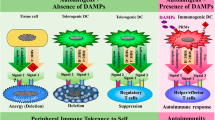
Abstract
The field of autoimmune neurology is rapidly evolving, and recent discoveries have advanced our understanding of disease aetiologies. In this article, we review the key pathogenic mechanisms underlying the development of CNS autoimmunity. First, we review non-modifiable risk factors, such as age, sex and ethnicity, as well as genetic factors such as monogenic variants, common variants in vulnerability genes and emerging HLA associations. Second, we highlight how interactions between environmental factors and epigenetics can modify disease onset and severity. Third, we review possible disease mechanisms underlying triggers that are associated with the loss of immune tolerance with consequent recognition of self-antigens; these triggers include infections, tumours and immune-checkpoint inhibitor therapies. Fourth, we outline how advances in our understanding of the anatomy of lymphatic drainage and neuroimmune interfaces are challenging long-held notions of CNS immune privilege, with direct relevance to CNS autoimmunity, and how disruption of B cell and T cell tolerance and the passage of immune cells between the peripheral and intrathecal compartments have key roles in initiating disease activity. Last, we consider novel therapeutic approaches based on our knowledge of the immunopathogenesis of autoimmune CNS disorders.
Key points
-
The immunopathogenesis of autoimmune CNS disorders involves complex interactions among a number of contributing factors.
-
Non-modifiable risk factors, such as age, sex, ethnicity and genetics (including monogenic disorders, common variants and polymorphisms in vulnerability genes, and HLA associations), might help to determine an individual’s predisposition to neurological autoimmunity.
-
Interactions between epigenetic and environmental factors can modify disease onset and severity.
-
‘Triggers’ for loss of immune tolerance, such as infections, neoplasms and iatrogenic drugs (for example, immune-checkpoint inhibitors), might precipitate neurological autoimmunity.
-
Disruption of B cell and T cell tolerance and the passage of immune cells from the peripheral circulation to the CNS have key roles in initiating disease activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pruss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 21, 798–813 (2021).
Sun, B., Ramberger, M., O’Connor, K. C., Bashford-Rogers, R. J. M. & Irani, S. R. The B cell immunobiology that underlies CNS autoantibody-mediated diseases. Nat. Rev. Neurol. 16, 481–492 (2020).
Ramanathan, S., Al-Diwani, A., Waters, P. & Irani, S. R. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J. Neurol. 268, 1689–1707 (2021).
McGinley, M. P., Goldschmidt, C. H. & Rae-Grant, A. D. Diagnosis and treatment of multiple sclerosis: a review. JAMA 325, 765–779 (2021).
Titulaer, M. J. et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165 (2013).
Florance, N. R. et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol. 66, 11–18 (2009).
Irani, S. R. et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133, 2734–2748 (2010).
Nosadini, M. et al. LGI1 and CASPR2 autoimmunity in children: systematic literature review and report of a young girl with Morvan syndrome. J. Neuroimmunol. 335, 577008 (2019).
Ramanathan, S. et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry 89, 127–137 (2018).
Simon, A. K., Hollander, G. A. & McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 282, 20143085 (2015).
Ray, D. & Yung, R. Immune senescence, epigenetics and autoimmunity. Clin. Immunol. 196, 59–63 (2018).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369 (2014).
Voskuhl, R. R. The effect of sex on multiple sclerosis risk and disease progression. Mult. Scler. 26, 554–560 (2020).
Dalmau, J. et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 18, 1045–1057 (2019).
Borisow, N. et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult. Scler. 23, 1092–1103 (2017).
Voskuhl, R. & Momtazee, C. Pregnancy: effect on multiple sclerosis, treatment considerations, and breastfeeding. Neurotherapeutics 14, 974–984 (2017).
Mousavi, M. J., Mahmoudi, M. & Ghotloo, S. Escape from X chromosome inactivation and female bias of autoimmune diseases. Mol. Med. 26, 127 (2020).
Flanagan, E. P. et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann. Neurol. 81, 298–309 (2017).
Marignier, R. et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 20, 762–772 (2021).
Jurynczyk, M. et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140, 3128–3138 (2017).
Thompson, J. et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141, 348–356 (2018).
Gadoth, A. et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann. Neurol. 82, 79–92 (2017).
