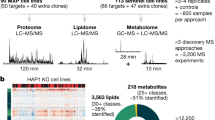
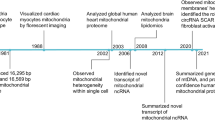
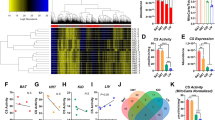
Abstract
Mitochondria are multifaceted organelles with key roles in anabolic and catabolic metabolism, bioenergetics, cellular signalling and nutrient sensing, and programmed cell death processes. Their diverse functions are enabled by a sophisticated set of protein components encoded by the nuclear and mitochondrial genomes. The extent and complexity of the mitochondrial proteome remained unclear for decades. This began to change 20 years ago when, driven by the emergence of mass spectrometry-based proteomics, the first draft mitochondrial proteomes were established. In the ensuing decades, further technological and computational advances helped to refine these ‘maps’, with current estimates of the core mammalian mitochondrial proteome ranging from 1,000 to 1,500 proteins. The creation of these compendia provided a systemic view of an organelle previously studied primarily in a reductionist fashion and has accelerated both basic scientific discovery and the diagnosis and treatment of human disease. Yet numerous challenges remain in understanding mitochondrial biology and translating this knowledge into the medical context. In this Roadmap, we propose a path forward for refining the mitochondrial protein map to enhance its discovery and therapeutic potential. We discuss how emerging technologies can assist the detection of new mitochondrial proteins, reveal their patterns of expression across diverse tissues and cell types, and provide key information on proteoforms. We highlight the power of an enhanced map for systematically defining the functions of its members. Finally, we examine the utility of an expanded, functionally annotated mitochondrial proteome in a translational setting for aiding both diagnosis of mitochondrial disease and targeting of mitochondria for treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hogeboom, G., Schneider, W. C. & Pallade, G. E. Cytochemical studies of mammalian tissues; isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J. Biol. Chem. 172, 619–635 (1948).
Lehninger, A. L. Esterification of inorganic phosphate coupled to electron transport between dihydrodiphosphopyridine nucleotide and oxygen. II. J. Biol. Chem. 178, 625–644 (1949).
Kennedy, E. P. & Lehninger, A. L. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J. Biol. Chem. 179, 957–972 (1949).
Barkulis, S. S. & Lehninger, A. L. Myokinase and the adenine nucleotide specificity in oxidative phosphorylations. J. Biol. Chem. 190, 339–344 (1951).
Lardy, H. A. & Adler, J. Synthesis of succinate from propionate and bicarbonate by soluble enzymes from liver mitochondria. J. Biol. Chem. 219, 933–942 (1956).
Ernster, L. & Schatz, G. Mitochondria: a historical review. J. Cell Biol. 91, 227s–255s (1981).
Rabilloud, T. et al. Two‐dimensional electrophoresis of human placental mitochondria and protein identification by mass spectrometry: toward a human mitochondrial proteome. Electrophoresis 19, 1006–1014 (1998).
Lopez, M. F. et al. High‐throughput profiling of the mitochondrial proteome using affinity fractionation and automation. Electrophoresis 21, 3427–3440 (2000).
Sickmann, A. et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl Acad. Sci. USA 100, 13207–13212 (2003).
Mootha, V. K. et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115, 629–640 (2003).
Taylor, S. W. et al. Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 21, 281–286 (2003).
Gaucher, S. P. et al. Expanded coverage of the human heart mitochondrial proteome using multidimensional liquid chromatography coupled with tandem mass spectrometry. J. Proteome Res. 3, 495–505 (2004).
Foster, L. J. et al. A mammalian organelle map by protein correlation profiling. Cell 125, 187–199 (2006).
Reinders, J., Zahedi, R. P., Pfanner, N., Meisinger, C. & Sickmann, A. Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J. Proteome Res. 5, 1543–1554 (2006).
Calvo, S. et al. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat. Genet. 38, 576–582 (2006).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Consortium, I. H. G. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–D1257 (2016).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, gkaa1011 (2020).
