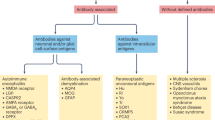
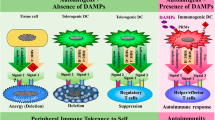
Abstract
The pathophysiology of complex neuroimmunological diseases, such as multiple sclerosis and autoimmune encephalitis, remains puzzling — various mechanisms that are difficult to dissect seem to contribute, hampering the understanding of the processes involved. Some rare neuroimmunological diseases are easier to study because their presentation and pathogenesis are more homogeneous. The investigation of these diseases can provide fundamental insights into neuroimmunological pathomechanisms that can in turn be applied to more complex diseases. In this Review, we summarize key mechanistic insights into three such rare but paradigmatic neuroimmunological diseases — Susac syndrome, Rasmussen encephalitis and narcolepsy type 1 — and consider the implications of these insights for the study of other neuroimmunological diseases. In these diseases, the combination of findings in humans, different modalities of investigation and animal models has enabled the triangulation of evidence to validate and consolidate the pathomechanistic features and to develop diagnostic and therapeutic strategies; this approach has provided insights that are directly relevant to other neuroimmunological diseases and applicable in other contexts. We also outline how next-generation technologies and refined animal models can further improve our understanding of pathomechanisms, including cell-specific and antigen-specific CNS immune responses, thereby paving the way for the development of targeted therapeutic approaches.
Key points
-
Susac syndrome, Rasmussen encephalitis and narcolepsy type 1 are more homogeneous than other more common neuroimmunological diseases and can therefore serve as paradigms for the study of fundamental neuroimmunological mechanisms.
-
The study of Susac syndrome, Rasmussen encephalitis and narcolepsy type 1 has demonstrated that cytotoxic CD8+ T cells play a major role in the pathophysiology of neuroinflammatory disease.
-
The triangulation of evidence from human and animal studies has provided insight into the pathomechanisms of Susac syndrome, Rasmussen encephalitis, and narcolepsy type 1 and could be applied to other neuroimmunological diseases.
-
Refined animal models and next-generation technologies are likely to provide further mechanistic insight into paradigmatic neuroimmunological diseases, enabling the development of innovative therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Matute-Blanch, C., Montalban, X. & Comabella, M. Multiple sclerosis, and other demyelinating and autoimmune inflammatory diseases of the central nervous system. Handb. Clin. Neurol. 146, 67–84 (2017).
Korn, T. & Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 17, 179–194 (2017).
Jarius, S., Wildemann, B. & Paul, F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 176, 149–164 (2014).
Dalmau, J. et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 7, 1091–1098 (2008).
Dalmau, J. & Graus, F. Antibody-mediated encephalitis. N. Engl. J. Med. 378, 840–851 (2018).
Lassmann, H. The changing concepts in the neuropathology of acquired demyelinating central nervous system disorders. Curr. Opin. Neurol. 32, 313–319 (2019).
Hohlfeld, R., Dornmair, K., Meinl, E. & Wekerle, H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 15, 198–209 (2016).
Hohlfeld, R., Dornmair, K., Meinl, E. & Wekerle, H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 15, 317–331 (2016).
Planas, R. et al. GDP-l-fucose synthase is a CD4+ T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci. Transl. Med. 10, eaat4301 (2018).
Jelcic, I. et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 175, 85–100.e23 (2018).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Lassmann, H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9, 3116 (2018).
Mayer, M. C. et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J. Immunol. 191, 3594–3604 (2013).
Meyer Zu Horste, G., Gross, C. C., Klotz, L., Schwab, N. & Wiendl, H. Next-generation neuroimmunology: new technologies to understand central nervous system autoimmunity. Trends Immunol. 41, 341–354 (2020).
Machado-Santos, J. et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082 (2018).
Sulzer, D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017).
Galiano-Landeira, J., Torra, A., Vila, M. & Bove, J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143, 3717–3733 (2020).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Seifert-Held, T. et al. Susac’s syndrome: clinical course and epidemiology in a Central European population. Int. J. Neurosci. 127, 776–780 (2017).
Dorr, J. et al. Characteristics of Susac syndrome: a review of all reported cases. Nat. Rev. Neurol. 9, 307–316 (2013).
Gross, C. C. et al. CD8+ T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat. Commun. 10, 5779 (2019).
