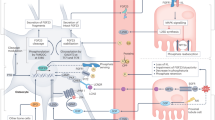
Abstract
The Klotho proteins, αKlotho and βKlotho, are essential components of endocrine fibroblast growth factor (FGF) receptor complexes, as they are required for the high-affinity binding of FGF19, FGF21 and FGF23 to their cognate FGF receptors (FGFRs). Collectively, these proteins form a unique endocrine system that governs multiple metabolic processes in mammals. FGF19 is a satiety hormone that is secreted from the intestine on ingestion of food and binds the βKlotho–FGFR4 complex in hepatocytes to promote metabolic responses to feeding. By contrast, under fasting conditions, the liver secretes the starvation hormone FGF21, which induces metabolic responses to fasting and stress responses through the activation of the hypothalamus–pituitary–adrenal axis and the sympathetic nervous system following binding to the βKlotho–FGFR1c complex in adipocytes and the suprachiasmatic nucleus, respectively. Finally, FGF23 is secreted by osteocytes in response to phosphate intake and binds to αKlotho–FGFR complexes, which are expressed most abundantly in renal tubules, to regulate mineral metabolism. Growing evidence suggests that the FGF–Klotho endocrine system also has a crucial role in the pathophysiology of ageing-related disorders, including diabetes, cancer, arteriosclerosis and chronic kidney disease. Therefore, targeting the FGF–Klotho endocrine axes might have therapeutic benefit in multiple systems; investigation of the crystal structures of FGF–Klotho–FGFR complexes is paving the way for the development of drugs that can regulate these axes.
Key points
-
The Klotho proteins αKlotho and βKlotho are essential components of endocrine fibroblast growth factor (FGF) receptor complexes, as they are required for the high-affinity binding of FGF19, FGF21 and FGF23 to their cognate FGF receptors.
-
FGF21 is a starvation hormone that induces stress responses by activating the sympathetic nervous system and the hypothalamus–pituitary–adrenal axis.
-
FGF19 is a satiety hormone that promotes metabolic responses to feeding.
-
FGF23 is a phosphaturic hormone; increased FGF23 levels in patients with early-stage chronic kidney disease or elderly individuals is indicative of excess phosphate intake relative to the residual nephron number.
-
Calciprotein particles are colloids of calcium phosphate adsorbed to fetuin A, which increase in concentration as renal function declines and that can induce innate immune responses and cell death, suggesting that they are mediators of phosphate-induced damage.
-
Solving the crystal structure of αKlotho and βKlotho will facilitate the development of agonists and antagonists of endocrine FGFs, which will be potentially useful for the treatment of various disorders, including chronic kidney disease and other ageing-related disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Notes
Ccr, creatinine clearance; Pcr, serum creatinine concentration; Pp, serum phosphate concentration; Ucr, urinary creatinine concentration; Up, urinary phosphate concentration; V, 24-hour urinary volume.
References
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997). This study reports the discovery of the αKlotho gene as a putative ‘ageing-suppressor’ gene.
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006). The first study to demonstrate that αKlotho forms complexes with FGFRs and functions as the obligate co-receptor for FGF23.
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Yu, X. et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146, 4647–4656 (2005).
Kuro-o, M. Ageing-related receptors resolved. Nature 553, 409–410 (2018).
Itoh, N. & Ornitz, D. M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121–130 (2011).
Jones, S. A. Physiology of FGF15/19. Adv. Exp. Med. Biol. 728, 171–182 (2012).
Hu, M. C., Shiizaki, K., Kuro-o, M. & Moe, O. W. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 75, 503–533 (2013).
Schlessinger, J. et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6, 743–750 (2000).
Ibrahimi, O. A. et al. Analysis of mutations in fibroblast growth factor (FGF) and a pathogenic mutation in FGF receptor (FGFR) provides direct evidence for the symmetric two-end model for FGFR dimerization. Mol. Cell. Biol. 25, 671–684 (2005).