Morgenstern, M. et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 33, 2464–2483.e18 (2021).
Smith, A. G. et al. A curated collection of human mitochondrial proteins — the Integrated Mitochondrial Protein Index (IMPI). Preprint at SSRN Electron. J. https://doi.org/10.2139/ssrn.4042282 (2022).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Schulte, U. et al. Mitochondrial complexome reveals quality-control pathways of protein import. Nature 614, 153–159 (2023).
Morgenstern, M. et al. Definition of a high-confidence mitochondrial proteome at quantitative scale. Cell Rep. 19, 2836–2852 (2017).
Vögtle, F.-N. et al. Landscape of submitochondrial protein distribution. Nat. Commun. 8, 290 (2017).
Sung, A. Y., Floyd, B. J. & Pagliarini, D. J. Systems biochemistry approaches to defining mitochondrial protein function. Cell Metab. 31, 669–678 (2020).
Hughes, C. S. et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 14, 68–85 (2019).
Muehlbauer, L. K. et al. Rapid multi-omics sample preparation for mass spectrometry. Anal. Chem. 95, 659–667 (2023).
Bayraktar, E. C. et al. MITO-tag mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proc. Natl Acad. Sci. USA 116, 303–312 (2019).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Benador, I. Y. et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885.e6 (2018).
Najt, C. P. et al. Organelle interactions compartmentalize hepatic fatty acid trafficking and metabolism. Cell Rep. 42, 112435 (2023).
Saki, M. & Prakash, A. DNA damage related crosstalk between the nucleus and mitochondria. Free Radic. Bio Med. 107, 216–227 (2017).
Hu, J. et al. Phosphorylation-dependent mitochondrial translocation of MAP4 is an early step in hypoxia-induced apoptosis in cardiomyocytes. Cell Death Dis. 5, e1424 (2014).
Li, X. et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016).
Rhee, H.-W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 55, 332–341 (2014).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801–810 (2012).
Liu, X., Salokas, K., Weldatsadik, R. G., Gawriyski, L. & Varjosalo, M. Combined proximity labeling and affinity purification−mass spectrometry workflow for mapping and visualizing protein interaction networks. Nat. Protoc. 15, 3182–3211 (2020).
Antonicka, H. et al. A high-density human mitochondrial proximity interaction network. Cell Metab. 32, 479–497.e9 (2020).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Cho, K. F. et al. Split-TurboID enables contact-dependent proximity labeling in cells. Proc. Natl Acad. Sci. USA 117, 12143–12154 (2020).
Bader, G. et al. Assigning mitochondrial localization of dual localized proteins using a yeast bi-genomic mitochondrial-split-GFP. eLife 9, e56649 (2020).
Mercer, T. R. et al. The human mitochondrial transcriptome. Cell 146, 645–658 (2011).
Hashimoto, Y. et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Aβ. Proc. Natl Acad. Sci. USA 98, 6336–6341 (2001).
Yen, K. et al. Humanin prevents age-related cognitive decline in mice and is associated with improved cognitive age in humans. Sci. Rep. 8, 14212 (2018).
Lee, C. et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454 (2015).
Olexiouk, V., Van Criekinge, W. & Menschaert, G. An update on sORFs.org: a repository of small ORFs identified by ribosome profiling. Nucleic Acids Res. 46, gkx1130 (2017).
Liang, C. et al. Mitochondrial microproteins link metabolic cues to respiratory chain biogenesis. Cell Rep. 40, 111204 (2022).
Chu, Q. et al. Regulation of the ER stress response by a mitochondrial microprotein. Nat. Commun. 10, 4883 (2019).
Rathore, A. et al. MIEF1 microprotein regulates mitochondrial translation. Biochemistry 57, 5564–5575 (2018).
Stein, C. S. et al. Mitoregulin: a lncRNA-encoded microprotein that supports mitochondrial supercomplexes and respiratory efficiency. Cell Rep. 23, 3710–3720.e8 (2018).