Trewin, B., Freeman, I., Ramanathan, S. & Irani, S. R. Immunotherapy in autoimmune encephalitis. Curr. Opin. Neurol. 35, 399–414 (2022).
Amezcua, L. & McCauley, J. L. Race and ethnicity on MS presentation and disease course. Mult. Scler. 26, 561–567 (2020).
Flanagan, E. P. et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 79, 775–783 (2016).
Mohammad, S. S. et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev. Med. Child Neurol. 58, 376–384 (2016).
van Schaarenburg, R. A. et al. C1q deficiency and neuropsychiatric systemic lupus erythematosus. Front. Immunol. 7, 647 (2016).
Chua, G. T. et al. A case report of complement C4B deficiency in a patient with steroid and IVIG-refractory anti-NMDA receptor encephalitis. BMC Neurol. 20, 339 (2020).
Bride, K. & Teachey, D. Autoimmune lymphoproliferative syndrome: more than a FAScinating disease. F1000Res 6, 1928 (2017).
Alburaiky, S. et al. Opsoclonus-myoclonus in Aicardi-Goutieres syndrome. Dev. Med. Child Neurol. 63, 1483–1486 (2021).
Hacohen, Y., Zuberi, S., Vincent, A., Crow, Y. J. & Cordeiro, N. Neuromyelitis optica in a child with Aicardi-Goutieres syndrome. Neurology 85, 381–383 (2015).
Zhang, X. et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 517, 89–93 (2015).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Tang, X. et al. STING-associated vasculopathy with onset in infancy in three children with new clinical aspect and unsatisfactory therapeutic responses to tofacitinib. J. Clin. Immunol. 40, 114–122 (2020).
McNaughton, P. et al. Naive B cells followed by aquaporin-4 antibodies characterise the onset of neuromyelitis optica: evidence from stem cell transplantation. J. Neurol. Neurosurg. Psychiatry 93, 1234–1236 (2022).
Armangue, T. et al. Toll-like receptor 3 deficiency in autoimmune encephalitis post-herpes simplex encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 6, e611 (2019).
Nishimura, S. et al. IRAK4 deficiency presenting with anti-NMDAR encephalitis and HHV6 reactivation. J. Clin. Immunol. 41, 125–135 (2021).
International Multiple Sclerosis Genetics Consortium et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188 (2019).
Sawcer, S., Franklin, R. J. & Ban, M. Multiple sclerosis genetics. Lancet Neurol. 13, 700–709 (2014).
Acosta-Ampudia, Y., Monsalve, D. M. & Ramirez-Santana, C. Identifying the culprits in neurological autoimmune diseases. J. Transl. Autoimmun. 2, 100015 (2019).
Estrada, K. et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat. Commun. 9, 1929 (2018).
Li, T. et al. Multi-level analyses of genome-wide association study to reveal significant risk genes and pathways in neuromyelitis optica spectrum disorder. Front. Genet. 12, 690537 (2021).
Zhong, X. et al. Whole-exome sequencing reveals the major genetic factors contributing to neuromyelitis optica spectrum disorder in Chinese patients with aquaporin 4-IgG seropositivity. Eur. J. Neurol. 28, 2294–2304 (2021).
Matsushita, T. et al. Genetic factors for susceptibility to and manifestations of neuromyelitis optica. Ann. Clin. Transl. Neurol. 7, 2082–2093 (2020).
Faraco, J. et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 9, e1003270 (2013).
Schmidt, H., Williamson, D. & Ashley-Koch, A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am. J. Epidemiol. 165, 1097–1109 (2007).
Zhang, Q., Lin, C. Y., Dong, Q., Wang, J. & Wang, W. Relationship between HLA-DRB1 polymorphism and susceptibility or resistance to multiple sclerosis in Caucasians: a meta-analysis of non-family-based studies. Autoimmun. Rev. 10, 474–481 (2011).
Oksenberg, J. R. et al. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am. J. Hum. Genet. 74, 160–167 (2004).
Matsuoka, T. et al. Association of the HLA-DRB1 alleles with characteristic MRI features of Asian multiple sclerosis. Mult. Scler. 14, 1181–1190 (2008).
Wang, J. et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183, 1264–1281.e20 (2020).
