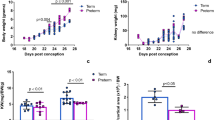
Abstract
In humans born at term, maximal nephron number is reached by the time nephrogenesis is completed — at approximately 36 weeks’ gestation. The number of nephrons does not increase further and subsequently remains stable until loss occurs through ageing or disease. Nephron endowment is key to the functional capacity of the kidney and its resilience to disease; hence, any processes that impair kidney development in the developing fetus can have lifelong adverse consequences for renal health and, consequently, for quality and length of life. The timing of nephrogenesis underlies the vulnerability of developing human kidneys to adverse early life exposures. Indeed, exposure of the developing fetus to a suboptimal intrauterine environment during gestation — resulting in intrauterine growth restriction (IUGR) — and/or preterm birth can impede kidney development and lead to reduced nephron endowment. Furthermore, emerging research suggests that IUGR and/or preterm birth is associated with an elevated risk of chronic kidney disease in later life. The available data highlight the important role of early life development in the aetiology of kidney disease and emphasize the need to develop strategies to optimize nephron endowment in IUGR and preterm infants.
Key points
-
Low birthweight, small for gestational age, intrauterine growth restriction (IUGR) and preterm birth are often overlapping terms used to describe infants that have not met their growth potential; however, it is important to recognize them as distinct conditions.
-
The majority of nephrons are formed in the third trimester; both preterm birth and IUGR — although temporally and mechanistically distinct processes — can adversely affect a considerable proportion of the nephrogenic period.
-
Strong clinical and experimental evidence indicates that a nephron deficit occurs in the kidneys of infants exposed to IUGR during development; the effect of preterm birth on nephron number is less clear.
-
Reduced nephron endowment at birth is associated with a reduction in total renal filtration surface area, and therefore diminished renal functional capacity and disease resilience across the life course.
-
A surge in epidemiological research has persuasively demonstrated a link between all-cause low birthweight, small for gestational age and preterm birth, on the one hand, and the later development of chronic kidney disease on the other.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blencowe, H. et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob. Health 7, e849–e860 (2019).
Hughes, M. M., Black, R. E. & Katz, J. 2500-g Low birth weight cutoff: history and implications for future research and policy. Matern. Child. Health J. 21, 283–289 (2017).
Hanson, M., Godfrey, K. M., Lillycrop, K. A., Burdge, G. C. & Gluckman, P. D. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog. Biophys. Mol. Biol. 106, 272–280 (2011).
Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298, 564–567 (1989).
Barker, D. J., Winter, P. D., Osmond, C., Margetts, B. & Simmonds, S. J. Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580 (1989).
Zohdi, V. et al. Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long-term renal health. Int. J. Nephrol. 2012, 1–13 (2012).
Blackmore, H. L. & Ozanne, S. E. Programming of cardiovascular disease across the life-course. J. Mol. Cell Cardiol. 83, 122–130 (2015).
Dasinger, J. H., Davis, G. K., Newsome, A. D. & Alexander, B. T. Developmental programming of hypertension. Hypertension 68, 826–831 (2016).
Bertagnolli, M., Luu, T. M., Lewandowski, A. J., Leeson, P. & Nuyt, A. M. Preterm birth and hypertension: is there a link? Curr. Hypertens. Rep. https://doi.org/10.1007/s11906-016-0637-6 (2016).
Luyckx, V. A. & Brenner, B. M. Clinical consequences of developmental programming of low nephron number. Anat. Rec. 303, 2613–2631 (2020).
Lumbers, E. R. et al. Programming of renal development and chronic disease in adult life. Front. Physiol. 11, 757 (2020).
Vanholder, R. et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin. Kidney J. 14, 1719–1730 (2021).
The Low Birth Weight and Nephron Number Working Group. The impact of kidney development on the life course: a consensus document for action. Nephron 136, 3–49 (2017).
World Health Organization. Global action plan for the prevention and control of NCDs, 2013–2020 (WHO Document Production Services, 2013).
Ingelfinger, J. R. et al. Averting the legacy of kidney disease — focus on childhood. Kidney Int. 89, 512–518 (2016).
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 384, 857–868 (2014).
Joseph, F. A. et al. New Australian birthweight centiles. Med. J. Aust. 213, 79–85 (2020).
Barros, F. C. et al. The distribution of clinical phenotypes of preterm birth syndrome. JAMA Pediatrics 169, 220 (2015).