Baker, M. J., Crameri, J. J., Thorburn, D. R., Frazier, A. E. & Stojanovski, D. Mitochondrial biology and dysfunction in secondary mitochondrial disease. Open Biol. 12, 220274 (2022).
Arroyo, J. D. et al. A genome-wide CRISPR death screen identifies genes essential for oxidative phosphorylation. Cell Metab. 24, 875–885 (2016).
Smith, L. M. et al. Proteoform: a single term describing protein complexity. Nat. Methods 10, 186–187 (2013).
Aebersold, R. et al. How many human proteoforms are there? Nat. Chem. Biol. 14, 206–214 (2018).
Smith, L. M. et al. The Human Proteoform Project: defining the human proteome. Sci. Adv. 7, eabk0734 (2021).
Schaffer, L. V. et al. Identification and quantification of murine mitochondrial proteoforms using an integrated top-down and intact-mass strategy. J. Proteome Res. 17, 3526–3536 (2018).
Catherman, A. D. et al. Large-scale top-down proteomics of the human proteome: membrane proteins, mitochondria, and senescence. Mol. Cell. Proteom. 12, 3465–3473 (2013).
Song, Z., Chen, H., Fiket, M., Alexander, C. & Chan, D. C. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 178, 749–755 (2007).
Anand, R. et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919–929 (2014).
Dotto, V. D., Fogazza, M., Carelli, V., Rugolo, M. & Zanna, C. Eight human OPA1 isoforms, long and short: what are they for? Biochim. Biophys. Acta 1859, 263–269 (2018).
Dotto, V. D. et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 19, 2557–2571 (2017).
Reane, D. V. et al. A MICU1 splice variant confers high sensitivity to the mitochondrial Ca2+ uptake machinery of skeletal muscle. Mol. Cell 64, 760–773 (2016).
Gang, H. et al. PDK2-mediated alternative splicing switches Bnip3 from cell death to cell survival. J. Cell Biol. 210, 1101–1115 (2015).
Morciano, G. et al. Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death. Mol. Biol. Cell 27, 20–34 (2016).
Sinitcyn, P. et al. Global detection of human variants and isoforms by deep proteome sequencing. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01714-x (2023).
Niemi, N. M. & Pagliarini, D. J. The extensive and functionally uncharacterized mitochondrial phosphoproteome. J. Biol. Chem. 297, 100880 (2021).
Hosp, F. et al. Lysine acetylation in mitochondria: from inventory to function. Mitochondrion 33, 58–71 (2017).
Anderson, K. A. & Hirschey, M. D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 52, 23–35 (2012).
Piantadosi, C. A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta 1820, 712–721 (2012).
Wieland, O. & Siess, E. Interconversion of phospho- and dephospho- forms of pig heart pyruvate dehydrogenase. Proc. Natl Acad. Sci. USA 65, 947–954 (1970).
Linn, T. C., Pettit, F. H. & Reed, L. J. α-Keto acid dehydrogenase complexes, X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc. Natl Acad. Sci. USA 62, 234–241 (1969).
Paxton, R. & Harris, R. A. Isolation of rabbit liver branched chain α-ketoacid dehydrogenase and regulation by phosphorylation. J. Biol. Chem. 257, 14433–14439 (1982).
Grimsrud, P. A. et al. A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab. 16, 672–683 (2012).
Hansen, F. M. et al. Mitochondrial phosphoproteomes are functionally specialized across tissues. Preprint at bioRxiv https://doi.org/10.1101/2022.03.23.485457 (2022).
Bak, S., León, I. R., Jensen, O. N. & Højlund, K. Tissue specific phosphorylation of mitochondrial proteins isolated from rat liver, heart muscle, and skeletal muscle. J. Proteome Res. 12, 4327–4339 (2013).
Kruse, R. & Højlund, K. Mitochondrial phosphoproteomics of mammalian tissues. Mitochondrion 33, 45–57 (2017).
Niemi, N. M. et al. Pptc7 is an essential phosphatase for promoting mammalian mitochondrial metabolism and biogenesis. Nat. Commun. 10, 3197 (2019).
