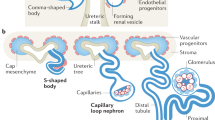
Abstract
The mechanisms underlying kidney development in mice and humans is an area of intense study. Insights into kidney organogenesis have the potential to guide our understanding of the origin of congenital anomalies and enable the assembly of genetic diagnostic tools. A number of studies have delineated signalling nodes that regulate positional identities and cell fates of nephron progenitor and precursor cells, whereas cross-species comparisons have markedly enhanced our understanding of conserved and divergent features of mammalian kidney organogenesis. Greater insights into the complex cellular movements that occur as the proximal–distal axis is established have challenged our understanding of nephron patterning and provided important clues to the elaborate developmental context in which human kidney diseases can arise. Studies of kidney development in vivo have also facilitated efforts to recapitulate nephrogenesis in kidney organoids in vitro, by providing a detailed blueprint of signalling events, cell movements and patterning mechanisms that are required for the formation of correctly patterned nephrons and maturation of physiologically functional apparatus that are responsible for maintaining human health.
Key points
-
The gradual recruitment of nephron progenitors prefigures positional identities in the developing nephron.
-
Differentiating cells positioned along the proximal–distal axis of the early nephron display characteristics of mature cell types, indicating the existence of precursor–progeny relationships.
-
The process of nephrogenesis is intricately controlled by a number of signalling pathways, including those involving Notch, WNT, BMP and FGF.
-
Nephrogenesis is highly conserved between human and mouse, indicating an ancient evolutionary origin.
-
Podocytes and epithelial tubular components of the nephron display distinct transcriptional signatures and form at different times during nephrogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schedl, A. Renal abnormalities and their developmental origin. Nat. Rev. Genet. 8, 791–802 (2007).
Short, K. M. et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 29, 188–202 (2014).
Hughson, M. D., Douglas-Denton, R., Bertram, J. F. & Hoy, W. E. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 69, 671–678 (2006).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Hoy, W. E., Hughson, M. D., Bertram, J. F., Douglas-Denton, R. & Amann, K. Nephron number, hypertension, renal disease, and renal failure. J. Am. Soc. Nephrol. 16, 2557–2564 (2005).
Luyckx, V. A., Shukha, K. & Brenner, B. M. Low nephron number and its clinical consequences. Rambam Maimonides Med. J. 2, e0061 (2011).
Luyckx, V. A. & Brenner, B. M. Clinical consequences of developmental programming of low nephron number. Anat. Rec. 303, 2613–2631 (2020).
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733 (2020).
Carney, E. F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 16, 251–251 (2020).
Saxen, L. Organogenesis of the Kidney (Cambridge Univ. Press, 1987).
Rumballe, B. A. et al. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev. Biol. 360, 110–122 (2011).
Lindström, N. O. et al. Conserved and divergent features of human and mouse kidney organogenesis. J. Am. Soc. Nephrol. 29, 785–805 (2018).
Cullen-McEwen, L., Sutherland, M. R. & Black, M. J. in Kidney Development, Disease, Repair and Regeneration (ed. Little, M.) 27–40 (Elsevier, 2016).
Jarmas, A. E., Brunskill, E. W., Chaturvedi, P., Salomonis, N. & Kopan, R. Progenitor translatome changes coordinated by Tsc1 increase perception of Wnt signals to end nephrogenesis. Nat. Commun. 12, 6332 (2021).
Davies, J. A. & Garrod, D. R. Induction of early stages of kidney tubule differentiation by lithium ions. Dev. Biol. 167, 50–60 (1995).
Moore, M. W. et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 (1996).
Barak, H. et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191–1207 (2012).
Das, A. et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 15, 1035–1044 (2013).
Brown, A. C., Muthukrishnan, S. D. & Oxburgh, L. A synthetic niche for nephron progenitor cells. Dev. Cell 34, 229–241 (2015).
Lindström, N. O., Carragher, N. O. & Hohenstein, P. The PI3K pathway balances self-renewal and differentiation of nephron progenitor cells through β-catenin signaling. Stem Cell Rep. 4, 551–560 (2015).
Mao, Y., Francis-West, P. & Irvine, K. D. Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142, 2574–2585 (2015).
McNeill, H. & Reginensi, A. Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J. Am. Soc. Nephrol. 28, 852–861 (2017).
Ihermann-Hella, A. et al. Dynamic MAPK/ERK activity sustains nephron progenitors through niche regulation and primes precursors for differentiation. Stem Cell Rep. 11, 912–928 (2018).