Harmer, N. J., Pellegrini, L., Chirgadze, D., Fernandez-Recio, J. & Blundell, T. L. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry 43, 629–640 (2004).
Mohammadi, M., Olsen, S. K. & Ibrahimi, O. A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 16, 107–137 (2005).
Goetz, R. et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 27, 3417–3428 (2007).
Ito, S. et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech. Dev. 98, 115–119 (2000).
Ogawa, Y. et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007). This study demonstrates that βKlotho forms complexes with FGFR1c and functions as the obligate co-receptor for FGF21.
Kurosu, H. et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007). This investigation showed that FGF19 binds to the βKlotho–FGFR4 complex to activate FGF signaling.
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Chen, G. et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553, 461–466 (2018). The first report on the crystal structure of the αKlotho–FGFR1c–FGF23 ternary complex.
Lee, S. et al. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505 (2018). The first report on the crystal structure of βKlotho.
Lan, T. et al. FGF19, FGF21, and an FGFR1/β-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718.e3 (2017).
Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
Liu, S. et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 (2006).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 (2006).
Fon Tacer, K. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010).
Kuro-o, M. Phosphate and Klotho. Kidney Int. 79, S20–S23 (2011).
Murer, H., Forster, I. & Biber, J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 447, 763–767 (2004).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Dominguez, J. R., Shlipak, M. G., Whooley, M. A. & Ix, J. H. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J. Am. Soc. Nephrol. 24, 647–654 (2013).
Barthel, T. K. et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J. Steroid Biochem. Mol. Biol. 103, 381–388 (2007).
Lavi-Moshayoff, V., Wasserman, G., Meir, T., Silver, J. & Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 (2010).
Inoue, Y. et al. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem. J. 390, 325–331 (2005).
Masuyama, R. et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Invest. 116, 3150–3159 (2006).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Meyer, M. B. et al. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J. Biol. Chem. 292, 17541–17558 (2017).
Olauson, H. et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLOS Genet. 9, e1003975 (2013).
Farrow, E. G., Davis, S. I., Summers, L. J. & White, K. E. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 20, 955–960 (2009).
Andrukhova, O. et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 6, 744–759 (2014).
Chen, S. Y. et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl Acad. Sci. USA 96, 2514–2519 (1999).
Cai, H. et al. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 69, 2162–2170 (2006).
Andrukhova, O. et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 33, 229–246 (2014).
Hall, J. E. in Guyton and Hall Textbook of Medical Physiology 13th edn 1001–1019 (Elsevier, 2016).
Yuan, Q. et al. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J. Bone Miner. Res. 26, 2026–2035 (2011).
Pitts, T. O. et al. Inhibitory effects of volume expansion performed in vivo on transport in the isolated rabbit proximal tubule perfused in vitro. J. Clin. Invest. 81, 997–1003 (1988).
Liput, J., Rose, M., Galya, C., Chen, T. C. & Puschett, J. B. Inhibition by volume expansion of phosphate uptake by the renal proximal tubule brush border membrane. Biochem. Pharmacol. 38, 321–325 (1989).
Brown, E. M., Pollak, M., Riccardi, D. & Hebert, S. C. Cloning and characterization of an extracellular Ca2+-sensing receptor from parathyroid and kidney: new insights into the physiology and pathophysiology of calcium metabolism. Nephrol. Dial Transplant 9, 1703–1706 (1994).
Quinn, S. J. et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am. J. Physiol. Endocrinol. Metab. 304, E310–E320 (2013).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
Zhang, B. et al. Up-regulation of FGF23 release by aldosterone. Biochem. Biophys. Res. Commun. 470, 384–390 (2016).
de Seigneux, S. & Martin, P. Y. Phosphate and FGF23 in the renoprotective benefit of RAAS inhibition. Pharmacol. Res. 106, 87–91 (2016).
Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect Biol. 1, a001651 (2009).
David, V., Francis, C. & Babitt, J. L. Ironing out the cross talk between FGF23 and inflammation. Am. J. Physiol. Renal Physiol. 312, F1–F8 (2017).
Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K. & Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003).
Forster, R. E. et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem. Biophys. Res. Commun. 414, 557–562 (2011).
Zhang, H. et al. Klotho is a target gene of PPAR-gamma. Kidney Int. 74, 732–739 (2008).
Tang, R. et al. Fosinopril and Losartan regulate Klotho gene and nicotinamide adenine dinucleotide phosphate oxidase expression in kidneys of spontaneously hypertensive rats. Kidney Blood Pressure Res. 34, 350–357 (2011).
de Borst, M. H., Vervloet, M. G., ter Wee, P. M. & Navis, G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J. Am. Soc. Nephrol. 22, 1603–1609 (2011).
Marsell, R. et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol. Dial Transplant 23, 827–833 (2008).
White, K. E. et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000). The first demonstration of a link between FGF23 and phosphate homeostasis in humans.
Kurosu, H. & Kuro-o, M. Endocrine fibroblast growth factors as regulators of metabolic homeostasis. Biofactors 35, 52–60 (2009).
Yu, X. & White, K. E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 16, 221–232 (2005).
The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 11, 130–136 (1995).
Garringer, H. J. et al. The role of mutant UDP-N-acetyl-α-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J. Clin. Endocrinol. Metab. 91, 4037–4042 (2006).
Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 (2006).
Ichikawa, S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2692–2701 (2007).
Brownstein, C. A. et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl Acad. Sci. USA 105, 3455–3460 (2008).
Smith, R. C. et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J. Clin. Invest. 122, 4710–4715 (2012).
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
Denic, A. et al. The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol. 28, 313–320 (2016).
Bacchetta, J. et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J. Clin. Endocrinol. Metab. 95, 1741–1748 (2010).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Mackay, E. M. & Oliver, J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J. Exp. Med. 61, 319–334 (1935).
Haut, L. L., Alfrey, A. C., Guggenheim, S., Buddington, B. & Schrier, N. Renal toxicity of phosphate in rats. Kidney Int. 17, 722–731 (1980).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Hu, M. C. et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 26, 1290–1302 (2015).
Kawaguchi, H. et al. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J. Clin. Invest. 104, 229–237 (1999).
Suga, T. et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell. Mol. Biol. 22, 26–33 (2000).
Kamemori, M. et al. Expression of Klotho protein in the inner ear. Hear Res. 171, 103–110 (2002).
Nagai, T. et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 17, 50–52 (2003).
Stubbs, J. R. et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 18, 2116–2124 (2007).
Kuro-o, M. A potential link between phosphate and aging — lessons from Klotho-deficient mice. Mech. Ageing Dev. 131, 270–275 (2010).
Stenvinkel, P. & Larsson, T. E. Chronic kidney disease: a clinical model of premature aging. Am. J. Kidney Dis. 62, 339–351 (2013).
Stenvinkel, P. et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat. Rev. Nephrol. 14, 265–284 (2018).
Heiss, A. et al. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 278, 13333–13341 (2003).
Heiss, A., Jahnen-Dechent, W., Endo, H. & Schwahn, D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2, 16–20 (2007).
Shuto, E. et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. 20, 1504–1512 (2009).
Yamada, H. et al. Daily variability in serum levels of calciprotein particles and their association with mineral metabolism parameters: a cross-sectional pilot study. Nephrology 23, 226–230 (2017).
Smith, E. R., Hanssen, E., McMahon, L. P. & Holt, S. G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLOS ONE 8, e60904 (2013).
Di Marco, G. S. et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Renal Physiol. 294, F1381–F1387 (2008).
Ewence, A. E. et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 103, e28–e34 (2008).
Sage, A. P., Lu, J., Tintut, Y. & Demer, L. L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 79, 414–422 (2011).
Reynolds, J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15, 2857–2867 (2004).
Villa-Bellosta, R. & Sorribas, V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb. Vasc. Biol. 29, 761–766 (2009).