Blencowe, H. et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health 10, S2 (2013).
Villar, J. et al. Association between preterm-birth phenotypes and differential morbidity, growth, and neurodevelopment at age 2 years. JAMA Pediatr. 175, 483 (2021).
Crump, C., Winkleby, M. A., Sundquist, J. & Sundquist, K. Prevalence of survival without major comorbidities among adults born prematurely. JAMA 322, 1580 (2019).
Moster, D., Lie, R. T. & Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 359, 262–273 (2008).
Beune, I. M. et al. Consensus based definition of growth restriction in the newborn. J. Pediatr. 196, 71–76.e71 (2018).
Zeve, D., Regelmann, M. O., Holzman, I. R. & Rapaport, R. Small at birth, but how small? The definition of SGA revisited. Horm. Res. Paediatr. 86, 357–360 (2016).
Gardosi, J., Kady, S. M., McGeown, P., Francis, A. & Tonks, A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ 331, 1113–1117 (2005).
Kristensen, S. et al. SGA subtypes and mortality risk among singleton births. Early Hum. Dev. 83, 99–105 (2007).
Katz, J. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 382, 417–425 (2013).
Lee, A. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Sharma, D., Shastri, S. & Sharma, P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. https://doi.org/10.4137/cmped.s40070 (2016).
Melamed, N. et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 152, 3–57 (2021).
Broere-Brown, Z. A., Schalekamp-Timmermans, S., Jaddoe, V. W. V. & Steegers, E. A. P. Deceleration of fetal growth rate as alternative predictor for childhood outcomes: a birth cohort study. BMC Pregnancy Childbirth 19, 216 (2019).
Sankaran, S. & Kyle, P. M. Aetiology and pathogenesis of IUGR. Best. Pract. Res. Clin. Obstet. Gynaecol. 23, 765–777 (2009).
Malhotra, A. et al. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. 10, 55 (2019).
von Beckerath, A. K. et al. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am. J. Obstet. Gynecol. 208, 130.e131–136 (2013).
Cohen, E., Wong, F. Y., Horne, R. S. C. & Yiallourou, S. R. Intrauterine growth restriction: impact on cardiovascular development and function throughout infancy. Pediatr. Res. 79, 821–830 (2016).
Cullen-McEwen, L., Sutherland, M. R. & Black, M. J. The human kidney: parallels in structure, spatial development, and timing of nephrogenesis. In Kidney Development, Disease, Repair and Regeneration (ed. Little, M. H.) 27–40 (Academic Press, 2016).
Osathanondh, V. & Potter, E. L. Development of human kidney as shown by microdissection. III. Formation and interrelationship of collecting tubules and nephrons. Arch. Pathol. 76, 290–302 (1963).
Little, M., Georgas, K., Pennisi, D. & Wilkinson, L. Kidney development: two tales of tubulogenesis. Curr. Top. Dev. Biol. 90, 193–229 (2010).
Dressler, G. R. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22, 509–529 (2006).
Schnell, J., Achieng, M. & Lindström, N. O. Principles of human and mouse nephron development. Nat. Rev. Nephrol. 18, 628–642 (2022).
Habara, K., Asakawa, M. & Ito, H. [Morphological studies on the renal papillae of the kidney in Japanese adults]. Kaibogaku Zasshi 69, 270–279 (1994).
Straus, W. L. The structure of the primate kidney. J. Anat. 69, 93–108 (1934).
Woolf, A. S., Winyard, P. J. D., Hermanns, M. H. & Welham, S. J. M. Maldevelopment of the human kidney and lower urinary tract: an overview. In The Kidney: From Normal Development to Congenital Disease (eds Vize, P. D., Woolf, A. S. & Bard, J. B. L.) 377–393 (Academic Press, 2003).
Hinchliffe, S. A., Sargent, P. H., Howard, C. V., Chan, Y. F. & van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Invest. 64, 777–784 (1991).
Ryan, D. et al. Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27, 275–283 (2018).
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374 (2011).
Rodríguez, M. M. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr. Dev. Pathol. 7, 17–25 (2004).
Stonestreet, B. S., Hansen, N. B., Laptook, A. R. & Oh, W. Glucocorticoid accelerates renal functional maturation in fetal lambs. Early Hum. Dev. 8, 331–341 (1983).