Niemi, N. M. et al. Pptc7 maintains mitochondrial protein content by suppressing receptor-mediated mitophagy. Preprint at bioRxiv https://doi.org/10.1101/2023.02.28.530351 (2023).
Kim, S. C. et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 (2006).
Park, J. et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 50, 919–930 (2013).
Colak, G. et al. Proteomic and biochemical studies of lysine malonylation suggest its malonic aciduria-associated regulatory role in mitochondrial function and fatty acid oxidation [S]. Mol. Cell Proteom. 14, 3056–3071 (2015).
Bharathi, S. S. et al. Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J. Biol. Chem. 288, 33837–33847 (2013).
Fan, J. et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol. Cell 53, 534–548 (2014).
Hallows, W. C., Lee, S. & Denu, J. M. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl Acad. Sci. USA 103, 10230–10235 (2006).
Horton, J. L. et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 1, e84897 (2016).
Hebert, A. S. et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 (2013).
Doulias, P.-T., Tenopoulou, M., Greene, J. L., Raju, K. & Ischiropoulos, H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal. 6, rs1 (2013).
Prime, T. A. et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia–reperfusion injury. Proc. Natl Acad. Sci. USA 106, 10764–10769 (2009).
Gu, Z., Nakamura, T. & Lipton, S. A. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol. Neurobiol. 41, 55–72 (2010).
Seim, G. L. et al. Nitric oxide-driven modifications of lipoic arm inhibit α-ketoacid dehydrogenases. Nat. Chem. Biol. 19, 265–274 (2023).
Gibson, Q. H. & Greenwood, C. Kinetic evidence for a short lived intermediate in the oxidation of cytochrome c oxidase by molecular oxygen. J. Biol. Chem. 240, 957–958 (1965).
Coles, S. J. et al. S-Nitrosoglutathione inactivation of the mitochondrial and cytosolic BCAT proteins: S-nitrosation and S-thiolation. Biochemistry 48, 645–656 (2009).
Cao, W. et al. Discovery and confirmation of O-GlcNAcylated proteins in rat liver mitochondria by combination of mass spectrometry and immunological methods. PLoS ONE 8, e76399 (2013).
Hu, Y. et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J. Biol. Chem. 284, 547–555 (2009).
Andrabi, S. A. et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl Acad. Sci. USA 111, 10209–10214 (2014).
Pagliarini, D. J. & Dixon, J. E. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem. Sci. 31, 26–34 (2006).
Gao, S. et al. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane a pentabasic amino acid sequence in the autoinhibitory domain of enos targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J. Biol. Chem. 279, 15968–15974 (2004).
Chatterjee, A. et al. MOF acetyl transferase regulates transcription and respiration in mitochondria. Cell 167, 722–738.e23 (2016).
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 (2012).
Luongo, T. S. et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 (2020).
Siddharth, T. & Lewis, N. Predicting pathways for old and new metabolites through clustering. Preprint at arXiv https://doi.org/10.48550/arxiv.2211.15720 (2022).
Wang, Y. et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 599, 136–140 (2021).
Shi, X. et al. Combinatorial GxGxE CRISPR screen identifies SLC25A39 in mitochondrial glutathione transport linking iron homeostasis to OXPHOS. Nat. Commun. 13, 2483 (2022).
Alston, C. L. et al. Bi-allelic mutations in NDUFA6 establish its role in early-onset isolated mitochondrial complex I deficiency. Am. J. Hum. Genet. 103, 592–601 (2018).
Floyd, B. J. et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol. Cell 63, 621–632 (2016).
Alston, C. L. et al. Pathogenic bi-allelic mutations in NDUFAF8 cause Leigh syndrome with an isolated complex I deficiency. Am. J. Hum. Genet. 106, 92–101 (2020).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Go, C. D. et al. A proximity-dependent biotinylation map of a human cell. Nature 595, 120–124 (2021).
Hung, V. et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. eLife 6, e24463 (2017).