Bank, N., Su, W. S. & Aynedjian, H. S. A micropuncture study of renal phosphate transport in rats with chronic renal failure and secondary hyperparathyroidism. J. Clin. Invest. 61, 884–894 (1978).
Ohyama, Y. et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 251, 920–925 (1998).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015).
Unger, R. H. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie 87, 57–64 (2005).
Miura, Y. et al. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 8, 1256 (2018).
Smith, E. R. et al. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol. Dial Transplant 27, 1957–1966 (2012).
Hamano, T. et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 21, 1998–2007 (2010).
Hamano, K., Nitta, A., Ohtake, T. & Kobayashi, S. Associations of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care 31, 1853–1857 (2008).
Cai, M. M., Smith, E. R., Brumby, C., McMahon, L. P. & Holt, S. G. Fetuin-A-containing calciprotein particle levels can be reduced by dialysis, sodium thiosulphate and plasma exchange. Potential therapeutic implications for calciphylaxis? Nephrology 18, 724–727 (2013).
Jurk, D. et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2, 4172 (2014).
Custodero, C. et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res. Rev. 46, 42–59 (2018).
Bloch, L. et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 583, 3221–3224 (2009).
Chen, C. D., Podvin, S., Gillespie, E., Leeman, S. E. & Abraham, C. R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl Acad. Sci. USA 104, 19796–19801 (2007).
Imura, A. et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 565, 143–147 (2004).
Hu, M. C. et al. Renal production, uptake, and handling of circulating αKlotho. J. Am. Soc. Nephrol. 27, 79–90 (2016).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Shiraki-Iida, T. et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 424, 6–10 (1998).
Barker, S. L. et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol. Dial Transplant 30, 223–233 (2015).
Yamazaki, Y. et al. Establishment of sandwich ELISA for soluble α-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 398, 513–518 (2010).
Mian, I. S. Sequence, structural, functional, and phylogenetic analyses of three glycosidase families. Blood Cells Mol. Dis. 24, 83–100 (1998).
Kretchmer, N. Lactose and lactase: a historical perspective. Gastroenterology 61, 805–813 (1971).
Ito, S., Fujimori, T., Hayashizaki, Y. & Nabeshima, Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim. Biophys. Acta 1576, 341–345 (2002).
Cha, S. K. et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl Acad. Sci. USA 105, 9805–9810 (2008).
Cha, S. K. et al. Regulation of ROMK1 channel and renal K+ excretion by Klotho. Mol. Pharmacol. 76, 38–46 (2009).
Ohtsubo, K. et al. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123, 1307–1321 (2005).
Partridge, E. A. et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 (2004).
Wright, J. D. et al. Modeled structural basis for the recognition of alpha2-3-sialyllactose by soluble Klotho. FASEB J. 31, 3574–3586 (2017).
Imura, A. et al. Alpha-Klotho as a regulator of calcium homeostasis. Science 316, 1615–1618 (2007).
Sugiura, H. et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol. Dial. Transplant 20, 2636–2645 (2005).
Wang, Y., Kuro-o, M. & Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 11, 410–417 (2012).
Haruna, Y. et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl Acad. Sci. USA 104, 2331–2336 (2007).
Hu, M. C. et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 22, 124–136 (2011).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005). This study confirmed that the αKlotho gene is an ageing-suppressor gene that can extend lifespan when overexpressed.
Hu, M. C. et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 78, 1240–1251 (2010).
Doi, S. et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 286, 8655–8665 (2011).
Hu, M. C. et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 91, 1104–1114 (2017).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Kim, J. H. et al. Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J. Am. Soc. Nephrol. 28, 140–151 (2017).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Hu, M. C. et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 24, 3438–3450 (2010).
Chang, Q. et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 (2005).
Hum, J. M. et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble Klotho. J. Am. Soc. Nephrol. 28, 1162–1174 (2016).
Saito, Y. et al. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 248, 324–329 (1998).
Leibrock, C. B. et al. NH4Cl treatment prevents tissue calcification in Klotho deficiency. J. Am. Soc. Nephrol. 26, 2423–2433 (2015).
