Abstract
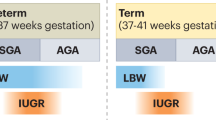
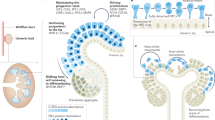
Low nephron number — resulting, for example, from prematurity or developmental anomalies — is a risk factor for the development of hypertension, chronic kidney disease and kidney failure. Considerable interest therefore exists in the mechanisms that regulate nephron endowment and contribute to the premature cessation of nephrogenesis following preterm birth. The cessation of nephrogenesis in utero or shortly after birth is synchronized across multiple niches in all mammals, and is coupled with the exhaustion of nephron progenitor cells. Consequently, no nephrons are formed after the cessation of developmental nephrogenesis, and lifelong renal function therefore depends on the complement of nephrons generated during gestation. In humans, a tenfold variation in nephron endowment between individuals contributes to differences in susceptibility to kidney disease; however, the mechanisms underlying this variation are not yet clear. Salient advances in our understanding of environmental inputs, and of intrinsic molecular mechanisms that contribute to the regulation of cessation timing or nephron progenitor cell exhaustion, have the potential to inform interventions to enhance nephron endowment and improve lifelong kidney health for susceptible individuals.
Key points
-
Nephron endowment in humans varies more than tenfold between individuals, with consequences for susceptibility to chronic kidney disease and kidney failure.
-
Nephron endowment can be reduced or increased by modulation of nutritional inputs including gestational protein, fat and vitamin intake. Adaptation to prenatal nutrition may prove detrimental when the offspring’s adult environment differs from gestational conditions.
-
Numerous genes are required for maintenance of the self-renewing population; the reduction or loss of these genes in embryonic nephron progenitor cells can result in premature depletion of this progenitor population with consequent reduced nephron numbers.
-
Nephrogenesis cessation timing in mice is determined by a community effect and is tunable by altering the perception of WNT signalling strength by nephron progenitor cells to favour self-renewal versus differentiation.
-
The twofold range in nephron number observed between mouse strains could be leveraged to identify genetic loci that regulate nephron number variation. Optimization of methods to accurately evaluate nephron numbers in vivo will be of great value to future efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Costantini, F. & Kopan, R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 (2010).
McMahon, A. P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 117, 31–64 (2016).
Little, M. H., Howden, S. E., Lawlor, K. T. & Vanslambrouck, J. M. Determining lineage relationships in kidney development and disease. Nat. Rev. Nephrol. 18, 8–21 (2022).
Short, K. M. & Smyth, I. M. Branching morphogenesis as a driver of renal development. Anat. Rec. 303, 2578–2587 (2020).
Dressler, G. R. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22, 509–529 (2006).
Oxburgh, L. Kidney nephron determination. Annu. Rev. Cell Dev. Biol. 34, 427–450 (2018).
Li, H., Hohenstein, P. & Kuure, S. Embryonic kidney development, stem cells and the origin of Wilms tumor. Genes https://doi.org/10.3390/genes12020318 (2021).
Hartman, H. A., Lai, H. L. & Patterson, L. T. Cessation of renal morphogenesis in mice. Dev. Biol. 310, 379–387 (2007).
Rumballe, B. A. et al. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev. Biol. 360, 110–122 (2011).
Al-Awqati, Q. & Goldberg, M. R. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 54, 1832–1842 (1998).
Schuh, M. P. et al. The rhesus macaque serves as a model for human lateral branch nephrogenesis. J. Am. Soc. Nephrol. 32, 1097–1112 (2021).
Short, K. M. et al. Branching morphogenesis in the developing kidney is not impacted by nephron formation or integration. Elife https://doi.org/10.7554/eLife.38992 (2018).
Cebrian, C., Borodo, K., Charles, N. & Herzlinger, D. A. Morphometric index of the developing murine kidney. Dev. Dyn. 231, 601–608 (2004).
Osathanondh, V. & Potter, E. L. Development of human kidney as shown by microdissection. V. Development of vascular pattern of glomerulus. Arch. Pathol. 82, 403–411 (1966).
Potter, E. L. Normal and Abnormal Development of the Kidney (Yearbook Medical, 1972).
Moritz, K. M., Wintour, E. M., Black, M. J., Bertram, J. F. & Caruana, G. Factors influencing mammalian kidney development: implications for health in adult life. Adv. Anat. Embryol. Cell Biol. 196, 1–78 (2008).