Hevler, J. F. et al. Selective cross‐linking of coinciding protein assemblies by in‐gel cross‐linking mass spectrometry. EMBO J. 40, e106174 (2021).
Liu, F., Lössl, P., Rabbitts, B. M., Balaban, R. S. & Heck, A. J. R. The interactome of intact mitochondria by cross-linking mass spectrometry provides evidence for coexisting respiratory supercomplexes. Mol. Cell Proteom. 17, 216–232 (2018).
Linden, A. et al. A cross-linking mass spectrometry approach defines protein interactions in yeast mitochondria. Mol. Cell Proteom. 19, 1161–1178 (2020).
Heide, H. et al. Complexome profiling identifies TMEM126B as a component of the mitochondrial complex I assembly complex. Cell Metab. 16, 538–549 (2012).
Gao, J. et al. CLUH regulates mitochondrial biogenesis by binding mRNAs of nuclear-encoded mitochondrial proteins. J. Cell Biol. 207, 213–223 (2014).
Schatton, D. et al. CLUH regulates mitochondrial metabolism by controlling translation and decay of target mRNAs. J. Cell Biol. 216, 675–693 (2017).
Meyer, J. G., Niemi, N. M., Pagliarini, D. J. & Coon, J. J. Quantitative shotgun proteome analysis by direct infusion. Nat. Methods 17, 1222–1228 (2020).
He, Y. et al. Multi-omic single-shot technology for integrated proteome and lipidome analysis. Anal. Chem. 93, 4217–4222 (2021).
Schäfer, J. A., Sutandy, F. X. R. & Münch, C. Omics-based approaches for the systematic profiling of mitochondrial biology. Mol. Cell 83, 911–926 (2023).
Lapointe, C. P. et al. Multi-omics reveal specific targets of the RNA-binding protein Puf3p and its orchestration of mitochondrial biogenesis. Cell Syst. 6, 125–135.e6 (2018).
Veling, M. T. et al. Multi-omic mitoprotease profiling defines a role for Oct1p in coenzyme Q production. Mol. Cell 68, 970–977.e11 (2017).
Stefely, J. A. et al. Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat. Biotechnol. 34, 1191–1197 (2016).
Rensvold, J. W. et al. Defining mitochondrial protein functions through deep multiomic profiling. Nature 606, 382–388 (2022).
Kathayat, R. S. et al. Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat. Commun. 9, 334 (2018).
Li, Y., Calvo, S. E., Gutman, R., Liu, J. S. & Mootha, V. K. Expansion of biological pathways based on evolutionary inference. Cell 158, 213–225 (2014).
Mühleip, A. et al. Structural basis of mitochondrial membrane bending by the I–II–III2–IV2 supercomplex. Nature 615, 934–938 (2023).
Szklarczyk, D. et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, gkaa1074 (2020).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021).
Franz, M. et al. GeneMANIA update 2018. Nucleic Acids Res. 46, W60–W64 (2018).
Escudero, S. et al. Dynamic regulation of long-chain fatty acid oxidation by a noncanonical interaction between the MCL-1 BH3 helix and VLCAD. Mol. Cell 69, 729–743.e7 (2018).
Chung, K.-P. et al. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat. Commun. 10, 3390 (2019).
Ferreira, C. R. et al. An international classification of inherited metabolic disorders (ICIMD). J. Inherit. Metab. Dis. 44, 164–177 (2021).
Rahman, J. & Rahman, S. Mitochondrial medicine in the omics era. Lancet 391, 2560–2574 (2018).
Schlieben, L. D. & Prokisch, H. The dimensions of primary mitochondrial disorders. Front. Cell Dev. Biol. 8, 600079 (2020).
Gorman, G. S. et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759 (2015).
Forny, P. et al. Diagnosing mitochondrial disorders remains challenging in the omics era. Neurol. Genet. 7, e597 (2021).
Wortmann, S. B., Koolen, D. A., Smeitink, J. A., Heuvel, L. & Rodenburg, R. J. Whole exome sequencing of suspected mitochondrial patients in clinical practice. J. Inherit. Metab. Dis. 38, 437–443 (2015).