Ramalingam, H. et al. Disparate levels of beta-catenin activity determine nephron progenitor cell fate. Dev. Biol. 440, 13–21 (2018).
Majumdar, A., Vainio, S., Kispert, A., McMahon, J. & McMahon, A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003).
Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. & McMahon, A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Levinson, R. S. et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132, 529–539 (2005).
Oxburgh, L. et al. BMP4 substitutes for loss of BMP7 during kidney development. Dev. Biol. 286, 637–646 (2005).
Kuure, S., Popsueva, A., Jakobson, M., Sainio, K. & Sariola, H. Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J. Am. Soc. Nephrol. 18, 1130–1139 (2007).
Park, J.-S., Valerius, M. T. & McMahon, A. P. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134, 2533–2539 (2007).
Boyle, S. C., Kim, M., Valerius, M. T., McMahon, A. P. & Kopan, R. Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138, 4245–4254 (2011).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Self, M. et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 (2006).
Narlis, M., Grote, D., Gaitan, Y., Boualia, S. K. & Bouchard, M. Pax2 and Pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J. Am. Soc. Nephrol. 18, 1121–1129 (2007).
Park, J.-S. et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell 23, 637–651 (2012).
Basta, J. M., Robbins, L., Kiefer, S. M., Dorsett, D. & Rauchman, M. Sall1 balances self-renewal and differentiation of renal progenitor cells. Development 141, 1047–1058 (2014).
Xu, J., Liu, H., Park, J. S., Lan, Y. & Jiang, R. Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis. Development 141, 1442–1452 (2014).
Xu, J. et al. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev. Cell 31, 434–447 (2014).
O’Brien, L. L. et al. Differential regulation of mouse and human nephron progenitors by the six family of transcriptional regulators. Development 143, 595–608 (2016).
O’Brien, L. L. et al. Transcriptional regulatory control of mammalian nephron progenitors revealed by multi-factor cistromic analysis and genetic studies. PLoS Genet. 14, e1007181 (2018).
Guo, Q. et al. A β-catenin-driven switch in TCF/LEF transcription factor binding to DNA target sites promotes commitment of mammalian nephron progenitor cells. eLife 10, e64444 (2021).
O’Brien, L. L. Nephron progenitor cell commitment: striking the right balance. Semin. Cell Dev. Biol. 91, 94–103 (2019).
Tomita, M. et al. Bmp7 maintains undifferentiated kidney progenitor population and determines nephron numbers at birth. PLoS ONE 8, e73554 (2013).
Dudley, A. T., Lyons, K. M. & Robertson, E. J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9, 2795–2807 (1995).
Fuchs, E. & Chen, T. A matter of life and death: self-renewal in stem cells. EMBO Rep. 14, 39–48 (2013).
Chen, S. et al. Intrinsic age-dependent changes and cell-cell contacts regulate nephron progenitor lifespan. Dev. Cell 35, 49–62 (2015).
Lindström, N. O. et al. Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J. Am. Soc. Nephrol. 29, 806–824 (2018).
Boyle, S. et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234–245 (2008).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Lindström, N. O. et al. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev. Cell 45, 651–660.e4 (2018).
Menon, R. et al. Single-cell analysis of progenitor cell dynamics and lineage specification in the human fetal kidney. Development 145, dev164038 (2018).
Combes, A. N. et al. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development 146, dev178673 (2019).
Hochane, M. et al. Single-cell transcriptomics reveals gene expression dynamics of human fetal kidney development. PLoS Biol. 17, e3000152 (2019).
Tran, T. et al. In vivo developmental trajectories of human podocyte inform in vitro differentiation of pluripotent stem cell-derived podocytes. Dev. Cell 50, 102–116.e6 (2019).
Little, M. H. et al. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr. Patterns 7, 680–699 (2007).
Georgas, K. et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 332, 273–286 (2009).
Lindström, N. O. et al. Conserved and divergent molecular and anatomic features of human and mouse nephron patterning. J. Am. Soc. Nephrol. 29, 825–840 (2018).
Brown, A. C. et al. Role for compartmentalization in nephron progenitor differentiation. Proc. Natl Acad. Sci. USA 110, 4640–4645 (2013).
Mugford, J. W., Yu, J., Kobayashi, A. & McMahon, A. P. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev. Biol. 333, 312–323 (2009).
O’Brien, L. L. et al. Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. eLife 7, e40392 (2018).
Packard, A. et al. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev. Cell 27, 319–330 (2013).