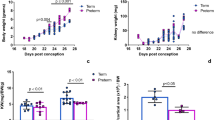
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374 (2011).
Abitbol, C. L. & Rodriguez, M. M. The long-term renal and cardiovascular consequences of prematurity. Nat. Rev. Nephrol. 8, 265–274 (2012).
Dyson, A. & Kent, A. L. The effect of preterm birth on renal development and renal health outcome. Neoreviews 20, e725–e736 (2019).
Rodriguez, M. M. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr. Dev. Pathol. 7, 17–25 (2004).
Brennan, S., Watson, D. L., Rudd, D. M. & Kandasamy, Y. Kidney growth following preterm birth: evaluation with renal parenchyma ultrasonography. Pediatr. Res. https://doi.org/10.1038/s41390-022-01970-8 (2022).
Gaitonde, D. Y., Cook, D. L. & Rivera, I. M. Chronic kidney disease: detection and evaluation. Am. Fam. Physician 96, 776–783 (2017).
Kusaba, T., Lalli, M., Kramann, R., Kobayashi, A. & Humphreys, B. D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl Acad. Sci. USA 111, 1527–1532 (2014).
Humphreys, B. D. et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl Acad. Sci. USA 108, 9226–9231 (2011).
Rinkevich, Y. et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 7, 1270–1283 (2014).
Asao, R. et al. Rac1 in podocytes promotes glomerular repair and limits the formation of sclerosis. Sci. Rep. 8, 5061 (2018).
Peired, A., Lazzeri, E., Lasagni, L. & Romagnani, P. Glomerular regeneration: when can the kidney regenerate from injury and what turns failure into success. Nephron Exp. Nephrol. 126, 70 (2014).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Prim. 3, 17088 (2017).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Puelles, V. G. et al. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 7–15 (2011).
Hoy, W. E. et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with physical and clinical characteristics and with ethnic group. Am. J. Nephrol. 33, 15–20 (2011).
Hoy, W. E. et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin. Nephrol. 74, S105–S112 (2010).
Hughson, M., Farris, A. B. III, Douglas-Denton, R., Hoy, W. E. & Bertram, J. F. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 63, 2113–2122 (2003).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Gurusinghe, S., Tambay, A. & Sethna, C. B. Developmental origins and nephron endowment in hypertension. Front. Pediatr. 5, 151 (2017).
Hoy, W. E., Hughson, M. D., Bertram, J. F., Douglas-Denton, R. & Amann, K. Nephron number, hypertension, renal disease, and renal failure. J. Am. Soc. Nephrol. 16, 2557–2564 (2005).
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237 (2020).
Calderon-Margalit, R. et al. History of childhood kidney disease and risk of adult end-stage renal disease. N. Engl. J. Med. 378, 428–438 (2018).
Leiba, A. et al. Association of adolescent hypertension with future end-stage renal disease. JAMA Intern. Med. 179, 517–523 (2019).
Alfandary, H. et al. Is the prognosis of congenital single functioning kidney benign? A population-based study. Pediatr. Nephrol. 36, 2837–2845 (2021).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Massie, A. B. et al. Quantifying postdonation risk of ESRD in living kidney donors. J. Am. Soc. Nephrol. 28, 2749–2755 (2017).
Reese, P. P., Boudville, N. & Garg, A. X. Living kidney donation: outcomes, ethics, and uncertainty. Lancet 385, 2003–2013 (2015).
Argeri, R. et al. Programmed adult kidney disease: importance of fetal environment. Front. Physiol. 11, 586290 (2020).
Charlton, J. R., Springsteen, C. H. & Carmody, J. B. Nephron number and its determinants in early life: a primer. Pediatr. Nephrol. 29, 2299–2308 (2014).
Lee, Y. Q., Collins, C. E., Gordon, A., Rae, K. M. & Pringle, K. G. The relationship between maternal nutrition during pregnancy and offspring kidney structure and function in humans: a systematic review. Nutrients https://doi.org/10.3390/nu10020241 (2018).
Schulz, L. C. The Dutch hunger winter and the developmental origins of health and disease. Proc. Natl Acad. Sci. USA 107, 16757–16758 (2010).
Painter, R. C. et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J. Am. Soc. Nephrol. 16, 189–194 (2005).