Mignot, E. et al. Extensive HLA class II studies in 58 non-DRB1*15 (DR2) narcoleptic patients with cataplexy. Tissue Antigens 49, 329–341 (1997).
Mignot, E. et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am. J. Hum. Genet. 68, 686–699 (2001).
Hong, S. C. et al. DQB1*0301 and DQB1*0601 modulate narcolepsy susceptibility in Koreans. Hum. Immunol. 68, 59–68 (2007).
Hor, H. et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat. Genet. 42, 786–789 (2010).
Kim, T. J. et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann. Neurol. 81, 183–192 (2017).
Binks, S. et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 141, 2263–2271 (2018).
Muniz-Castrillo, S. et al. Clinical and prognostic value of immunogenetic characteristics in anti-LGI1 encephalitis. Neurol. Neuroimmunol. Neuroinflamm 8, e974 (2021).
Peris Sempere, V. et al. Human leukocyte antigen association study reveals DRB1*04:02 effects additional to DRB1*07:01 in anti-LGI1 encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 9, e1140 (2022).
Ramanathan, S. et al. Leucine-rich glioma-inactivated 1 versus contactin-associated protein-like 2 antibody neuropathic pain: clinical and biological comparisons. Ann. Neurol. 90, 683–690 (2021).
Muniz-Castrillo, S. et al. Anti-CASPR2 clinical phenotypes correlate with HLA and immunological features. J. Neurol. Neurosurg. Psychiatry 91, 1076–1084 (2020).
Sabater, L. et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 13, 575–586 (2014).
Honorat, J. A. et al. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol. Neuroimmunol. Neuroinflamm. 4, e385 (2017).
Cabezudo-Garcia, P., Mena-Vazquez, N., Estivill Torrus, G. & Serrano-Castro, P. Response to immunotherapy in anti-IgLON5 disease: a systematic review. Acta Neurol. Scand. 141, 263–270 (2020).
Shambrook, P. et al. Delayed benefit from aggressive immunotherapy in waxing and waning anti-IgLON5 disease. Neurol. Neuroimmunol. Neuroinflamm. 8, e1009 (2021).
Gaig, C. et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 88, 1736–1743 (2017).
Gaig, C. et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol. Neuroimmunol. Neuroinflamm. 6, e605 (2019).
Grant-Peters, M. et al. No strong HLA association with MOG antibody disease in the UK population. Ann. Clin. Transl. Neurol. 8, 1502–1507 (2021).
Olsson, T., Barcellos, L. F. & Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017).
Salzer, J. et al. Smoking as a risk factor for multiple sclerosis. Mult. Scler. 19, 1022–1027 (2013).
Simpson, S. Jr., Blizzard, L., Otahal, P., Van der Mei, I. & Taylor, B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 82, 1132–1141 (2011).
Munger, K. L., Levin, L. I., Hollis, B. W., Howard, N. S. & Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296, 2832–2838 (2006).
Bjornevik, K. et al. Sun exposure and multiple sclerosis risk in Norway and Italy: the EnvIMS study. Mult. Scler. 20, 1042–1049 (2014).
Staples, J., Ponsonby, A. L. & Lim, L. Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis. BMJ 340, c1640 (2010).
Munger, K. L., Chitnis, T. & Ascherio, A. Body size and risk of MS in two cohorts of US women. Neurology 73, 1543–1550 (2009).
Probstel, A. K. & Baranzini, S. E. The Role of the gut microbiome in multiple sclerosis risk and progression: towards characterization of the “MS microbiome”. Neurotherapeutics 15, 126–134 (2018).
Greer, J. M. & McCombe, P. A. The role of epigenetic mechanisms and processes in autoimmune disorders. Biologics 6, 307–327 (2012).
GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Kular, L. et al. DNA methylation as a mediator of HLA-DRB1*15:01 and a protective variant in multiple sclerosis. Nat. Commun. 9, 2397 (2018).
Souren, N. Y. et al. DNA methylation signatures of monozygotic twins clinically discordant for multiple sclerosis. Nat. Commun. 10, 2094 (2019).
Shimada, M., Miyagawa, T., Toyoda, H., Tokunaga, K. & Honda, M. Epigenome-wide association study of DNA methylation in narcolepsy: an integrated genetic and epigenetic approach. Sleep https://doi.org/10.1093/sleep/zsy019 (2018).