Combes, A. N., Lefevre, J. G., Wilson, S., Hamilton, N. A. & Little, M. H. Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Dev. Biol. 418, 297–306 (2016).
Lawlor, K. T. et al. Nephron progenitor commitment is a stochastic process influenced by cell migration. eLife 8, e41156 (2019).
Carl Huber, G. On the development and shape of uriniferous tubules of certain of the higher mammals. Am. J. Anat. 4, 1–98 (1905).
Rienhoff, W. F. Development and growth of the metanephros or permanent kidney in chick embryos (eight to ten days’ incubation). Johns Hopkins Hosp. Bull. 33, 392–405 (1922).
Lindström, N. O. et al. Spatial transcriptional mapping of the human nephrogenic program. Dev. Cell 56, 2381–2398.e6 (2021).
Lindström, N. O. et al. Integrated β-catenin, BMP, PTEN, and Notch signalling patterns the nephron. eLife 3, e04000 (2015).
Miao, Z. et al. Single cell regulatory landscape of the mouse kidney highlights cellular differentiation programs and disease targets. Nat. Commun. 12, 2277 (2021).
Naganuma, H. et al. Molecular detection of maturation stages in the developing kidney. Dev. Biol. 470, 62–73 (2021).
Cannoodt, R., Saelens, W. & Saeys, Y. Computational methods for trajectory inference from single-cell transcriptomics. Eur. J. Immunol. 46, 2496–2506 (2016).
McCormick, J. A. & Ellison, D. H. Distal convoluted tubule. Compr. Physiol. 5, 45–98 (2015).
Palmer, L. G. & Frindt, G. Na+ and K+ transport by the renal connecting tubule. Curr. Opin. Nephrol. Hypertens. 16, 477–483 (2007).
Dantzler, W. H., Layton, A. T., Layton, H. E. & Pannabecker, T. L. Urine-concentrating mechanism in the inner medulla: function of the thin limbs of the loops of Henle. Clin. J. Am. Soc. Nephrol. 9, 1781–1789 (2014).
Bell, P. D., Lapointe, J. Y. & Peti-Peterdi, J. Macula densa cell signaling. Annu. Rev. Physiol. 65, 481–500 (2003).
Peti-Peterdi, J. & Harris, R. C. Macula densa sensing and signaling mechanisms of renin release. J. Am. Soc. Nephrol. 21, 1093–1096 (2010).
Sparks, M. A., Crowley, S. D., Gurley, S. B., Mirotsou, M. & Coffman, T. M. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4, 1201–1228 (2014).
Ransick, A. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev. Cell 51, 399–413.e7 (2019).
Chen, L., Chou, C.-L. & Knepper, M. A. Targeted single-cell RNA-seq identifies minority cell types of kidney distal nephron. J. Am. Soc. Nephrol. 32, 886–896 (2021).
Stark, K., Vainio, S., Vassileva, G. & McMahon, A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Tanigawa, S. et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev. Biol. 352, 58–69 (2011).
Burn, S. F. et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev. Biol. 352, 288–298 (2011).
Kobayashi, A. et al. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132, 2809–2823 (2005).
Grieshammer, U. et al. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847–3857 (2005).
Chung, E., Deacon, P., Marable, S., Shin, J. & Park, J.-S. Notch signaling promotes nephrogenesis by downregulating Six2. Development 143, 3907–3913 (2016).
Estrach, S., Ambler, C. A., Lo Celso, C., Hozumi, K. & Watt, F. M. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 (2006).
Katoh, M. & Katoh, M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 17, 681–685 (2006).
Liu, Z. et al. The extracellular domain of notch2 increases its cell-surface abundance and ligand responsiveness during kidney development. Dev. Cell 25, 585–598 (2013).
Schneider, J., Arraf, A. A., Grinstein, M., Yelin, R. & Schultheiss, T. M. Wnt signaling orients the proximal-distal axis of chick kidney nephrons. Development 142, 2686–2695 (2015).
Karner, C. M. et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 41, 793–799 (2009).
Deacon, P., Concodora, C. W., Chung, E. & Park, J.-S. β-catenin regulates the formation of multiple nephron segments in the mouse kidney. Sci. Rep. 9, 15915 (2019).
Farin, H. F. et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530, 340–343 (2016).
Sagner, A. & Briscoe, J. Establishing neuronal diversity in the spinal cord: a time and a place. Development 146, dev182154 (2019).
Heliot, C. et al. HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development 140, 873–885 (2013).
Lindner, T. H. et al. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum. Mol. Genet. 8, 2001–2008 (1999).