Whitsett, J. A. & Matsuzaki, Y. Transcriptional regulation of perinatal lung maturation. Pediatr. Clin. North. Am. 53, 873–887 (2006).
Gubhaju, L. et al. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am. J. Physiol. Renal Physiol. 297, F1668–F1677 (2009).
Perl, A. J., Schuh, M. P. & Kopan, R. Regulation of nephron progenitor cell lifespan and nephron endowment. Nat. Rev. Nephrol. 18, 683–695 (2022).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Zhang, Z. et al. A common RET variant is associated with reduced newborn kidney size and function. J. Am. Soc. Nephrol. 19, 2027–2034 (2008).
El Kares, R. et al. A human ALDH1A2 gene variant is associated with increased newborn kidney size and serum retinoic acid. Kidney Int. 78, 96–102 (2010).
Singh, R. R., Cuffe, J. S. & Moritz, K. M. Short- and long-term effects of exposure to natural and synthetic glucocorticoids during development. Clin. Exp. Pharmacol. Physiol. 39, 979–989 (2012).
Stelloh, C. et al. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl. Res. 159, 80–89 (2012).
Wlodek, M. E. et al. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J. Am. Soc. Nephrol. 18, 1688–1696 (2007).
Makrakis, J., Zimanyi, M. A. & Black, M. J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr. Nephrol. 22, 1861–1867 (2007).
Gray, S. P., Denton, K. M., Cullen-McEwen, L., Bertram, J. F. & Moritz, K. M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 21, 1891–1902 (2010).
Hokke, S. et al. Maternal fat feeding augments offspring nephron endowment in mice. PLoS One 11, e0161578 (2016).
Walton, S. L. et al. Prenatal hypoxia leads to hypertension, renal renin-angiotensin system activation and exacerbates salt-induced pathology in a sex-specific manner. Sci. Rep. https://doi.org/10.1038/s41598-017-08365-4 (2017).
Hinchliffe, S. A., Lynch, M. R., Sargent, P. H., Howard, C. V. & Van Velzen, D. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol. 99, 296–301 (1992).
Mañalich, R., Reyes, L., Herrera, M., Melendi, C. & Fundora, I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 58, 770–773 (2000).
Sato, A., Yamaguchi, Y., Liou, S. M., Sato, M. & Suzuki, M. Growth of the fetal kidney assessed by real-time ultrasound. Gynecol. Obstet. Invest. 20, 1–5 (1985).
Deutinger, J., Bartl, W., Pfersmann, C., Neumark, J. & Bernaschek, G. Fetal kidney volume and urine production in cases of fetal growth retardation. J. Perinat. Med. 15, 307–315 (1987).
Konje, J. C., Bell, S. C., Morton, J. J., de Chazal, R. & Taylor, D. J. Human fetal kidney morphometry during gestation and the relationship between weight, kidney morphometry and plasma active renin concentration at birth. Clin. Sci. 91, 169–175 (1996).
Konje, J. C., Okaro, C. I., Bell, S. C., De Chazal, R. & Taylor, D. J. A cross-sectional study of changes in fetal renal size with gestation in appropriate- and small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 10, 22–26 (1997).
Silver, L. E., Decamps, P. J., Korst, L. M., Platt, L. D. & Castro, L. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am. J. Obstet. Gynecol. 188, 1320–1325 (2003).
Verburg, B. O. et al. Fetal kidney volume and its association with growth and blood flow in fetal life: the Generation R Study. Kidney Int. 72, 754–761 (2007).
Senra, J. C. et al. Kidney impairment in fetal growth restriction: three-dimensional evaluation of volume and vascularization. Prenat. Diagn. 40, 1408–1417 (2020).
Sutherland, M. R., Vojisavljevic, D. & Black, M. J. A practical guide to the stereological assessment of glomerular number, size, and cellular composition. Anat. Rec. 303, 2679–2692 (2020).
Merlet-Bénichou, C., Leroy, B., Gilbert, T. & Lelièvre-Pégorier, M. Retard de croissance intra-utérin et déficit en néphrons. Méd. Sci. 9, 777–780 (1993).
Schreuder, M., Delemarre-van de Waal, H. & van Wijk, A. Consequences of intrauterine growth restriction for the kidney. Kidney Blood Press. Res. 29, 108–125 (2006).
