475–481 (2019).
Underwood, J. & Winston, A. Guidelines for evaluation and management of cognitive disorders in HIV-positive individuals. Curr. HIV/AIDS Rep. 13, 235–240 (2016).
Lucas, S. B., Wong, K. T., Nightingale, S. & Miller, R. F. HIV-associated CD8 encephalitis: a UK case series and review of histopathologically confirmed cases. Front. Neurol. 12, 628296 (2021).
Lescure, F. X. et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin. Infect. Dis. 57, 101–108 (2013).
Johnson, T. & Nath, A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr. Opin. Neurol. 24, 284–290 (2011).
Venkataramana, A. et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67, 383–388 (2006).
Ringelstein, A. et al. Severe aseptic leucoencephalopathy as immune reconstitution inflammatory syndrome in Caucasian and African patients. AIDS 23, 1435–1437 (2009).
Chang, K. et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 34, 203–213 (2020).
Clifford, K. M. et al. Progressive brain atrophy despite persistent viral suppression in HIV patients older than 60 years. J. Acquir. Immune Defic. Syndr. 76, 289–297 (2017).
Sheppard, D. P. et al. Accelerated and accentuated neurocognitive aging in HIV infection. J. Neurovirol. 23, 492–500 (2017).
Oliveira, N. L. et al. Longitudinal 5-year prediction of cognitive impairment among men with HIV disease. AIDS 35, 889–898 (2021).
Vera, J. H. et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 86, 1425–1432 (2016).
Eden, A. et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS ONE 11, e0157160 (2016).
Hagberg, L. et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 7, 15 (2010).
Milanini, B. et al. Longitudinal brain atrophy patterns and neuropsychological performance in older adults with HIV-associated neurocognitive disorder compared with early Alzheimer’s disease. Neurobiol. Aging 82, 69–76 (2019).
McLaurin, K. A., Booze, R. M. & Mactutus, C. F. Diagnostic and prognostic biomarkers for HAND. J. Neurovirol. 25, 686–701 (2019).
Robertson, J. et al. Increased immune activation and signs of neuronal injury in HIV-negative people on preexposure prophylaxis. AIDS 35, 2129–2136 (2021).
Yilmaz, A. et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert. Rev. Mol. Diagn. 17, 761–770 (2017).
Bandera, A. et al. HIV-associated neurocognitive impairment in the modern ART era: are we close to discovering reliable biomarkers in the setting of virological suppression? Front. Aging Neurosci. 11, 187 (2019).
Ellis, R. J. et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin. Infect. Dis. 58, 1015–1022 (2014).
Decloedt, E. H. et al. Moderate to severe HIV-associated neurocognitive impairment: a randomized placebo-controlled trial of lithium. Medicine 95, e5401 (2016).
Sacktor, N. et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J. Neurovirol. 24, 16–27 (2018).
Sacktor, N. et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77, 1135–1142 (2011).
Robbins, R. N. et al. Exploring the utility of the Montreal Cognitive Assessment to detect HIV-associated neurocognitive disorder: the challenge and need for culturally valid screening tests in South Africa. Clin. Neuropsychol. 27, 437–454 (2013).
Cysique, L. A. et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J. Clin. Exp. Neuropsychol. 33, 505–522 (2011).
Robertson, K. et al. International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J. Neurovirol. 22, 472–478 (2016).
Pellowski, J. A., Kalichman, S. C., Matthews, K. A. & Adler, N. A pandemic of the poor: social disadvantage and the US HIV epidemic. Am. Psychol. 68, 197–209 (2013).
Ward-Peterson, M. et al. Using multilevel models to evaluate the influence of contextual factors on HIV/AIDS, sexually transmitted infections, and risky sexual behavior in sub-Saharan Africa: a systematic review. Ann. Epidemiol. 28, 119–134 (2018).
Dreyer, A. J. et al. Rates of cognitive impairment in a South African cohort of people with HIV: variation by definitional criteria and lack of association with neuroimaging biomarkers. J. Neurovirol. 27, 579–594 (2021).