Weng, J. P. et al. Hepatocyte nuclear factor-1 beta (MODY5) gene mutations in Scandinavian families with early-onset diabetes or kidney disease or both. Diabetologia 43, 131–132 (2000).
Nakai, S. et al. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development 130, 4751–4759 (2003).
Massa, F. et al. Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140, 886–896 (2013).
Reggiani, L., Raciti, D., Airik, R., Kispert, A. & Brändli, A. W. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 21, 2358–2370 (2007).
Corkins, M. E. et al. A comparative study of cellular diversity between the Xenopus pronephric and mouse metanephric nephron. Preprint at bioRxiv https://doi.org/10.1101/2022.01.11.475739 (2022).
Lebel, M. et al. The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol. Cell. Biol. 23, 8216–8225 (2003).
Marneros, A. G. AP-2β/KCTD1 control distal nephron differentiation and protect against renal fibrosis. Dev. Cell 54, 348–366.e5 (2020).
Li, Y., Cheng, C. N., Verdun, V. A. & Wingert, R. A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 386, 111–122 (2014).
Grote, D., Souabni, A., Busslinger, M. & Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61 (2006).
Grote, D. et al. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 4, e1000316 (2008).
Chen, L. et al. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc. Natl Acad. Sci. USA 114, E9989–E9998 (2017).
Howden, S. E. et al. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28, 671–684.e6 (2021).
Barker, N. et al. Lgr5+ve stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2, 540–552 (2012).
Yu, J. et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161–171 (2009).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Portilla, D. et al. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 69, 2194–2204 (2006).
Liu, J. et al. Renoprotective and immunomodulatory effects of GDF15 following AKI Invoked by Ischemia-reperfusion injury. J. Am. Soc. Nephrol. 31, 701–715 (2020).
Chevalier, R. L. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 311, F145–F161 (2016).
Marable, S. S., Chung, E., Adam, M., Potter, S. S. & Park, J.-S. S. Hnf4a deletion in the mouse kidney phenocopies Fanconi renotubular syndrome. JCI Insight 3, e97497 (2018).
Marable, S. S., Chung, E. & Park, J.-S. Hnf4a is required for the development of Cdh6-expressing progenitors into proximal tubules in the mouse kidney. J. Am. Soc. Nephrol. 31, 2543–2558 (2020).
Hansen, S. K. et al. Genetic evidence that HNF-1α-dependent transcriptional control of HNF-4α is essential for human pancreatic β cell function. J. Clin. Invest. 110, 827–833 (2002).
Hamilton, A. J. et al. The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a β cell phenotype. J. Med. Genet. 51, 165–169 (2014).
de Boer, I. H. et al. Rationale and design of the kidney precision medicine project. Kidney Int. 99, 498–510 (2021).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Preprint at bioRxiv https://doi.org/10.1101/2021.07.28.454201 (2021).
Tian, J. M. & Schibler, U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 5, 2225–2234 (1991).
Eeckhoute, J., Formstecher, P. & Laine, B. Hepatocyte nuclear factor 4alpha enhances the hepatocyte nuclear factor 1α-mediated activation of transcription. Nucleic Acids Res. 32, 2586–2593 (2004).
Chen, L. et al. HNF4 factors control chromatin accessibility and are redundantly required for maturation of the fetal intestine. Development 146, dev179432 (2019).
Dudley, A. T. & Robertson, E. J. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 208, 349–362 (1997).
Luo, G. et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820 (1995).
Blank, U. et al. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev. Biol. 8, 86 (2008).
Miyazaki, Y., Oshima, K., Fogo, A., Hogan, B. L. M. & Ichikawa, I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 105, 863–873 (2000).
Oxburgh, L., Brown, A. C., Fetting, J. & Hill, B. BMP signaling in the nephron progenitor niche. Pediatr. Nephrol. 26, 1491–1497 (2011).
Nishinakamura, R. & Sakaguchi, M. BMP signaling and its modifiers in kidney development. Pediatr. Nephrol. 29, 681–686 (2014).
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for notch1. Nat. Genet. 16, 243–251 (1997).
Spinner, N. B. et al. Jagged1 mutations in Alagille syndrome. Hum. Mutat. 17, 18–33 (2001).
Penton, A. L., Leonard, L. D. & Spinner, N. B. Notch signaling in human development and disease. Semin. Cell Dev. Biol. 23, 450–457 (2012).