Hardy, T. A. et al. Brain histopathology in three cases of Susac’s syndrome: implications for lesion pathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 86, 582–584 (2015).
Magro, C. M., Poe, J. C., Lubow, M. & Susac, J. O. Susac syndrome: an organ-specific autoimmune endotheliopathy syndrome associated with anti-endothelial cell antibodies. Am. J. Clin. Pathol. 136, 903–912 (2011).
Li, R., Patterson, K. R. & Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 19, 696–707 (2018).
Jarius, S. et al. Clinical, paraclinical and serological findings in Susac syndrome: an international multicenter study. J. Neuroinflammation 11, 46 (2014).
Varadkar, S. et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 13, 195–205 (2014).
Bien, C. G., Elger, C. E. & Wiendl, H. Advances in pathogenic concepts and therapeutic agents in Rasmussen’s encephalitis. Expert Opin. Invest. Drugs 11, 981–989 (2002).
Bien, C. G. et al. Diagnosis and staging of Rasmussen’s encephalitis by serial MRI and histopathology. Neurology 58, 250–257 (2002).
Bien, C. G. et al. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia 54, 543–550 (2013).
Andermann, F. & Farrell, K. Early onset Rasmussen’s syndrome: a malignant, often bilateral form of the disorder. Epilepsy Res. 70 (Suppl. 1), S259–262 (2006).
Villani, F. et al. Adult-onset Rasmussen’s encephalitis: anatomical-electrographic-clinical features of 7 Italian cases. Epilepsia 47 (Suppl. 5), 41–46 (2006).
Bien, C. G. et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann. Neurol. 51, 311–318 (2002).
Schwab, N. et al. CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain 132, 1236–1246 (2009).
Bauer, J. et al. Astrocytes are a specific immunological target in Rasmussen’s encephalitis. Ann. Neurol. 62, 67–80 (2007).
Schneider-Hohendorf, T. et al. CD8+ T-cell pathogenicity in Rasmussen encephalitis elucidated by large-scale T-cell receptor sequencing. Nat. Commun. 7, 11153 (2016).
Troscher, A. R. et al. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 137, 619–635 (2019).
Kebir, H. et al. Humanized mouse model of Rasmussen’s encephalitis supports the immune-mediated hypothesis. J. Clin. Invest. 128, 2000–2009 (2018).
Farrell, M. A. et al. Neuropathologic findings in cortical resections (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol. 83, 246–259 (1992).
Bien, C. G. et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128, 454–471 (2005).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Takahashi, Y., Mine, J., Kubota, Y., Yamazaki, E. & Fujiwara, T. A substantial number of Rasmussen syndrome patients have increased IgG, CD4+ T cells, TNFα, and Granzyme B in CSF. Epilepsia 50, 1419–1431 (2009).
Owens, G. C. et al. Differential expression of interferon-gamma and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J. Neuroinflammation 10, 56 (2013).
Owens, G. C. et al. Evidence for the involvement of gamma delta T cells in the immune response in Rasmussen encephalitis. J. Neuroinflammation 12, 134 (2015).
Steinman, L. Blocking immune intrusion into the brain suppresses epilepsy in Rasmussen’s encephalitis model. J. Clin. Invest. 128, 1724–1726 (2018).
Di Liberto, G. et al. Neurons under T cell attack coordinate phagocyte-mediated synaptic stripping. Cell 175, 458–471.e19 (2018).
Shah, J. R. et al. Rasmussen encephalitis associated with Parry-Romberg syndrome. Neurology 61, 395–397 (2003).
Carreno, M. et al. Parry Romberg syndrome and linear scleroderma in coup de sabre mimicking Rasmussen encephalitis. Neurology 68, 1308–1310 (2007).
Straube, A., Padovan, C. S. & Seelos, K. Parry-Romberg syndrome and Rasmussen syndrome: only an incidental similarity? Nervenarzt 72, 641–646 (2001).
Longo, D. et al. Parry-Romberg syndrome and Rasmussen encephalitis: possible association. Clinical and neuroimaging features. J. Neuroimaging 21, 188–193 (2011).
Wilson, E. H., Weninger, W. & Hunter, C. A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379 (2010).
Hall, M. A., Reid, J. L. & Lanchbury, J. S. The distribution of human TCR junctional region lengths shifts with age in both CD4 and CD8 T cells. Int. Immunol. 10, 1407–1419 (1998).