Dai, R. & Ahmed, S. A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 157, 163–179 (2011).
Keller, A. et al. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PLoS One 4, e7440 (2009).
Waschbisch, A. et al. Glatiramer acetate treatment normalizes deregulated microRNA expression in relapsing remitting multiple sclerosis. PLoS One 6, e24604 (2011).
Keller, A. et al. Next-generation sequencing identifies altered whole blood microRNAs in neuromyelitis optica spectrum disorder which may permit discrimination from multiple sclerosis. J. Neuroinflamm. 12, 196 (2015).
Vaknin-Dembinsky, A. et al. Circulating microRNAs as biomarkers for rituximab therapy, in neuromyelitis optica (NMO). J. Neuroinflamm. 13, 179 (2016).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Yuki, N., Yoshino, H., Sato, S. & Miyatake, T. Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology 40, 1900–1902 (1990).
Yuki, N. et al. A bacterium lipopolysaccharide that elicits Guillain-Barre syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178, 1771–1775 (1993).
Yuki, N. et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc. Natl Acad. Sci. USA 101, 11404–11409 (2004).
Rhodes, K. M. & Tattersfield, A. E. Guillain-Barre syndrome associated with Campylobacter infection. Br. Med. J. 285, 173–174 (1982).
Kirvan, C. A., Swedo, S. E., Heuser, J. S. & Cunningham, M. W. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 9, 914–920 (2003).
Yaddanapudi, K. et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol. Psychiatry 15, 712–726 (2010).
Brimberg, L. et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 37, 2076–2087 (2012).
Partinen, M. et al. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 13, 600–613 (2014).
Juvet, L. K., Robertson, A. H., Laake, I., Mjaaland, S. & Trogstad, L. Safety of influenza A H1N1pdm09 vaccines: an overview of systematic reviews. Front. Immunol. 12, 740048 (2021).
Szakacs, A., Darin, N. & Hallbook, T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology 80, 1315–1321 (2013).
Dauvilliers, Y. et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 136, 2486–2496 (2013).
Han, F. et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann. Neurol. 70, 410–417 (2011).
Sarkanen, T. O., Alakuijala, A. P. E., Dauvilliers, Y. A. & Partinen, M. M. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med. Rev. 38, 177–186 (2018).
Bernard-Valnet, R. et al. Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy. Brain 145, 2018–2030 (2022).
Latorre, D. et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 562, 63–68 (2018).
Handel, A. E. et al. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 5, e12496 (2010).
Belbasis, L., Bellou, V., Evangelou, E., Ioannidis, J. P. & Tzoulaki, I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 14, 263–273 (2015).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Abrahamyan, S. et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 91, 681–686 (2020).
Jacobs, B. M., Giovannoni, G., Cuzick, J. & Dobson, R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Mult. Scler. 26, 1281–1297 (2020).
Hedstrom, A. K., Lima Bomfim, I., Hillert, J., Olsson, T. & Alfredsson, L. Obesity interacts with infectious mononucleosis in risk of multiple sclerosis. Eur. J. Neurol. 22, 578–e38 (2015).
Lanz, T. V. et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327 (2022).
Pruss, H. et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann. Neurol. 72, 902–911 (2012).
Titulaer, M. J., Leypoldt, F. & Dalmau, J. Antibodies to N-methyl-D-aspartate and other synaptic receptors in choreoathetosis and relapsing symptoms post-herpes virus encephalitis. Mov. Disord. 29, 3–6 (2014).
Hacohen, Y. et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov. Disord. 29, 90–96 (2014).
Mohammad, S. S. et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2 receptor. Mov. Disord. 29, 117–122 (2014).
Armangue, T. et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 17, 760–772 (2018).
Linnoila, J. et al. Mouse model of anti-NMDA receptor post-herpes simplex encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 6, e529 (2019).
Liu, B. et al. Autoimmune encephalitis after Japanese encephalitis in children: a prospective study. J. Neurol. Sci. 424, 117394 (2021).
Dalmau, J. & Graus, F. Antibody-mediated encephalitis. N. Engl. J. Med. 378, 840–851 (2018).
Al-Diwani, A. et al. Cervical lymph nodes and ovarian teratomas as germinal centres in NMDA receptor-antibody encephalitis. Brain 145, 2742–2754 (2022).