Lucas, S. R. R., Silva, V. L. C., Miraglia, S. M. & Gil, F. Z. Functional and morphometric evaluation of offspring kidney after intrauterine undernutrition. Pediatr. Nephrol. 11, 719–723 (1997).
Zimanyi, M. A. et al. A developmental nephron deficit in rats is associated with increased susceptibility to a secondary renal injury due to advanced glycation end-products. Diabetologia 49, 801–810 (2006).
Boubred, F. et al. The magnitude of nephron number reduction mediates intrauterine growth-restriction-induced long term chronic renal disease in the rat. A comparative study in two experimental models. J. Transl. Med. 14, 331 (2016).
Gonçalves, G. D. et al. Maternal hypoxia developmentally programs low podocyte endowment in male, but not female offspring. Anat. Rec. 303, 2668–2678 (2020).
Philipson, E. H., Sokol, R. J. & Williams, T. Oligohydramnios: clinical associations and predictive value for intrauterine growth retardation. Am. J. Obstet. Gynecol. 146, 271–278 (1983).
Sutherland, M. R. et al. Renal dysfunction is already evident within the first month of life in Australian Indigenous infants born preterm. Kidney Int. 96, 1205–1216 (2019).
Heuchel, K. M. et al. Blood pressure and kidney function in neonates and young infants with intrauterine growth restriction. Pediatr. Nephrol. https://doi.org/10.1007/s00467-022-05713-z (2022).
Aly, H. et al. Renal function in small for gestational age preterm infants. J. Perinatol. 39, 1263–1267 (2019).
Zhu, J., Xing, Y. & Wang, X. L. [A preliminary study of renal function in small-for-gestational-age infants at early stage after birth]. Zhongguo Dang Dai Er Ke Za Zhi 19, 389–392 (2017).
Robinson, D., Weiner, C., Nakamura, K. & Robillard, J. Effect of intrauterine growth retardation on renal function on day one of life. Am. J. Perinatol. 7, 343–346 (1990).
Pachi, A. et al. Renal tubular damage in fetuses with intrauterine growth retardation. Fetal Diagn. Ther. 8, 109–113 (1993).
Kamianowska, M., Szczepański, M., Kulikowska, E. E., Bebko, B. & Wasilewska, A. The tubular damage markers: neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in newborns with intrauterine growth restriction. Neonatology 115, 169–174 (2019).
Chevalier, R. L. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 311, F145–F161 (2016).
Fowden, A. L., Ward, J. W. & Forhead, A. J. in Developmental Origins of Health and Disease (eds Gluckman, P. & Hanson, M.) 143–158 (Cambridge University Press, 2006).
Chevalier, R. L. Bioenergetic evolution explains prevalence of low nephron number at birth: risk factor for CKD. Kidney360 1, 863–879 (2020).
Nijland, M. J., Schlabritz-Loutsevitch, N. E., Hubbard, G. B., Nathanielsz, P. W. & Cox, L. A. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. J. Physiol. 579, 643–656 (2007).
Wang, Y.-P. et al. Effects of a restricted fetal growth environment on human kidney morphology, cell apoptosis and gene expression. J. Renin Angiotensin Aldosterone Syst. 16, 1028–1035 (2015).
Welham, S. J. M., Wade, A. & Woolf, A. S. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 61, 1231–1242 (2002).
Wilkinson, L. J. et al. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int. 87, 975–983 (2015).
Xia, S. et al. Prenatal exposure to hypoxia induced beclin 1 signaling-mediated renal autophagy and altered renal development in rat fetuses. Reprod. Sci. 22, 156–164 (2015).
Moritz, K. M. et al. Review: sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 31 (Suppl), S40–S46 (2010).
Batourina, E. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet. 27, 74–78 (2001).
Lelièvre-Pégorier, M. et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 54, 1455–1462 (1998).
Gray, S. P., Cullen-McEwen, L. A., Bertram, J. F. & Moritz, K. M. Mechanism of alcohol-induced impairment in renal development: could it be reduced by retinoic acid? Clin. Exp. Pharmacol. Physiol. 39, 807–813 (2012).
Gibson, I. W., Downie, T. T., More, I. A. & Lindop, G. B. Atubular glomeruli and glomerular cysts — a possible pathway for nephron loss in the human kidney? J. Pathol. 179, 421–426 (1996).
Marcussen, N. Tubulointerstitial damage leads to atubular glomeruli: significance and possible role in progression. Nephrol. Dial. Transplant. 15, 74–75 (2000).