Taylor, R. W. et al. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA 312, 68–77 (2014).
Pronicka, E. et al. New perspective in diagnostics of mitochondrial disorders: two years’ experience with whole-exome sequencing at a national paediatric centre. J. Transl. Med. 14, 174 (2016).
Theunissen, T. E. J. et al. Whole exome sequencing is the preferred strategy to identify the genetic defect in patients with a probable or possible mitochondrial cause. Front. Genet. 9, 400 (2018).
Schon, K. R. et al. Use of whole genome sequencing to determine genetic basis of suspected mitochondrial disorders: cohort study. BMJ 375, e066288 (2021).
Kremer, L. S. et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat. Commun. 8, 15824 (2017).
Mertes, C. et al. Detection of aberrant splicing events in RNA-seq data using FRASER. Nat. Commun. 12, 529 (2021).
Brechtmann, F. et al. OUTRIDER: a statistical method for detecting aberrantly expressed genes in rna sequencing data. Am. J. Hum. Genet. 103, 907–917 (2018).
Yépez, V. A. et al. Clinical implementation of RNA sequencing for Mendelian disease diagnostics. Genome Med. 14, 38 (2022).
Smirnov, D., Schlieben, L. D., Peymani, F., Berutti, R. & Prokisch, H. Guidelines for clinical interpretation of variant pathogenicity using RNA phenotypes. Hum. Mutat. 43, 1056–1070 (2022).
Parikh, S. et al. Diagnosis of ‘possible’ mitochondrial disease: an existential crisis. J. Med. Genet. 56, 123 (2019).
Frazier, A. E., Thorburn, D. R. & Compton, A. G. Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology. J. Biol. Chem. 294, 5386–5395 (2019).
Tsang, M. H. Y. et al. Delineation of molecular findings by whole-exome sequencing for suspected cases of paediatric-onset mitochondrial diseases in the southern Chinese population. Hum. Genomics 14, 28 (2020).
Calvo, S. E. et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 42, 851–858 (2010).
Köhler, S. et al. The Human Phenotype Ontology in 2021. Nucleic Acids Res. 49, gkaa1043 (2020).
Simon, M. T. et al. Novel mutations in the mitochondrial complex I assembly gene NDUFAF5 reveal heterogeneous phenotypes. Mol. Genet. Metab. 126, 53–63 (2019).
Shaikh, S. S., Nahorski, M. S., Rai, H. & Woods, C. G. Before progressing from “exomes” to “genomes” … don’t forget splicing variants. Eur. J. Hum. Genet. 26, 1–4 (2018).
Sahni, N. et al. Widespread macromolecular interaction perturbations in human genetic disorders. Cell 161, 647–660 (2015).
Helman, G. et al. Multiomic analysis elucidates complex I deficiency caused by a deep intronic variant in NDUFB10. Hum. Mutat. 42, 19–24 (2021).
Wortmann, S. B. et al. How to proceed after “negative” exome: a review on genetic diagnostics, limitations, challenges, and emerging new multiomics techniques. J. Inherit. Metab. Dis. 45, 663–681 (2022).
Stenton, S. L., Kremer, L. S., Kopajtich, R., Ludwig, C. & Prokisch, H. The diagnosis of inborn errors of metabolism by an integrative “multi‐omics” approach: a perspective encompassing genomics, transcriptomics, and proteomics. J. Inherit. Metab. Dis. 43, 25–35 (2020).
Lynch, D. R. et al. Efficacy of omaveloxolone in Friedreich’s ataxia: delayed‐start analysis of the MOXIe extension. Mov. Disord. 38, 313–320 (2023).
Carelli, V. et al. Idebenone treatment in Leber’s hereditary optic neuropathy. Brain 134, e188 (2011).
Murray, N. H. et al. Small-molecule inhibition of the archetypal UbiB protein COQ8. Nat. Chem. Biol. 19, 230–238 (2023).