Stanner, S. A. & Yudkin, J. S. Fetal programming and the Leningrad Siege study. Twin Res. 4, 287–292 (2001).
Barker, D. J., Shiell, A. W., Barker, M. E. & Law, C. M. Growth in utero and blood pressure levels in the next generation. J. Hypertens. 18, 843–846 (2000).
Alshamrani, A. et al. Long-term but not short-term maternal fasting reduces nephron number and alters the glomerular filtration barrier in rat offspring. Life https://doi.org/10.3390/life11040318 (2021).
Awazu, M. & Hida, M. Maternal nutrient restriction inhibits ureteric bud branching but does not affect the duration of nephrogenesis in rats. Pediatr. Res. 77, 633–639 (2015).
Howell, J. J. & Manning, B. D. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 22, 94–102 (2011).
Chen, S. et al. Intrinsic age-dependent changes and cell-cell contacts regulate nephron progenitor lifespan. Dev. Cell 35, 49–62 (2015).
Volovelsky, O. et al. Hamartin regulates cessation of mouse nephrogenesis independently of Mtor. Proc. Natl Acad. Sci. USA 115, 5998–6003 (2018).
Makayes, Y. et al. Increasing mTORC1 pathway activity or methionine supplementation during pregnancy reverses the negative effect of maternal malnutrition on the developing kidney. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2020091321 (2021).
Li, J. et al. Ouabain protects against adverse developmental programming of the kidney. Nat. Commun. 1, 42 (2010).
Rabadi, M. M. et al. Maternal malnourishment induced upregulation of fetuin-B blunts nephrogenesis in the low birth weight neonate. Dev. Biol. 443, 78–91 (2018).
Yu, M. et al. Intrauterine low-protein diet disturbs metanephric gene expression and induces urinary tract developmental abnormalities in mice. Biochem. Biophys. Res. Commun. 513, 732–739 (2019).
Swali, A. et al. Cell cycle regulation and cytoskeletal remodelling are critical processes in the nutritional programming of embryonic development. PLoS One 6, e23189 (2011).
Swali, A. et al. Processes underlying the nutritional programming of embryonic development by iron deficiency in the rat. PLoS One 7, e48133 (2012).
Lelievre-Pegorier, M. et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 54, 1455–1462 (1998).
Gilbert, T. Vitamin A and kidney development. Nephrol. Dial. Transpl. 17, 78–80 (2002).
Goodyer, P. et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr. Nephrol. 22, 209–214 (2007).
Makrakis, J., Zimanyi, M. A. & Black, M. J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr. Nephrol. 22, 1861–1867 (2007).
Rosselot, C. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010).
Batourina, E. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet. 27, 74–78 (2001).
Levinson, R. & Mendelsohn, C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Semin. Cell Dev. Biol. 14, 225–231 (2003).
Sutherland, M. R., Gubhaju, L., Yoder, B. A., Stahlman, M. T. & Black, M. J. The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr. Res. 65, 397–402 (2009).
Maka, N. et al. Vitamin D deficiency during pregnancy and lactation stimulates nephrogenesis in rat offspring. Pediatr. Nephrol. 23, 55–61 (2008).
Vyas, S. P. & Goswami, R. Calcitriol and retinoic acid antagonize each other to suppress the production of IL-9 by Th9 cells. J. Nutr. Biochem. 96, 108788 (2021).
Jimenez-Lara, A. M. & Aranda, A. Interaction of vitamin D and retinoid receptors on regulation of gene expression. Horm. Res. 54, 301–305 (2000).
Hokke, S. et al. Maternal fat feeding augments offspring nephron endowment in mice. PLoS One 11, e0161578 (2016).
Boubred, F. et al. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am. J. Physiol. Renal Physiol. 293, F1944–F1949 (2007).
Belfort, M. B., Martin, C. R., Smith, V. C., Gillman, M. W. & McCormick, M. C. Infant weight gain and school-age blood pressure and cognition in former preterm infants. Pediatrics 125, e1419–e1426 (2010).
Wlodek, M. E. et al. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J. Am. Soc. Nephrol. 18, 1688–1696 (2007).
Mansano, R., Desai, M., Garg, A., Choi, G. Y. & Ross, M. G. Enhanced nephrogenesis in offspring of water-restricted rat dams. Am. J. Obstet. Gynecol. 196, 480 e481–480 e486 (2007).