Scammell, T. E. Narcolepsy. N. Engl. J. Med. 373, 2654–2662 (2015).
Jennum, P., Ibsen, R., Knudsen, S. & Kjellberg, J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep 36, 835–840 (2013).
Peyron, C. et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997 (2000).
Thannickal, T. C. et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000).
Tafti, M. et al. DQB1 locus alone explains most of the risk and protection in narcolepsy with cataplexy in Europe. Sleep 37, 19–25 (2014).
Faraco, J. et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 9, e1003270 (2013).
Han, F. et al. HLA-DQ association and allele competition in Chinese narcolepsy. Tissue Antigens 80, 328–335 (2012).
Ollila, H. M. et al. HLA-DPB1 and HLA class I confer risk of and protection from narcolepsy. Am. J. Hum. Genet. 96, 136–146 (2015).
Han, F. et al. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS Genet. 9, e1003880 (2013).
Han, F. et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann. Neurol. 70, 410–417 (2011).
Heier, M. S. et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 14, 867–871 (2013).
Nguyen, X. H., Saoudi, A. & Liblau, R. S. Vaccine-associated inflammatory diseases of the central nervous system: from signals to causation. Curr. Opin. Neurol. 29, 362–371 (2016).
Sarkanen, T. O., Alakuijala, A. P. E., Dauvilliers, Y. A. & Partinen, M. M. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med. Rev. 38, 177–186 (2018).
Nishino, S., Ripley, B., Overeem, S., Lammers, G. J. & Mignot, E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40 (2000).
Clark, M., Kroger, C. J. & Tisch, R. M. Type 1 diabetes: a chronic anti-self-inflammatory response. Front. Immunol. 8, 1898 (2017).
Pugliese, A. Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 127, 2881–2891 (2017).
Bernard-Valnet, R. et al. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice. Proc. Natl Acad. Sci. USA 113, 10956–10961 (2016).
Dauvilliers, Y. et al. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol. 70, 1305–1310 (2013).
Pedersen, N. W. et al. CD8+ T cells from patients with narcolepsy and healthy controls recognize hypocretin neuron-specific antigens. Nat. Commun. 10, 837 (2019).
Latorre, D. et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 562, 63–68 (2018).
Hartmann, F. J. et al. High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J. Exp. Med. 213, 2621–2633 (2016).
Nguyen, X. H. et al. Circulating follicular helper T cells exhibit reduced ICOS expression and impaired function in narcolepsy type 1 patients. J. Autoimmun. 94, 134–142 (2018).
Luo, G. et al. Autoimmunity to hypocretin and molecular mimicry to flu in type 1 narcolepsy. Proc. Natl Acad. Sci. USA 115, E12323–E12332 (2018).
Ramberger, M. et al. CD4+ T-cell reactivity to orexin/hypocretin in patients with narcolepsy type 1. Sleep 40, zsw070 (2017).
Cogswell, A. C. et al. Children with narcolepsy type 1 have increased T-cell responses to orexins. Ann. Clin. Transl. Neurol. 6, 2566–2572 (2019).
Siebold, C. et al. Crystal structure of HLA-DQ0602 that protects against type 1 diabetes and confers strong susceptibility to narcolepsy. Proc. Natl Acad. Sci. USA 101, 1999–2004 (2004).
Liblau, R. S. Put to sleep by immune cells. Nature 562, 46–48 (2018).
Ahrends, T. et al. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 47, 848–861.e5 (2017).
Yshii, L. et al. IFN-γ is a therapeutic target in paraneoplastic cerebellar degeneration. JCI Insight 4, e127001 (2019).
Pardo, C. A. et al. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia 45, 516–526 (2004).
Renia, L., Grau, G. E. & Wassmer, S. C. CD8+ T cells and human cerebral malaria: a shifting episteme. J. Clin. Invest. 130, 1109–1111 (2020).
Konradt, C. et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat. Microbiol. 1, 16001 (2016).
Hasadsri, L., Lee, J., Wang, B. H., Yekkirala, L. & Wang, M. Anti-yo associated paraneoplastic cerebellar degeneration in a man with large cell cancer of the lung. Case Rep. Neurol. Med. 2013, 725936 (2013).