Pesini, A., Hidalgo-Gutierrez, A. & Quinzii, C. M. Mechanisms and therapeutic effects of benzoquinone ring analogs in primary CoQ deficiencies. Antioxidants 11, 665 (2022).
Pierrel, F. Impact of chemical analogs of 4-hydroxybenzoic acid on coenzyme Q biosynthesis: from inhibition to bypass of coenzyme Q deficiency. Front. Physiol. 8, 436 (2017).
Freyer, C. et al. Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J. Med. Genet. 52, 779 (2015).
Guerra, R. M. & Pagliarini, D. J. Coenzyme Q biochemistry and biosynthesis. Trends Biochem. Sci. 48, 463–476 (2023).
Baker, P. R. et al. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain 137, 366–379 (2014).
Head, P. E. et al. Aberrant methylmalonylation underlies methylmalonic acidemia and is attenuated by an engineered sirtuin. Sci. Transl. Med. 14, eabn4772 (2022).
Yahata, N., Matsumoto, Y., Omi, M., Yamamoto, N. & Hata, R. TALEN-mediated shift of mitochondrial DNA heteroplasmy in MELAS-iPSCs with m.13513G>A mutation. Sci. Rep. 7, 15557 (2017).
Hashimoto, M. et al. MitoTALEN: a general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol. Ther. 23, 1592–1599 (2015).
Gammage, P. A., Rorbach, J., Vincent, A. I., Rebar, E. J. & Minczuk, M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large‐scale deletions or point mutations. EMBO Mol. Med. 6, 458–466 (2014).
Liu, Z., Sun, Y., Qi, Z., Cao, L. & Ding, S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 12, 66 (2022).
Nitzan, K. et al. Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in Alzheimer’s disease mice. J. Alzheimers Dis. 72, 587–604 (2019).
Chang, J.-C. et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res. 170, 40–56.e3 (2016).
Ripcke, J., Zarse, K., Ristow, M. & Birringer, M. Small‐molecule targeting of the mitochondrial compartment with an endogenously cleaved reversible tag. Chembiochem 10, 1689–1696 (2009).
Steenberge, L. H., Sung, A. Y., Fan, J. & Pagliarini, D. J. Coenyzme Q4 is a functional substitute for coenyzme Q10 and can be targeted to the mitochondria. Preprint at bioRxiv https://doi.org/10.1101/2023.07.20.549963 (2023).
Esveld, S. Lvan et al. A prioritized and validated resource of mitochondrial proteins in plasmodium identifies unique biology. mSphere 6, e00614–e00621 (2021).
Basu, A. et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell 154, 1151–1161 (2013).
Smith, A. C. & Robinson, A. J. MitoMiner v4.0: an updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 47, gky1072 (2018).
Brandina, I. et al. Enolase takes part in a macromolecular complex associated to mitochondria in yeast. Biochim. Biophys. Acta 1757, 1217–1228 (2006).
Wisnovsky, S., Sack, T., Pagliarini, D. J., Laposa, R. R. & Kelley, S. O. DNA polymerase θ increases mutational rates in mitochondrial DNA. ACS Chem. Biol. 13, 900–908 (2018).
Hao, Z. et al. N6-deoxyadenosine methylation in mammalian mitochondrial DNA. Mol. Cell 78, 382–395.e8 (2020).
Chen, W. W., Freinkman, E. & Sabatini, D. M. Rapid immunopurification of mitochondria for metabolite profiling and absolute quantification of matrix metabolites. Nat. Protoc. 12, 2215–2231 (2017).
Fijalkowski, I., Peeters, M. K. R. & Damme, P. V. Small protein enrichment improves proteomics detection of sORF encoded polypeptides. Front. Genet. 12, 713400 (2021).
Giansanti, P., Tsiatsiani, L., Low, T. Y. & Heck, A. J. R. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc. 11, 993–1006 (2016).
Swaney, D. L. & Villén, J. Enrichment of modified peptides via immunoaffinity precipitation with modification-specific antibodies. Cold Spring Harb. Protoc. 2016, pdb.prot088013 (2016).