Varrin-Doyer, M. et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 72, 53–64 (2012).
Cree, B. A., Spencer, C. M., Varrin-Doyer, M., Baranzini, S. E. & Zamvil, S. S. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann. Neurol. 80, 443–447 (2016).
Pandit, L. et al. Clostridium bolteae is elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol. Neuroimmunol. Neuroinflamm. 8, e907 (2021).
Graus, F., Elkon, K. B., Cordon-Cardo, C. & Posner, J. B. Sensory neuronopathy and small cell lung cancer. Antineuronal antibody that also reacts with the tumor. Am. J. Med. 80, 45–52 (1986).
Graus, F. et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol. Neuroimmunol. Neuroinflamm. 8, e1014 (2021).
Zekeridou, A. & Lennon, V. A. Neurologic autoimmunity in the era of checkpoint inhibitor cancer immunotherapy. Mayo Clin. Proc. 94, 1865–1878 (2019).
Dubey, D. et al. Expanded clinical phenotype, oncological associations, and immunopathologic insights of paraneoplastic Kelch-like protein-11 encephalitis. JAMA Neurol. 77, 1420–1429 (2020).
Dubey, D. et al. Amphiphysin-IgG autoimmune neuropathy: a recognizable clinicopathologic syndrome. Neurology 93, e1873–e1880 (2019).
Meglic, B., Graus, F. & Grad, A. Anti-Yo-associated paraneoplastic cerebellar degeneration in a man with gastric adenocarcinoma. J. Neurol. Sci. 185, 135–138 (2001).
Simabukuro, M. M. et al. GABAA receptor and LGI1 antibody encephalitis in a patient with thymoma. Neurol. Neuroimmunol. Neuroinflamm. 2, e73 (2015).
Makuch, M. et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann. Neurol. 83, 553–561 (2018).
Guasp, M., Modena, Y., Armangue, T., Dalmau, J. & Graus, F. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 7, e659 (2020).
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373, eabf9277 (2021).
Mayes, P. A., Hance, K. W. & Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov. 17, 509–527 (2018).
Sechi, E. et al. Neurologic autoimmunity and immune checkpoint inhibitors: autoantibody profiles and outcomes. Neurology 95, e2442–e2452 (2020).
Vogrig, A. et al. Central nervous system complications associated with immune checkpoint inhibitors. J. Neurol. Neurosurg. Psychiatry 91, 772–778 (2020).
Marini, A. et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology 96, 754–766 (2021).
Iwama, S. et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra245 (2014).
Yshii, L. M. et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 139, 2923–2934 (2016).
Mammen, A. L. et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann. Rheum. Dis. 78, 150–152 (2019).
Vogrig, A. et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 6, e604 (2019).
Chang, H. et al. HLA-B27 association of autoimmune encephalitis induced by PD-L1 inhibitor. Ann. Clin. Transl. Neurol. 7, 2243–2250 (2020).
Gerdes, L. A. et al. CTLA4 as Immunological checkpoint in the development of multiple sclerosis. Ann. Neurol. 80, 294–300 (2016).
Das, S. & Johnson, D. B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 7, 306 (2019).
Maddison, P., Newsom-Davis, J., Mills, K. R. & Souhami, R. L. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet 353, 117–118 (1999).
Wirtz, P. W. et al. P/Q-type calcium channel antibodies, Lambert-Eaton myasthenic syndrome and survival in small cell lung cancer. J. Neuroimmunol. 164, 161–165 (2005).
Rudnick, E. et al. Opsoclonus-myoclonus-ataxia syndrome in neuroblastoma: clinical outcome and antineuronal antibodies-a report from the Children’s Cancer Group Study. Med. Pediatr. Oncol. 36, 612–622 (2001).
Wenke, N. K. et al. N-methyl-D-aspartate receptor dysfunction by unmutated human antibodies against the NR1 subunit. Ann. Neurol. 85, 771–776 (2019).
Kornau, H. C. et al. Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann. Neurol. 87, 405–418 (2020).
Kowarik, M. C. et al. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations. J. Neuroinflamm. 12, 19 (2015).
Zrzavy, T. et al. Neuropathological variability within a spectrum of NMDAR-encephalitis. Ann. Neurol. 90, 725–737 (2021).