Fuller, C. E. in Atlas of Pediatric Brain Tumors (eds Adesina, A. M., Tihan, T., Fuller, C. E, & Poussaint, T. Y.) 303-306 (Springer International Publishing, 2016).
van der Valk, P. & Amor, S. Preactive lesions in multiple sclerosis. Curr. Opin. Neurol. 22, 207–213 (2009).
Singh, S. et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 125, 595–608 (2013).
van Horssen, J. et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J. Neuroinflammation 9, 156 (2012).
Bassetti, C. L. A. et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 15, 519–539 (2019).
Bittner, S. et al. Rasmussen encephalitis treated with natalizumab. Neurology 81, 395–397 (2013).
Friedman, H., Ch’ien, L. & Parham, D. Virus in brain of child with hemiplegia, hemiconvulsions, and epilepsy. Lancet 2, 666 (1977).
Walter, G. F. & Renella, R. R. Epstein-Barr virus in brain and Rasmussen’s encephalitis. Lancet 1, 279–280 (1989).
Jay, V. et al. Chronic encephalitis and epilepsy (Rasmussen’s encephalitis): detection of cytomegalovirus and herpes simplex virus 1 by the polymerase chain reaction and in situ hybridization. Neurology 45, 108–117 (1995).
Power, C., Poland, S. D., Blume, W. T., Girvin, J. P. & Rice, G. P. Cytomegalovirus and Rasmussen’s encephalitis. Lancet 336, 1282–1284 (1990).
Casanova, J. L. & Abel, L. Lethal infectious diseases as inborn errors of immunity: toward a synthesis of the germ and genetic theories. Annu. Rev. Pathol. 16, 23–50 (2021).
Seifinejad, A. et al. Molecular codes and in vitro generation of hypocretin and melanin concentrating hormone neurons. Proc. Natl Acad. Sci. USA 116, 17061–17070 (2019).
Beltran, E. et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J. Clin. Invest. 129, 4758–4768 (2019).
Schafflick, D. et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 11, 247 (2020).
Kula, T. et al. T-scan: a genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028.e13 (2019).
Kim, S. M. et al. Analysis of the paired TCR alpha- and beta-chains of single human T cells. PLoS One 7, e37338 (2012).
Siewert, K. et al. Unbiased identification of target antigens of CD8+ T cells with combinatorial libraries coding for short peptides. Nat. Med. 18, 824–828 (2012).
Arakawa, A. et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 212, 2203–2212 (2015).
Bahi-Buisson, N. et al. [Recent advances in pathogenic concepts and therapeutic strategies in Rasmussen’s encephalitis]. Rev. Neurol. 161, 395–405 (2005).
Agamanolis, D. P. et al. Brain microvascular pathology in Susac syndrome: an electron microscopic study of five cases. Ultrastruct. Pathol. 43, 229–236 (2019).
Kleffner, I. et al. Diagnostic criteria for Susac syndrome. J. Neurol. Neurosurg. Psychiatry 87, 1287–1295 (2016).
Petty, G. W., Matteson, E. L., Younge, B. R., McDonald, T. J. & Wood, C. P. Recurrence of Susac syndrome (retinocochleocerebral vasculopathy) after remission of 18 years. Mayo Clin. Proc. 76, 958–960 (2001).
Lucchinetti, C. F. et al. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 24, 83–97 (2014).
Hoftberger, R. et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 139, 875–892 (2020).
Kortvelyessy, P. et al. Complement-associated neuronal loss in a patient with CASPR2 antibody-associated encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2, e75 (2015).
Bien, C. G. et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135, 1622–1638 (2012).
Kuehn, J. C. et al. A 64-year-old patient with a mesiotemporal mass and symptomatic epilepsy. Brain Pathol. 30, 413–414 (2020).
Bracher, A. et al. An expanded parenchymal CD8+ T cell clone in GABAA receptor encephalitis. Ann. Clin. Transl. Neurol. 7, 239–244 (2020).
Popkirov, S. et al. Rho-associated protein kinase 2 (ROCK2): a new target of autoimmunity in paraneoplastic encephalitis. Acta Neuropathol. Commun. 5, 40 (2017).
Pitsch, J. et al. Drebrin autoantibodies in patients with seizures and suspected encephalitis. Ann. Neurol. 87, 869–884 (2020).