Briffa, J. F., Wlodek, M. E. & Moritz, K. M. Transgenerational programming of nephron deficits and hypertension. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2018.05.025 (2018).
Harrison, M. & Langley-Evans, S. C. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br. J. Nutr. 101, 1020–1030 (2009).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child. Adolesc. Health 1, 184–194 (2017).
Askenazi, D. J., Griffin, R., McGwin, G., Carlo, W. & Ambalavanan, N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr. Nephrol. 24, 991–997 (2009).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Harer, M. W., Pope, C. F., Conaway, M. R. & Charlton, J. R. Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr. Nephrol. 32, 1067–1076 (2017).
Viswanathan, S., Manyam, B., Azhibekov, T. & Mhanna, M. M. J. Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr. Nephrol. 27, 303–311 (2012).
Koralkar, R. et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr. Res. 69, 354–358 (2011).
Menendez-Castro, C. et al. Neonatal nephron loss during active nephrogenesis — detrimental impact with long-term renal consequences. Sci. Rep. 8, 4542 (2018).
Charlton, J. R. et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr. Res. 87, 1185–1192 (2020).
Sutherland, M. R. et al. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am. J. Physiol. Renal Physiol. 302, F1286–F1292 (2012).
Lindle, K. A. et al. Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatr. Cardiol. 35, 499–506 (2014).
Yosypiv, I. V. Renin-angiotensin system in mammalian kidney development. Pediatr. Nephrol. 36, 479–489 (2021).
Tufro-McReddie, A., Romano, L. M., Harris, J. M., Ferder, L. & Gomez, R. A. Angiotensin II regulates nephrogenesis and renal vascular development. Am. J. Physiol. 269, F110–F115 (1995).
Yosypiv, I. V., Sequeira-Lopez, M. L. S., Song, R. & De Goes Martini, A. Stromal prorenin receptor is critical for normal kidney development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R640–R650 (2019).
Song, R., Kidd, L., Janssen, A. & Yosypiv, I. V. Conditional ablation of the prorenin receptor in nephron progenitor cells results in developmental programming of hypertension. Physiol. Rep. 6, e13644 (2018).
McGoldrick, E., Stewart, F., Parker, R. & Dalziel, S. R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 12, CD004454 (2020).
Singh, R. R., Moritz, K. M., Bertram, J. F. & Cullen-McEwen, L. A. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am. J. Physiol. Renal Physiol. 293, F548–F554 (2007).
Aisa, M. C. et al. Renal consequences of gestational diabetes mellitus in term neonates: a multidisciplinary approach to the DOHaD perspective in the prevention and early recognition of neonates of GDM mothers at risk of hypertension and chronic renal diseases in later life. J. Clin. Med. https://doi.org/10.3390/jcm8040429 (2019).
Ding, A., Walton, S. L., Moritz, K. M. & Phillips, J. K. Impact of prenatal and postnatal maternal environment on nephron endowment, renal function and blood pressure in the Lewis polycystic kidney rat. J. Dev. Orig. Health Dis. 10, 154–163 (2019).
Hilliard, S. et al. Defining the dynamic chromatin landscape of mouse nephron progenitors. Biol. Open https://doi.org/10.1242/bio.042754 (2019).
Wanner, N. et al. DNA methyltransferase 1 controls nephron progenitor cell renewal and differentiation. J. Am. Soc. Nephrol. 30, 63–78 (2019).
Zhang, L. et al. EED, a member of the polycomb group, is required for nephron differentiation and the maintenance of nephron progenitor cells. Development https://doi.org/10.1242/dev.157149 (2018).
Liu, H. et al. The polycomb proteins EZH1 and EZH2 co-regulate chromatin accessibility and nephron progenitor cell lifespan in mice. J. Biol. Chem. https://doi.org/10.1074/jbc.RA120.013348 (2020).
O’Brien, L. L. et al. Differential regulation of mouse and human nephron progenitors by the Six family of transcriptional regulators. Development 143, 595–608 (2016).
Li, J. et al. Chromatin remodelers interact with Eya1 and Six2 to target enhancers to control nephron progenitor cell maintenance. J. Am. Soc. Nephrol. 32, 2815–2833 (2021).
Basta, J. M. et al. The core SWI/SNF catalytic subunit Brg1 regulates nephron progenitor cell proliferation and differentiation. Dev. Biol. 464, 176–187 (2020).