Gao, Q., Lu, C., Tian, X., Zheng, J. & Ding, F. Urine podocyte mRNA loss in preterm infants and related perinatal risk factors. Pediatr. Nephrol. https://doi.org/10.1007/s00467-022-05663-6 (2022).
Ashraf, U. M., Hall, D. L., Rawls, A. Z. & Alexander, B. T. Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin. Sci. 135, 2307–2327 (2021).
Jain, V. G., Willis, K. A., Jobe, A. & Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 91, 289–296 (2022).
Galinsky, R. et al. Effect of intra-amniotic lipopolysaccharide on nephron number in preterm fetal sheep. Am. J. Physiol. Renal Physiol. 301, F280–F285 (2011).
Hoogenboom, L. A. et al. Chorioamnionitis causes kidney inflammation, podocyte damage, and pro-fibrotic changes in fetal lambs. Front. Pediatr. 10, 796702 (2022).
Sorokina, I., Ospanova, T., Myroshnychenko, M. & Korneyko, I. Macroscopic features of the kidneys of fetuses and newborns in preeclampsia: postmortem observational study. Int. J. Reprod. Biomed. 16, 115–118 (2018).
Antenatal Corticosteroid Clinical Practice Guidelines Panel. Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines (Liggins Institute, The University of Auckland, 2015).
Moritz, K. M. et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R500–R509 (2011).
Satlin, L. M., Woda, C. B. & Schwartz, G. J. in The Kidney: From Normal Development to Congenital Disease (eds Vize, P. D., Woolf, A. & Bard, J. B. L.) 267–325 (Academic Press, 2003).
Popescu, C. R. et al. Hyperoxia exposure impairs nephrogenesis in the neonatal rat: role of HIF-1α. PLoS One 8, e82421 (2013).
Sutherland, M. R., Ryan, D., Dahl, M. J., Albertine, K. H. & Black, M. J. Effects of preterm birth and ventilation on glomerular capillary growth in the neonatal lamb kidney. J. Hypertens. 34, 1988–1997 (2016).
Sutherland, M. R. et al. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am. J. Physiol. Renal Physiol. 302, F1286–F1292 (2012).
Kent, A. L. et al. Renal glomeruli and tubular injury following indomethacin, ibuprofen, and gentamicin exposure in a neonatal rat model. Pediatr. Res. 62, 307–312 (2007).
Charlton, J. R. et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr. Res. 87, 1185–1192 (2020).
Gubhaju, L. et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am. J. Physiol. Renal Physiol. 307, F149–F158 (2014).
Gallini, F., Maggio, L., Romagnoli, C., Marrocco, G. & Tortorolo, G. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr. Nephrol. 15, 119–124 (2000).
Go, H. et al. Neonatal and maternal serum creatinine levels during the early postnatal period in preterm and term infants. PLoS One 13, e0196721 (2018).
Vieux, R., Hascoet, J. M., Merdariu, D., Fresson, J. & Guillemin, F. Glomerular filtration rate reference values in very preterm infants. Pediatr 125, e1186–e1192 (2010).
Awad, H., el-Safty, I., el-Barbary, M. & Imam, S. Evaluation of renal glomerular and tubular functional and structural integrity in neonates. Am. J. Med. Sci. 324, 261–266 (2002).
Tsukahara, H. et al. Renal handling of albumin and β-2-microglobulin in neonates. Nephron 68, 212–216 (1994).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child. Adolesc. Health 1, 184–194 (2017).
Stapleton, F. B., Jones, D. P. & Green, R. S. Acute renal failure in neonates: incidence, etiology and outcome. Pediatr. Nephrol. 1, 314–320 (1987).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Mammen, C. et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am. J. Kidney Dis. 59, 523–530 (2012).
Menon, S., Kirkendall, E. S., Nguyen, H. & Goldstein, S. L. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J. Pediatr. 165, 522–527.e2 (2014).
Nenov, V. D., Taal, M. W., Sakharova, O. V. & Brenner, B. M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 9, 85–97 (2000).
Luyckx, V. A. et al. Nephron overload as a therapeutic target to maximize kidney lifespan. Nat. Rev. Nephrol. 18, 171–183 (2022).