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Law, L. Y. et al. The spectrum of immune-mediated and inflammatory lesions of the brainstem: clues to diagnosis. Neurology 93, 390–405 (2019).
Susac, J. O. et al. MRI findings in Susac’s syndrome. Neurology 61, 1783–1787 (2003).
White, M. L., Zhang, Y. & Smoker, W. R. Evolution of lesions in Susac syndrome at serial MR imaging with diffusion-weighted imaging and apparent diffusion coefficient values. AJNR 25, 706–713 (2004).
Kleffner, I. et al. Diffusion tensor imaging demonstrates fiber impairment in Susac syndrome. Neurology 70, 1867–1869 (2008).
Susac, J. O. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology 44, 591–593 (1994).
O’Halloran, H. S., Pearson, P. A., Lee, W. B., Susac, J. O. & Berger, J. R. Microangiopathy of the brain, retina, and cochlea (Susac syndrome). A report of five cases and a review of the literature. Ophthalmology 105, 1038–1044 (1998).
Foldvary-Schaefer, N., Grigg-Damberger, M. & Mehra, R. Sleep disorders – A Case a Week from the Cleveland Clinic. 2nd Edn (Oxford University Press, 2019).
Acknowledgements
The authors thank Prof. Christian G. Bien (Epilepsy Center Bethel, Krankenhaus Mara, Bielefeld Germany) for providing an MRI scan of a patient with Rasmussen encephalitis and Prof. Brigitte Wildemann (University Hospital Heidelberg, Germany), Prof. Nicholas Schwab and Dr. Nico Melzer (both University Hospital Münster, Germany) for discussions on AQP4+ neuromyelitis optica spectrum disorders, MOG antibody-associated disease, Rasmussen encephalitis, and autoimmune encephalitis. The authors are supported by the German Research Foundation (DFG grants CRC SFB TR-128 A09 to H.W. and C.C.G., SFB1009 A03 to H.W., and GR3946-3/1 to C.C.G.) and the Interdisciplinary Center for Clinical Studies (IZKF grant Kl3/010/19 to C.C.G.), the Austrian Science Fund (FWF Project P26936-B27) to J.B., and the Narcomics ERA-Net, ImmunitySleep ANR, RHU BETPSY and ARSEP grants to R.L.
Author information
Authors and Affiliations
Contributions
H.W. provided the initial idea, outlined the content of the manuscript, wrote sections of the manuscript and designed Fig. 2. C.C.G. wrote sections of the manuscript, designed and created Box 1, Fig. 3, Fig. 4, Fig. 5, Table 1, and Supplementary Tables 1 and 2, and edited the manuscript. J.B. created Fig. 2 and Table 1 and provided histology data for Fig. 1. R.L. wrote sections of the manuscript. All authors contributed to the development of the manuscript, critically revised the manuscript, approved the final version, and are responsible for the content.
Corresponding author
Ethics declarations
Competing interests
H.W. receives honoraria for acting as a member of Scientific Advisory Boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma, and Sanofi-Aventis and receives speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche, Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma, Genzyme, Teva, and WebMD Global. He is also a paid consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis and the Swiss Multiple Sclerosis Society. His research is funded by Biogen, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi-Genzyme. C.C.G. has received speaker honoraria from Bayer Health Care, MyLan and Genzyme, and travel expenses for attending meetings from Bayer Health Care, Biogen, Euroimmun, Genzyme, MyLan and Novartis Pharma. She recieves research funding from Biogen, Roche, and Novartis. R.L. has received grant support from BMS, GlaxoSmithKline and Pierre Fabre. He has received speaker or scientific board honoraria from Biogen, Novartis, Sanofi-Genzyme, and Servier and currently has grants from ANR, ARSEP, Cancer Research Institute, French Cancer research foundation (ARC), ERA-Net Narcomics, GlaxoSmithKline, Rare Diseases Foundation, and RHU BETPSY.
Additional information
Peer review information
Nature Reviews Neurology thanks J. Kira, M. Reindl and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Public T cell clones
-
T cell clones that target a specific epitope and that are shared by different individuals.
Rights and permissions
About this article
Cite this article
Wiendl, H., Gross, C.C., Bauer, J. et al. Fundamental mechanistic insights from rare but paradigmatic neuroimmunological diseases. Nat Rev Neurol 17, 433–447 (2021). https://doi.org/10.1038/s41582-021-00496-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00496-7