Damato, V. et al. Rituximab abrogates aquaporin-4-specific germinal center activity in patients with neuromyelitis optica spectrum disorders. Proc. Natl Acad. Sci. USA 119, e2121804119 (2022).
Irani, S. R. et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133, 1655–1667 (2010).
Ramberger, M. et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 143, 1731–1745 (2020).
Wilson, R. et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain 141, 1063–1074 (2018).
Winklmeier, S. et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol. Neuroimmunol. Neuroinflamm. 6, 625 (2019).
Cotzomi, E. et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 142, 1598–1615 (2019).
Hara, M. et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology 90, e1386–e1394 (2018).
Pruss, H. et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 75, 1735–1739 (2010).
Pruss, H. et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology 78, 1743–1753 (2012).
Burt, R. K. et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology 93, e1732–e1741 (2019).
Kothur, K. et al. Utility of CSF Cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One 11, e0161656 (2016).
Kothur, K. et al. B cell, Th17, and neutrophil related cerebrospinal fluid cytokine/chemokines are elevated in MOG antibody associated demyelination. PLoS One 11, e0149411 (2016).
Kothur, K., Wienholt, L., Brilot, F. & Dale, R. C. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 77, 227–237 (2016).
Giovannelli, J. et al. A meta-analysis comparing first-line immunosuppressants in neuromyelitis optica. Ann. Clin. Transl. Neurol. 8, 2025–2037 (2021).
Thaler, F. S. et al. Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: real-world evidence from the GENERATE Registry. Neurol. Neuroimmunol. Neuroinflamm. 8, e1088 (2021).
Nosadini, M. et al. Use and safety of immunotherapeutic management of N-methyl-d-aspartate receptor antibody encephalitis: a meta-analysis. JAMA Neurol. 78, 1333–1344 (2021).
Pittock, S. J. et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 12, 554–562 (2013).
Pittock, S. J. et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 381, 614–625 (2019).
Jun, J. S., Lee, S. T., Kim, R., Chu, K. & Lee, S. K. Tocilizumab treatment for new onset refractory status epilepticus. Ann. Neurol. 84, 940–945 (2018).
Kothur, K. et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology 90, 289–291 (2018).
Lee, W. J. et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics 13, 824–832 (2016).
Hanna, J., Hossain, G. S. & Kocerha, J. The potential for microRNA therapeutics and clinical research. Front. Genet. 10, 478 (2019).
Ramanathan, S., Dale, R. C. & Brilot, F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun. Rev. 15, 307–324 (2016).
Houzen, H. et al. Prevalence and clinical features of neuromyelitis optica spectrum disorders in northern Japan. Neurology 89, 1995–2001 (2017).
Viswanathan, S. & Wah, L. M. A nationwide epidemiological study on the prevalence of multiple sclerosis and neuromyelitis optica spectrum disorder with important multi-ethnic differences in Malaysia. Mult. Scler. 25, 1452–1461 (2019).
Simpson, A., Mowry, E. M. & Newsome, S. D. Early aggressive treatment approaches for multiple sclerosis. Curr. Treat. Options Neurol. 23, 19 (2021).
Velasco, M. et al. Effectiveness of treatments in neuromyelitis optica to modify the course of disease in adult patients. Systematic review of literature. Mult. Scler. Relat. Disord. 50, 102869 (2021).
Kim, S. H. et al. Less frequent rituximab retreatment maintains remission of neuromyelitis optica spectrum disorder, following long-term rituximab treatment. J. Neurol. Neurosurg. Psychiatry 90, 486–487 (2019).
Kim, Y. et al. Functional impairment of CD19+CD24hiCD38hi B cells in neuromyelitis optica spectrum disorder is restored by B cell depletion therapy. Sci. Transl. Med. 13, eabk2132 (2021).
Belot, A. et al. Protein kinase cdelta deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 65, 2161–2171 (2013).
Atallah, I. et al. Immune deficiency, autoimmune disease and intellectual disability: a pleiotropic disorder caused by biallelic variants in the TPP2 gene. Clin. Genet. 99, 780–788 (2021).
Briggs, T. A. et al. Spondyloenchondrodysplasia due to mutations in ACP5: a comprehensive survey. J. Clin. Immunol. 36, 220–234 (2016).