Mukherjee, M., Fogarty, E., Janga, M. & Surendran, K. Notch signaling in kidney development, maintenance, and disease. Biomolecules 9, 1–23 (2019).
Duvall, K. et al. Revisiting the role of notch in nephron segmentation confirms a role for proximal fate selection during mouse and human nephrogenesis. Development 149, dev200446 (2022).
Cheng, H.-T. et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 (2007).
Chung, E., Deacon, P. & Park, J.-S. Notch is required for the formation of all nephron segments and primes nephron progenitors for differentiation. Development 144, 4530–4539 (2017).
Chen, L. & Al-Awqati, Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am. J. Physiol. Renal Physiol. 288, F939–F952 (2005).
Liu, Z. et al. The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development 142, 2452–2463 (2015).
Ong, C.-T. et al. Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J. Biol. Chem. 281, 5106–5119 (2006).
Cheng, H.-T. et al. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130, 5031–5042 (2003).
Baker, C. M., Verstuyf, A., Jensen, K. B. & Watt, F. M. Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Dev. Biol. 343, 40–50 (2010).
Sjöqvist, M. & Andersson, E. R. Do as I say, Not(ch) as I do: lateral control of cell fate. Dev. Biol. 447, 58–70 (2019).
Seymour, P. A. et al. Jag1 modulates an oscillatory Dll1-Notch-Hes1 signaling module to coordinate growth and fate of pancreatic progenitors. Dev. Cell 52, 731–747.e8 (2020).
Shimojo, H. et al. Oscillatory control of Delta-like1 in cell interactions regulates dynamic gene expression and tissue morphogenesis. Genes Dev. 30, 102–116 (2016).
Kageyama, R., Shimojo, H. & Isomura, A. Oscillatory control of Notch signaling in development. Adv. Exp. Med. Biol. 1066, 265–277 (2018).
Hicks, C. et al. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2, 515–520 (2000).
Maezawa, Y. et al. Loss of the podocyte-expressed transcription factor Tcf21/Pod1 results in podocyte differentiation defects and FSGS. J. Am. Soc. Nephrol. 25, 2459–2470 (2014).
Kann, M. et al. Genome-wide analysis of Wilms’ tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J. Am. Soc. Nephrol. 26, 2097–2104 (2015).
Moriguchi, T. et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26, 5715–5727 (2006).
Berry, R. L. et al. Deducing the stage of origin of Wilms’ tumours from a developmental series of Wt1-mutant mice. Dis. Model. Mech. 8, 903–917 (2015).
Oliver, J. Nephrons and Kidneys (Hoeber, 1968).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Freedman, B. S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Acknowledgements
The authors are grateful to all members past and present in the Lindström laboratory, and to A. McMahon for his mentorship. J.S. is funded by a T32 training grant. M.A.A. and N.O.L. are funded by the University of Southern California, USA.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Kieran Short and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Filopodia
-
Dynamic, thin cell-membrane protrusions associated with cell sensing and migration.
- Lamellipodia
-
Dynamic, broad cell-membrane protrusions associated with cell migration.
- Planar cell polarity
-
An axial dimension associated with the plane within an epithelium and elsewhere used in association with a pathway.
- Maturity-onset diabetes of the young
-
A rare form of genetically linked diabetes with an early onset.
- Chromatin immunoprecipitation
-
(ChIP). A laboratory technique used to isolate DNA bound by transcription factors and other DNA-binding proteins using antibodies against these proteins.
- Fanconi renotubular syndrome
-
(FRTS). A rare disorder associated with nephron proximal tubules and their loss of function.
- ChIP sequencing
-
A DNA sequencing analysis method for characterizing putative cis-regulatory DNA sequences bound by transcription factors and other DNA-binding proteins.
- Lateral inhibition
-
A cell signalling process whereby a cell inhibits a neighbouring cell from activating a signalling event.
- Lateral induction
-
A cell signalling process where a cell activates or allows a signal event to take place in a neighbouring cell.
Rights and permissions
About this article
Cite this article
Schnell, J., Achieng, M. & Lindström, N.O. Principles of human and mouse nephron development. Nat Rev Nephrol 18, 628–642 (2022). https://doi.org/10.1038/s41581-022-00598-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00598-5
This article is cited by
-
Installation of the developing nephron in the fetal human kidney during advanced pregnancy
Molecular and Cellular Pediatrics (2023)
-
The genetics and pathogenesis of CAKUT
Nature Reviews Nephrology (2023)
-
The impact of intrauterine growth restriction and prematurity on nephron endowment
Nature Reviews Nephrology (2023)