Nabeshima, Y. et al. Calpain 1 inhibitor BDA-410 ameliorates alpha-klotho-deficiency phenotypes resembling human aging-related syndromes. Sci. Rep. 4, 5847 (2014).
Wirrig, E. E., Gomez, M. V., Hinton, R. B. & Yutzey, K. E. COX2 inhibition reduces aortic valve calcification in vivo. Arterioscler. Thromb. Vasc. Biol. 35, 938–947 (2015).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005). This study characterized FGF21 as an anti-diabetic hormone.
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007).
Inagaki, T. et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8, 77–83 (2008).
Potthoff, M. J. et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl Acad. Sci. USA 106, 10853–10858 (2009).
Owen, B. M., Mangelsdorf, D. J. & Kliewer, S. A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metabolism 26, 22–29 (2015).
Adams, A. C. et al. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab. 2, 31–37 (2012).
Fisher, F. M. et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152, 2996–3004 (2011).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Hsuchou, H., Pan, W. & Kastin, A. J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386 (2007).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Med. 19, 1147–1152 (2013).
Anuwatmatee, S., Tang, S., Wu, B. J., Rye, K. A. & Ong, K. L. Fibroblast growth factor 21 in chronic kidney disease. Clin. Chim. Acta. https://doi.org/10.1016/j.cca.2017.11.002 (2017).
van der Pluijm, I. et al. Impaired genome maintenance suppresses the growth hormone — insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLOS Biol. 5, e2 (2007).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Schumacher, B. et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLOS Genet. 4, e1000161 (2008).
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012).
Cohen, D. L., Huan, Y. & Townsend, R. R. Ambulatory blood pressure in chronic kidney disease. Curr. Hypertension Rep. 15, 160–166 (2013).
McClung, C. A. How might circadian rhythms control mood? Let me count the ways. Biol. Psychiatry 74, 242–249 (2013).
Farrokhi, F., Abedi, N., Beyene, J., Kurdyak, P. & Jassal, S. V. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am. J. Kidney Dis. 63, 623–635 (2014).
Kohara, M. et al. Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLOS ONE 12, e0178971 (2017).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005). This study identified FGF15 as a regulator of bile acid synthesis.
Badman, M. K. et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 (2007).
Kuro-o, M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol. Metab. 19, 239–245 (2008).
Ito, S. et al. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J. Clin. Invest. 115, 2202–2208 (2005).
Yu, C. et al. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 275, 15482–15489 (2000).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Tomlinson, E. et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143, 1741–1747 (2002).
Fu, L. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004).
Johansson, H. et al. Circulating fibroblast growth factor 19 in portal and systemic blood. J. Clin. Exp. Hepatol. 8, 162–168 (2018).
Nicholes, K. et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 160, 2295–2307 (2002).
Desnoyers, L. R. et al. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 27, 85–97 (2008).
Wang, H. et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J. Clin. Invest. 121, 3220–3232 (2011).
Walters, J. R. et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin. Gastroenterol. Hepatol. 7, 1189–1194 (2009).
Cosola, C., Rocchetti, M. T., Cupisti, A. & Gesualdo, L. Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 130, 132–142 (2018).
Wahlstrom, A., Kovatcheva-Datchary, P., Stahlman, M., Backhed, F., & Marschall, H.-U. Crosstalk between bile acids and gut microbiota and its impact on farnesoid X receptor signalling. 35, 246–250 (2017).
Li, M., Qureshi, A. R., Ellis, E. & Axelsson, J. Impaired postprandial fibroblast growth factor (FGF)-19 response in patients with stage 5 chronic kidney diseases is ameliorated following antioxidative therapy. Nephrol. Dial. Transplant 28 (Suppl. 4), 212–219 (2013).
Morishita, K. et al. The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J. Nutr. 131, 3182–3188 (2001).
Segawa, H. et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am. J. Physiol. Renal Physiol. 292, F769–F779 (2007).
Azuma, M. et al. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 26, 4264–4274 (2012).