Toubiana, J. et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 127, 3154–3164 (2016).
Schuh, E. et al. Expanding spectrum of neurologic manifestations in patients with NLRP3 low-penetrance mutations. Neurol. Neuroimmunol. Neuroinflamm. 2, e109 (2015).
Zhou, Q. et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 (2016).
Duan, R. et al. A De novo frameshift mutation in TNFAIP3 impairs A20 deubiquitination function to cause neuropsychiatric systemic lupus erythematosus. J. Clin. Immunol. 39, 795–804 (2019).
Moseley, N. et al. Anti-voltage-gated potassium channel (VGKC) antibodies and acquired neuromyotonia in patients with immune dysregulation, polyendocrinopathy, enteropathy X-lined (IPEX) syndrome. J. Clin. Immunol. 41, 1972–1974 (2021).
Schindler, M. K. et al. Haploinsufficiency of immune checkpoint receptor CTLA4 induces a distinct neuroinflammatory disorder. J. Clin. Invest. 130, 5551–5561 (2020).
Kopczak, A. et al. GAD antibody-associated limbic encephalitis in a young woman with APECED. Endocrinol. Diabetes Metab. Case Rep. 2017, 17–0010 (2017).
van Sonderen, A. et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann. Neurol. 81, 193–198 (2017).
Sun, X. et al. Anti-SOX1 antibodies in paraneoplastic neurological syndrome. J. Clin. Neurol. 16, 530–546 (2020).
McKeon, A. et al. Purkinje cell cytoplasmic autoantibody type 1 accompaniments: the cerebellum and beyond. Arch. Neurol. 68, 1282–1289 (2011).
Venkatraman, A. & Opal, P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies — a review. Ann. Clin. Transl. Neurol. 3, 655–663 (2016).
Lorusso, L. et al. Immunophenotypical characterization of paraneoplastic neurological syndrome patients: a multicentric study. J. Biosci. 46, 13 (2021).
de Graaff, E. et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann. Neurol. 71, 815–824 (2012).
Dubey, D. et al. Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology 90, e103–e110 (2018).
Jitprapaikulsan, J. et al. Phenotypic presentations of paraneoplastic neuropathies associated with MAP1B-IgG. J. Neurol. Neurosurg. Psychiatry 91, 328–330 (2020).
Hammami, M. B. et al. Paraneoplastic cochleovestibulopathy: clinical presentations, oncological and serological associations. J. Neurol. Neurosurg. Psychiatry 92, 1181–1185 (2021).
Mandel-Brehm, C. et al. Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N. Engl. J. Med. 381, 47–54 (2019).
Valencia-Sanchez, C. et al. Characterisation of TRIM46 autoantibody-associated paraneoplastic neurological syndrome. J. Neurol. Neurosurg. Psychiatry 93, 196–200 (2022).
Hoftberger, R., Rosenfeld, M. R. & Dalmau, J. Update on neurological paraneoplastic syndromes. Curr. Opin. Oncol. 27, 489–495 (2015).
Simard, C. et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol. Neuroimmunol. Neuroinflamm. 7, e699 (2020).
Joubert, B. et al. Clinical spectrum of encephalitis associated with antibodies against the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor: case series and review of the literature. JAMA Neurol. 72, 1163–1169 (2015).
McKay, J. H., Dimberg, E. L. & Lopez Chiriboga, A. S. A systematic review of gamma-aminobutyric acid receptor type B autoimmunity. Neurol. Neurochir. Pol. 53, 1–7 (2019).
Spatola, M. et al. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology 90, e1964–e1972 (2018).
Zalewski, N. L. et al. P/Q- and N-type calcium-channel antibodies: oncological, neurological, and serological accompaniments. Muscle Nerve 54, 220–227 (2016).
Bost, C. et al. Malignant tumors in autoimmune encephalitis with anti-NMDA receptor antibodies. J. Neurol. 265, 2190–2200 (2018).
Christ, M. et al. Autoimmune encephalitis associated with antibodies against the metabotropic glutamate receptor type 1: case report and review of the literature. Ther. Adv. Neurol. Disord. 12, 1756286419847418 (2019).
Petit-Pedrol, M. et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 13, 276–286 (2014).
Spatola, M. et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology 88, 1012–1020 (2017).
Irani, S. R. et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann. Neurol. 72, 241–255 (2012).