Muthukrishnan, S. D., Ryzhov, S., Karolak, M. & Oxburgh, L. Nephron progenitor cell death elicits a limited compensatory response associated with interstitial expansion in the neonatal kidney. Dis. Model. Mech. https://doi.org/10.1242/dmm.030544 (2018).
Cebrian, C., Asai, N., D’Agati, V. & Costantini, F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 7, 127–137 (2014).
Short, K. M. et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 29, 188–202 (2014).
Combes, A. N., Lefevre, J. G., Wilson, S., Hamilton, N. A. & Little, M. H. Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Dev. Biol. 418, 297–306 (2016).
O’Brien, L. L. et al. Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. Elife https://doi.org/10.7554/eLife.40392 (2018).
Barak, H. et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191–1207 (2012).
Gallegos, T. F., Kamei, C. N., Rohly, M. & Drummond, I. A. Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney. Dev. Biol. 454, 44–51 (2019).
Lawlor, K. T. et al. Nephron progenitor commitment is a stochastic process influenced by cell migration. Elife https://doi.org/10.7554/eLife.41156 (2019).
Krause, M., Rak-Raszewska, A., Pietila, I., Quaggin, S. E. & Vainio, S. Signaling during kidney development. Cells 4, 112–132 (2015).
Kopan, R., Chen, S. & Little, M. Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr. Top. Dev. Biol. 107C, 293–331 (2014).
Cain, J. E., Di Giovanni, V., Smeeton, J. & Rosenblum, N. D. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr. Res. 68, 91–98 (2010).
Kuure, S. & Sariola, H. Mouse models of congenital kidney anomalies. Adv. Exp. Med. Biol. 1236, 109–136 (2020).
O’Brien, L. L. Nephron progenitor cell commitment: striking the right balance. Semin. Cell Dev. Biol. 91, 94–103 (2019).
Sauer, B. Inducible gene targeting in mice using the Cre/lox system. Methods 14, 381–392 (1998).
Huh, S. H., Warchol, M. E. & Ornitz, D. M. Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. Elife https://doi.org/10.7554/eLife.05921 (2015).
Combes, A. N. et al. Haploinsufficiency for the Six2 gene increases nephron progenitor proliferation promoting branching and nephron number. Kidney Int. 93, 589–598 (2018).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
McNeill, H. & Reginensi, A. Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J. Am. Soc. Nephrol. 28, 852–861 (2017).
Lindstrom, N. O., Carragher, N. O. & Hohenstein, P. The PI3K pathway balances self-renewal and differentiation of nephron progenitor cells through beta-catenin signaling. Stem Cell Rep. 4, 551–560 (2015).
Ihermann-Hella, A. et al. Dynamic MAPK/ERK activity sustains nephron progenitors through niche regulation and primes precursors for differentiation. Stem Cell Rep. 11, 912–928 (2018).
Costantini, F. & Shakya, R. GDNF/Ret signaling and the development of the kidney. Bioessays 28, 117–127 (2006).
Magella, B. et al. Cross-platform single cell analysis of kidney development shows stromal cells express Gdnf. Dev. Biol. 434, 36–47 (2018).
Chatterjee, R. et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum. Genet. 131, 1725–1738 (2012).
Kaczmarczyk, M. et al. Association between RET (rs1800860) and GFRA1 (rs45568534, rs8192663, rs181595401, rs7090693, and rs2694770) variants and kidney size in healthy newborns. Genet. Test. Mol. Biomark. 20, 624–628 (2016).
Hwang, D. Y. et al. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. 85, 1429–1433 (2014).
Cullen-McEwen, L. A., Kett, M. M., Dowling, J., Anderson, W. P. & Bertram, J. F. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41, 335–340 (2003).
Cullen-McEwen, L. A., Drago, J. & Bertram, J. F. Nephron endowment in glial cell line-derived neurotrophic factor (GDNF) heterozygous mice. Kidney Int. 60, 31–36 (2001).
Pichel, J. G. et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382, 73–76 (1996).
Li, H. et al. Postnatal prolongation of mammalian nephrogenesis by excess fetal GDNF. Development https://doi.org/10.1242/dev.197475 (2021).
Hatini, V., Huh, S. O., Herzlinger, D., Soares, V. C. & Lai, E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 10, 1467–1478 (1996).