Hoogenboom, L. A., Wolfs, T. G. A. M., Hütten, M. C., Peutz-Kootstra, C. J. & Schreuder, M. F. Prematurity, perinatal inflammatory stress, and the predisposition to develop chronic kidney disease beyond oligonephropathy. Pediatr. Nephrol. 36, 1673–1681 (2021).
Voggel, J. et al. Translational insights into mechanisms and preventative strategies after renal injury in neonates. Semin. Fetal Neonatal Med. 27, 101245 (2022).
Brenner, B. M. & Chertow, G. M. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am. J. Kidney Dis. 23, 171–175 (1994).
Fong, D., Denton, K. M., Moritz, K. M., Evans, R. & Singh, R. R. Compensatory responses to nephron deficiency: adaptive or maladaptive? Nephrology 19, 119–128 (2014).
Crump, C., Sundquist, J. & Sundquist, K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 63, 508–518 (2020).
Barker, D. J. P. et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36, 62–67 (1993).
Huxley, R. R., Shiell, A. W. & Law, C. M. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J. Hypertens. 18, 815–831 (2000).
De Jong, F., Monuteaux, M. C., Van Elburg, R. M., Gillman, M. W. & Belfort, M. B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59, 226–234 (2012).
Morrison, J. L., Duffield, J. A., Muhlhausler, B. S., Gentili, S. & McMillen, I. C. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr. Nephrol. 25, 669–677 (2010).
Abitbol, C. L. et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr. Nephrol. 24, 1363–1370 (2009).
Ou-Yang, M. C. et al. Accelerated weight gain, prematurity, and the risk of childhood obesity: a meta-analysis and systematic review. PLoS One 15, e0232238 (2020).
White, S. L. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 54, 248–261 (2009).
Gielen, M. et al. Birth weight and creatinine clearance in young adult twins: influence of genetic, prenatal, and maternal factors. J. Am. Soc. Nephrol. 16, 2471–2476 (2005).
Gjerde, A., Reisæter, A. V., Skrunes, R., Marti, H.-P. & Vikse, B. E. Intrauterine growth restriction and risk of diverse forms of kidney disease during the first 50 years of life. Clin. J. Am. Soc. Nephrol. 15, 1413–1423 (2020).
Ruggajo, P. et al. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am. J. Kidney Dis. 67, 601–608 (2016).
Vikse, B. E., Irgens, L. M., Leivestad, T., Hallan, S. & Iversen, B. M. Low birth weight increases risk for end-stage renal disease. J. Am. Soc. Nephrol. 19, 151–157 (2008).
Eriksson, J. G., Salonen, M. K., Kajantie, E. & Osmond, C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki birth cohort study, 1924–1944. Am. J. Kidney Dis. 71, 20–26 (2018).
Hirano, D. et al. Association between low birth weight and childhood-onset chronic kidney disease in Japan: a combined analysis of a nationwide survey for paediatric chronic kidney disease and the National Vital Statistics Report. Nephrol. Dial. Transpl. 31, 1895–1900 (2016).
Hsu, C. W., Yamamoto, K. T., Henry, R. K., De Roos, A. J. & Flynn, J. T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 25, 2105–2111 (2014).
Australian Institute of Health and Welfare. Cardiovascular disease, diabetes and chronic kidney disease — Australian facts: Aboriginal and Torres Strait Islander people. Series 5. Cat. CDK 5 Canberra (AIHW, 2015).
Hoy, W. E., Swanson, C. E. & Mott, S. A. Birthweight and the prevalence, progression, and incidence of CKD in a multideterminant model in a high-risk Australian Aboriginal community. Kidney Int. Rep. 6, 2782–2793 (2021).
Hoy, W. E. et al. The influence of birthweight, past poststreptococcal glomerulonephritis and current body mass index on levels of albuminuria in young adults: the multideterminant model of renal disease in a remote Australian Aboriginal population with high rates of renal disease and renal failure. Nephrol. Dial. Transpl. 31, 971–977 (2014).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Luyckx, V. A. et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390, 424–428 (2017).
Author information
Authors and Affiliations
Contributions
M.R.S. wrote the manuscript and created the figures. Both authors contributed substantially to discussion of the manuscript content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Kai-Dietrich Nüsken, Kirsty Pringle, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sutherland, M.R., Black, M.J. The impact of intrauterine growth restriction and prematurity on nephron endowment. Nat Rev Nephrol 19, 218–228 (2023). https://doi.org/10.1038/s41581-022-00668-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00668-8