Ohnishi, M., Nakatani, T., Lanske, B. & Razzaque, M. S. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ. Cardiovasc. Genet. 2, 583–590 (2009).
Mencke, R. & Hillebrands, J. L. The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res. Rev. 35, 124–146 (2016).
Lindberg, K. et al. Arterial Klotho expression and FGF23 effects on vascular calcification and function. PLOS ONE 8, e60658 (2013).
Koh, N. et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 280, 1015–1020 (2001).
Andrukhova, O. et al. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51, 621–628 (2012).
Kuro-o, M. Klotho in health and disease. Curr. Opin. Nephrol. Hypertens. 21, 362–368 (2012).
Olauson, H. et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 23, 1641–1651 (2012).
Ide, N. et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int. 90, 348–362 (2016).
Young, A. et al. Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am. J. Kidney Dis. 59, 761–769 (2011).
Westerberg, P. A., Ljunggren, O., Larsson, T. E., Wadstrom, J. & Linde, T. Fibroblast growth factor-23 and mineral metabolism after unilateral nephrectomy. Nephrol. Dial Transplant 25, 4068–4071 (2010).
Patterson, R. et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur. J. Epidemiol. 33, 811–829 (2018).
Xie, T. & Leung, P. S. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am. J. Physiol. Endocrinol. Metab. 313, E292–E302 (2017).
Arking, D. E. et al. Association of human aging with a functional variant of klotho. Proc. Natl Acad. Sci. USA 99, 856–861 (2002).
Dyson, H. J. & Wright, P. E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 (2005).
Wright, P. E. & Dyson, H. J. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J. Mol. Biol. 293, 321–331 (1999).
Yamada, H. et al. The urinary phosphate to serum fibroblast growth factor 23 ratio is a useful marker of atherosclerosis in early-stage chronic kidney disease. PLOS ONE 11, e0160782 (2016).
Yamada, H. et al. The urinary phosphate to serum fibroblast growth factor 23 ratio, deemed the nephron index, is a useful clinical index for early stage chronic kidney disease in patients with type 2 diabetes: an observational pilot study. Int. J. Nephrol. 2018, 4 (2018).
Chopra, A. & Lineweaver, C. H. in Proc. 8th Australian Space Science Conf. (eds Short, W. & Cairns, I.) 49–55 (National Space Society of Australia Ltd, 2008).
Kuro-o, M. & Moe, O. W. FGF23-alphaKlotho as a paradigm for a kidney-bone network. Bone 100, 4–18 (2016).
Kuro-o, M. Klotho and endocrine fibroblast growth factors: marker of chronic kidney disease progression and cardiovascular complications? Nephrol. Dial. Transplant https://doi.org/10.1093/ndt/gfy126 (2018).
Acknowledgements
The author’s work is supported by the Japan Agency for Medical Research and Development (AMED) Core Research for Evolutionary Medical Science and Technology (CREST), AMED (JP18gm0610012) and ACT-MS (18im0210806h0001), the Japan Society for the Promotion of Science (16H05302, 16K15470) and the Japan Aerospace Exploration Agency (JAXA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author has received research funds from Bayer, Astellas, Bristol-Myer-Squibb, Kyowa-Hakko-Kirin and Kissei Pharmaceutical Co., Ltd.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hereditary phosphate-wasting syndromes
-
Inherited disorders in which the disease symptoms are related to the depletion of phosphorus in the body owing to increased urinary phosphate excretion and resulting in hypophosphataemia and disrupted bone mineralization.
- Inappropriately normal
-
Normal protein serum levels when they should be outside of the normal range owing to concurrent levels of other serum proteins. This effect is indicative of impaired physiological responses.
- Rickets
-
A condition characterized by impaired (low) bone mineralization that results in weak, soft bones with increased osteoid (unmineralized bone matrix) in children. In adults, this condition is termed osteomalacia.
- Breakpoint
-
Location in a chromosome where a genomic DNA sequence has been disrupted by deletion, translocation or insertion.