Hum, S., Rymer, C., Schaefer, C., Bushnell, D. & Sims-Lucas, S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9, e88400 (2014).
Zhang, H. et al. FAT4 fine-tunes kidney development by regulating RET signaling. Dev. Cell 48, 780–792 e784 (2019).
Phelep, A. et al. MITF — a controls branching morphogenesis and nephron endowment. PLoS Genet. 13, e1007093 (2017).
Grieshammer, U. et al. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847–3857 (2005).
Huh, S. H., Ha, L. & Jang, H. S. Nephron progenitor maintenance is controlled through fibroblast growth factors and Sprouty1 interaction. J. Am. Soc. Nephrol. 31, 2559–2572 (2020).
Sharma, A. et al. FGF8 induces chemokinesis and regulates condensation of mouse nephron progenitor cells. Preprint at bioRxiv https://doi.org/10.1101/2022.02.07.478973 (2022).
Michos, O. et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 6, e1000809 (2010).
Miyamoto, R. et al. Loss of Sprouty2 partially rescues renal hypoplasia and stomach hypoganglionosis but not intestinal aganglionosis in Ret Y1062F mutant mice. Dev. Biol. 349, 160–168 (2011).
Rozen, E. J. et al. Loss of Sprouty1 rescues renal agenesis caused by Ret mutation. J. Am. Soc. Nephrol. 20, 255–259 (2009).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Ramalingam, H. et al. Disparate levels of beta-catenin activity determine nephron progenitor cell fate. Dev. Biol. 440, 13–21 (2018).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Dickinson, K. K. et al. Molecular determinants of WNT9b responsiveness in nephron progenitor cells. PLoS One 14, e0215139 (2019).
Kuure, S., Popsueva, A., Jakobson, M., Sainio, K. & Sariola, H. Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J. Am. Soc. Nephrol. 18, 1130–1139 (2007).
Guo, Q. et al. A β-catenin-driven switch in TCF/LEF transcription factor binding to DNA target sites promotes commitment of mammalian nephron progenitor cells. Elife 10, e64444 (2021).
Vidal, V. P. et al. R-spondin signalling is essential for the maintenance and differentiation of mouse nephron progenitors. Elife https://doi.org/10.7554/eLife.53895 (2020).
Lebensohn, A. M. & Rohatgi, R. R-spondins can potentiate WNT signaling without LGRs. Elife https://doi.org/10.7554/eLife.33126 (2018).
Pan, X., Karner, C. M. & Carroll, T. J. Myc cooperates with beta-catenin to drive gene expression in nephron progenitor cells. Development 144, 4173–4182 (2017).
Park, J.-S. et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell 23, 637–651 (2012).
Self, M. et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 (2006).
Jarmas, A. E., Brunskill, W. E., Chaturvedi, P., Salomonis, N. & Kopan, R. Progenitor translatome changes coordinated by Tsc1 increase perception of Wnt signals to end nephrogenesis. Nat. Commun. https://doi.org/10.1038/s41467-021-26626-9 (2021).
McCoy, K. E., Zhou, X. & Vize, P. D. Non-canonical wnt signals antagonize and canonical wnt signals promote cell proliferation in early kidney development. Dev. Dyn. 240, 1558–1566 (2011).
Chiba, T. et al. Transforming growth factor-beta1 suppresses bone morphogenetic protein-2-induced mesenchymal-epithelial transition in HSC-4 human oral squamous cell carcinoma cells via Smad1/5/9 pathway suppression. Oncol. Rep. 37, 713–720 (2017).
Kim, S. et al. Micellized protein transduction domain-bone morphogenetic protein-7 efficiently blocks renal fibrosis via inhibition of transforming growth factor-beta-mediated epithelial-mesenchymal transition. Front. Pharmacol. 11, 591275 (2020).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Fetting, J. L. et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 142, 17–27 (2014).
Brown, A. C., Muthukrishnan, S. D. & Oxburgh, L. A synthetic niche for nephron progenitor cells. Dev. Cell 34, 229–241 (2015).
Maeshima, A. et al. Number of glomeruli is increased in the kidney of transgenic mice expressing the truncated type II activin receptor. Biochem. Biophys. Res. Commun. 268, 445–449 (2000).
Sims-Lucas, S., Caruana, G., Dowling, J., Kett, M. M. & Bertram, J. F. Augmented and accelerated nephrogenesis in TGF-β2 heterozygous mutant mice. Pediatr. Res. 63, 607–612 (2008).