Binder, L. M., Iverson, G. L. & Brooks, B. L. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch. Clin. Neuropsychol. 24, 31–46 (2009).
MacCallum, R. C., Zhang, S., Preacher, K. J. & Rucker, D. D. On the practice of dichotomization of quantitative variables. Psychol. Methods 7, 19–40 (2002).
Sacktor, N. et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86, 334–340 (2016).
Jak, A. J. et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17, 368–375 (2009).
Loewenstein, D. A. et al. Using different memory cutoffs to assess mild cognitive impairment. Am. J. Geriatr. Psychiatry 14, 911–919 (2006).
Schinka, J. A. et al. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am. J. Geriatr. Psychiatry 18, 684–691 (2010).
Maki, P. M. et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84, 231–240 (2015).
Watson, C. W. et al. Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychol. 38, 33–42 (2019).
Burch, L. S. et al. Socioeconomic status and treatment outcomes for individuals with HIV on antiretroviral treatment in the UK: cross-sectional and longitudinal analyses. Lancet Public Health 1, e26–e36 (2016).
Bartels, C., Wegrzyn, M., Wiedl, A., Ackermann, V. & Ehrenreich, H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 11, 118 (2010).
Jongsiriyanyong, S. & Limpawattana, P. Mild cognitive impairment in clinical practice: a review article. Am. J. Alzheimers Dis. Other Demen. 33, 500–507 (2018).
Faure-Delage, A. et al. Socio-cultural perceptions and representations of dementia in Brazzaville, Republic of Congo: the EDAC survey. Dement. Geriatr. Cogn. Dis. Extra 2, 84–96 (2012).
Mushi, D. et al. Social representation and practices related to dementia in Hai district of Tanzania. BMC Public Health 14, 260 (2014).
Cacciamani, F. et al. Awareness of cognitive decline trajectories in asymptomatic individuals at risk for AD. Alzheimers Res. Ther. 12, 129 (2020).
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 (2004).
Lukasik, K. M., Waris, O., Soveri, A., Lehtonen, M. & Laine, M. The relationship of anxiety and stress with working memory performance in a large non-depressed sample. Front. Psychol. 10, 4 (2019).
Storbeck, J. Performance costs when emotion tunes inappropriate cognitive abilities: implications for mental resources and behavior. J. Exp. Psychol. Gen. 141, 411–416 (2012).
De Francesco, D. et al. Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med. 20, 274–285 (2019).
Hammond, E. R. et al. Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms; a prospective cohort study. J. Neurovirol. 22, 479–487 (2016).
Rubin, L. H. & Maki, P. M. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr. HIV/AIDS Rep. 16, 82–95 (2019).
Woods, S. P. et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J. Clin. Exp. Neuropsychol. 26, 759–778 (2004).
Maritz, J. et al. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve 41, 599–606 (2010).
[No authors listed] Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 41, 778–785 (1991).
Joska, J. A. et al. Prevalence of HIV-1 Infection in an elderly rural population and associations with neurocognitive impairment. AIDS 33, 1765–1771 (2019).
Benjamin, L. A. et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 11, 878–890 (2012).
Chilunda, V., Calderon, T. M., Martinez-Aguado, P. & Berman, J. W. The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era. Brain Res. 1724, 146426 (2019).
Acknowledgements
The authors dedicate this manuscript to Christopher Sandford, a tireless advocate for the HIV community who died shortly before its publication. His insightful input and enthusiasm for the project were invaluable to this manuscript’s development.
Author information
Authors and Affiliations
Contributions
S.N. wrote the article. All authors contributed substantially to discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks J. McArthur, T. Barber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nightingale, S., Ances, B., Cinque, P. et al. Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nat Rev Neurol 19, 424–433 (2023). https://doi.org/10.1038/s41582-023-00813-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00813-2
This article is cited by
-
Reply to ‘Cognitive criteria in HIV: greater consensus is needed’
Nature Reviews Neurology (2024)
-
Cognitive criteria in HIV: greater consensus is needed
Nature Reviews Neurology (2024)
-
Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management
Nature Reviews Neurology (2023)