- CKD–MBD
-
Denotes chronic kidney disease (CKD) complications that are associated with and are likely caused by disturbed calcium and phosphate metabolism and by abnormal serum levels of fibroblast growth factor 23 (FGF23), vitamin D, and parathyroid hormone. The term CDK–mineral and bone disorder (CKD–MBD) is used to describe cardiovascular and bone disorders in CKD, including vascular calcification, cardiac hypertrophy and renal osteodystrophy.
- Postprandial
-
The period that follows the ingestion of food.
- Sarcopenia
-
The degenerative decline in skeletal muscle volume and strength with ageing.
- Osteopenia
-
A condition of low bone mineral density. Osteoporosis is a clinical diagnosis of osteopenia that is associated with a decrease in both bone matrix and bone mineral density, as well as altered bone microarchitecture.
- Emphysematous lung
-
Lung tissue that is affected by pulmonary emphysema, which is characterized by enlarged alveolar spaces and damaged alveolar walls.
- Colloids
-
Uniform mixtures of small particles (dispersoids) in the dispersion medium. Dispersoids are not dissolved but are evenly distributed in the dispersion medium.
- Arteriosclerosis
-
A condition that includes two distinct pathologies, atherosclerosis and vascular calcification. Atherosclerosis is characterized by the accumulation of foam cells (macrophages laden with lipids) in the tunica intima, potentially causing obstruction of blood flow. By contrast, vascular calcification occurs in the tunica media and minimally obstructs the blood flow but increases vascular stiffness.
- Flow-mediated dilatation
-
Clinical test in which the expansion rate of the brachial artery is calculated to evaluate vascular endothelial function.
- Torpor
-
Short-term hibernation-like state that is associated with a low body temperature and inactivity.
- Suprachiasmatic nucleus
-
(SCN). Cluster of neurons in the hypothalamus that function as the master circadian pacemaker. Some of these neurons have a direct projection to corticotropin-releasing factor-producing neurons in the paraventricular nucleus.
- Nucleus of the solitary tract
-
(NTS). Cluster of sensory neurons in the medulla oblongata that are innervated from some cranial nerves, including vagus nerves, and project to various nuclei in the brainstem and parasympathetic neurons. It is also known as the central relay for the baroreflex that maintains blood pressure.
- Progeroid syndrome
-
Hereditary disorder in which there are multiple signs and symptoms of ageing in individuals in the early stages of life. Examples include Werner syndrome, Hutchinson–Gilford syndrome, xeroderma pigmentosum and Cockayne syndrome. Patients with progeroid syndromes have defects in DNA repair systems.
- Bile acid pool
-
Amount of bile acids held in the intestine, portal circulation, liver and gall bladder. Bile acids secreted into the intestine are mostly reabsorbed and returned to the liver to be secreted into the intestine again (enterohepatic circulation). The liver synthesizes the same amount of bile acids that are lost in the faeces to maintain the bile acid pool size.
Rights and permissions
About this article
Cite this article
Kuro-o, M. The Klotho proteins in health and disease. Nat Rev Nephrol 15, 27–44 (2019). https://doi.org/10.1038/s41581-018-0078-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0078-3
This article is cited by
-
Analysis of the correlation between serum Klotho and FeNO: a cross-sectional study from NHANES (2007–2012)
BMC Pulmonary Medicine (2024)
-
Role of klotho and fibroblast growth factor 23 in arterial calcification, thickness, and stiffness: a meta-analysis of observational studies
Scientific Reports (2024)
-
PKC regulates αKlotho gene expression in MDCK and NRK-52E cells
Pflügers Archiv - European Journal of Physiology (2024)
-
Exercise-induced changes in plasma S-Klotho levels are associated with the obtained enhancements of heart rate variability in sedentary middle-aged adults: the FIT-AGEING study
Journal of Physiology and Biochemistry (2024)
-
Association between remnant cholesterol and anti-aging soluble α-klotho protein: New perspective on anti-aging from a NHANES study
Irish Journal of Medical Science (1971 -) (2024)