Walker, K. A. et al. Betaglycan is required for the establishment of nephron endowment in the mouse. PLoS One 6, e18723 (2011).
Nagalakshmi, V. K. et al. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int. 79, 317–330 (2011).
Cerqueira, D. M. et al. Bim gene dosage is critical in modulating nephron progenitor survival in the absence of microRNAs during kidney development. FASEB J. 31, 3540–3554 (2017).
Urbach, A. et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev. 28, 971–982 (2014).
Yermalovich, A. V. et al. Lin28 and let-7 regulate the timing of cessation of murine nephrogenesis. Nat. Commun. 10, 168 (2019).
Sene, L. B., Scarano, W. R., Zapparoli, A., Gontijo, J. A. R. & Boer, P. A. Impact of gestational low-protein intake on embryonic kidney microRNA expression and in nephron progenitor cells of the male fetus. PLoS One 16, e0246289 (2021).
Liu, J. et al. Regulation of nephron progenitor cell self-renewal by intermediary metabolism. J. Am. Soc. Nephrol. 28, 3323–3335 (2017).
Walton, S. L. et al. Prenatal hypoxia leads to hypertension, renal renin-angiotensin system activation and exacerbates salt-induced pathology in a sex-specific manner. Sci. Rep. 7, 8241 (2017).
Wilkinson, L. J. et al. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric beta-catenin signaling. Kidney Int. 87, 975–983 (2015).
Leelahavanichkul, A. et al. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 78, 1136–1153 (2010).
Ma, L. J. & Fogo, A. B. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 64, 350–355 (2003).
Ellis-Hutchings, R. G. et al. Altered health outcomes in adult offspring of Sprague Dawley and Wistar rats undernourished during early or late pregnancy. Birth Defects Res. B Dev. Reprod. Toxicol. 89, 396–407 (2010).
Baldelomar, E. J., Charlton, J. R., deRonde, K. A. & Bennett, K. M. In vivo measurements of kidney glomerular number and size in healthy and Os/+ mice using MRI. Am. J. Physiol. Renal Physiol. 317, F865–F873 (2019).
Baldelomar, E. J. et al. Mapping nephron mass in vivo using positron emission tomography. Am. J. Physiol. Renal Physiol. 320, F183–F192 (2021).
Eirini Papathanasiou, A. et al. Perinatal lipocalin-2 profile at the extremes of fetal growth. J. Matern. Fetal Neonatal Med. 34, 2166–2172 (2021).
Ardissino, G. et al. Serum creatinine during physiological perinatal dehydration may estimate individual nephron endowment. Eur. J. Pediatr. 177, 1383–1388 (2018).
Bagherie-Lachidan, M. et al. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 142, 2564–2573 (2015).
Das, A. et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 15, 1035–1044 (2013).
Diep, C. Q. et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470, 95–100 (2011).
Camarata, T. et al. Postembryonic nephrogenesis and persistence of Six2-expressing nephron progenitor cells in the reptilian kidney. PLoS One 11, e0153422 (2016).
Author information
Authors and Affiliations
Contributions
A.J.P. and R.K. contributed equally to all aspects of the article. M.P.S. contributed to the review of clinical topics and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Satu Kuure, Elena Torban and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Uteroplacental insufficiency
-
A condition of inadequate placental exchange of oxygen, nutrients and waste products.
- Patent ductus arteriosus
-
Abnormal persistent connection between the aorta and pulmonary artery after birth.
- Heterochronic transplantation
-
Experimental method involving co-injection of nephron progenitor cells isolated from mouse kidneys of different embryonic ages into the same young niche in kidney explants.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perl, A.J., Schuh, M.P. & Kopan, R. Regulation of nephron progenitor cell lifespan and nephron endowment. Nat Rev Nephrol 18, 683–695 (2022). https://doi.org/10.1038/s41581-022-00620-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00620-w
This article is cited by
-
Installation of the developing nephron in the fetal human kidney during advanced pregnancy
Molecular and Cellular Pediatrics (2023)
-
The impact of intrauterine growth restriction and prematurity on nephron endowment
Nature Reviews Nephrology (2023)
-
Predicting outcomes in children with congenital anomalies of the kidney and urinary tract
Pediatric Nephrology (2023)