Dubey, D. et al. Autoimmune GFAP astrocytopathy: prospective evaluation of 90 patients in 1 year. J. Neuroimmunol. 321, 157–163 (2018).
Budhram, A. et al. Clinical spectrum of high-titre GAD65 antibodies. J. Neurol. Neurosurg. Psychiatry 92, 645–654 (2021).
van Sonderen, A. et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87, 1449–1456 (2016).
Tobin, W. O. et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 83, 1797–1803 (2014).
Carvajal-Gonzalez, A. et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 137, 2178–2192 (2014).
Sepulveda, M. et al. Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin-4 antibodies. Mult. Scler. 24, 1753–1759 (2018).
Wildemann, B. et al. MOG-expressing teratoma followed by MOG-IgG-positive optic neuritis. Acta Neuropathol. 141, 127–131 (2021).
Gresa-Arribas, N. et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology 86, 2235–2242 (2016).
Dale, R. C. et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 135, 3453–3468 (2012).
Murphy, K. P., Kennedy, M. P., Barry, J. E., O’Regan, K. N. & Power, D. G. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol. Res. Treat. 37, 351–353 (2014).
Bukhari, W. et al. NMOSD and MS prevalence in the Indigenous populations of Australia and New Zealand. J. Neurol. 269, 836–845 (2022).
Papp, V. et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology 96, 59–77 (2021).
van Sonderen, A. et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87, 521–528 (2016).
Syrbe, S. et al. CASPR2 autoimmunity in children expanding to mild encephalopathy with hypertension. Neurology 94, e2290–e2301 (2020).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 13, 280 (2016).
Wegener-Panzer, A. et al. Clinical and imaging features of children with autoimmune encephalitis and MOG antibodies. Neurol. Neuroimmunol. Neuroinflamm. 7, e731 (2020).
Medawar, P. B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29, 58–69 (1948).
Engelhardt, B. et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 132, 317–338 (2016).
Schlager, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Carare, R. O., Hawkes, C. A. & Weller, R. O. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav. Immun. 36, 9–14 (2014).
Hughes, E. G. et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 30, 5866–5875 (2010).
Haselmann, H. et al. Human autoantibodies against the AMPA receptor subunit GluA2 induce receptor reorganization and memory dysfunction. Neuron 100, 91–105.e9 (2018).
Petit-Pedrol, M. et al. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain 141, 3144–3159 (2018).
Author information
Authors and Affiliations
Contributions
S.R. and R.C.D. researched data for the article and wrote the article. All authors contributed substantially to discussion of the content, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.R. has received research funding from the National Health and Medical Research Council (NHMRC, Australia), the Petre Foundation, the Brain Foundation (Australia), the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for Biogen, Excemed and Limbic Neurology. F.B. has received research funding from NSW Health, MS Australia, the NHMRC (Australia), the Medical Research Future Fund (Australia) and Novartis. She was on an advisory board for Novartis and Merck, and has been an invited speaker for Biogen, Novartis and Limbic Neurology. S.R.I. is supported by the Wellcome Trust (104079/Z/14/Z) and has received research funding from the UCB–Oxford University Alliance, BMA Research Grants — Vera Down (2013) and Margaret Temple (2017), Fulbright UK-US commission (MS Society research award), and Epilepsy Research UK (P1201). S.R.I. is a co-applicant on ‘Diagnostic Strategy to improve specificity of CASPR2 antibody detection’ (PCT/G82019 /051257) and receives royalties on a licenced patent application WO/210/046716 (PCT/GB2009/051441) entitled Neurological Autoimmune Disorders. S.R.I. has received honoraria from UCB, Immunovant, MedImmun, Roche, Cerebral therapeutics, ADC therapeutics, Brain and Medlink Neurology, and has received research support from CSL Behring, UCB and ONO Pharma. R.C.D. has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation and the NHMRC (Australia; Investigator Grant). He has also received honoraria from Biogen Idec as an invited speaker.
Peer review
Peer review information
Nature Reviews Neurology thanks J. Dalmau, A. McKeon and S. Vernino for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramanathan, S., Brilot, F., Irani, S.R. et al. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat Rev Neurol 19, 172–190 (2023). https://doi.org/10.1038/s41582-023-00776-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00776-4