Brandi, J., Noberini, R., Bonaldi, T. & Cecconi, D. Advances in enrichment methods for mass spectrometry-based proteomics analysis of post-translational modifications. J. Chromatogr. A 1678, 463352 (2022).
Acknowledgements
The authors thank R. Guerra for critical feedback on this manuscript. This work was funded by National Institutes of Health (NIH) grants R35GM131795 and R01DK098672 and funds from the BJC Investigator Program (to D.J.P.), and fellowships from the European Molecular Biology Organization (ALTF 263-2022) and the Swiss National Science Foundation (P500PB_211038) (to P.F.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Michael Ryan who co-reviewed with Matthew Challis, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cancer Therapeutics Response Portal: https://portals.broadinstitute.org/ctrp.v2.1/
Human Phenotype Ontology: https://hpo.jax.org/
protein–protein BLAST: https://blast.ncbi.nlm.nih.gov/
Saccharomyces Genome Database: https://www.yeastgenome.org/
Glossary
- Activity-based protein profiling
-
A proteomic method that uses enzyme-specific chemical probes to elucidate protein–small molecule interactions. Probes can be fluorescent species, biotin, alkynes or other small molecules that bind to their target proteins and enable enrichment for downstream mass spectrometry analyses.
- Affinity-enrichment mass spectrometry
-
A technique that couples affinity purification of a protein or protein complex with mass spectrometry analysis to identify protein–protein interactions (PPIs).
- Crosslinking mass spectrometry
-
A common technique for identifying protein–protein interactions (PPIs). Small molecules with reactive head groups interact with proteins in close proximity and ‘crosslink’ them together. The crosslinked proteins, and the sites of crosslinking, can then be identified using liquid chromatography–mass spectrometry.
- Discovery proteomics
-
An open-ended proteomics analysis that aims to identify as many proteins in a sample as possible without directly targeting any particular protein species. Often used as the first step in defining a particular proteome.
- False discovery rate
-
(FDR). A value that estimates the proportion of false positives among all positive findings in a statistical analysis where FDR = false positives / (false positives + true positives). This value can be cross-validated by withholding a portion of a training set and measuring the rate at which the algorithm can correctly assign values.
- Importomics
-
A method used in the mitochondrial high-confidence proteome (MitoCoP) study to identify proteins that are typically imported into mitochondria by quantifying their degradation following disruption of the mitochondrial import machinery.
- Inborn errors of metabolism
-
A group of monogenic diseases caused by pathogenic variants in genes that code for proteins involved in metabolism.
- Iterative support vector machine learning
-
Iterative support vector machine is a supervised machine learning algorithm that uses a set of training data to build a model that can then be used to classify new data. The algorithm seeks to define a boundary within dimensional space that is used to separate and define classes. This boundary is defined by maximizing the distance between it and the closest individual data points (support vectors) within the training set and is then used by the support vector machine model to make further classification decisions.
- Mass spectrometry
-
An analytical method capable of identifying and quantifying diverse chemical species, including proteins and peptides, by virtue of their mass and related properties.
- Monogenic pathogenic variants
-
Genetic lesions that can cause or increase the risk of developing an inherited disease.
- Naive Bayesian classifier
-
Naive Bayes is a probabilistic machine learning algorithm that uses Bayes’ theorem to classify data points. Bayes theorem defines the probability of an outcome or class (for example, ‘mitochondrial’) being true based on prior knowledge of information related to the outcome. The naive Bayes model will assume that the predictors of the model are independent of one another and assign weights to each predictor.
- Nonsense-mediated mRNA decay
-
A cellular process that degrades mRNAs containing premature stop codons, thereby helping to prevent the production of truncated proteins.
- Primary mitochondrial diseases
-
A group of genetic disorders that are caused by pathogenic mutations in genes that code for proteins that affect mitochondrial function.
- Proximity labelling approaches
-
A class of techniques used to analyse macromolecular complexes, protein interaction networks and subcellular protein localization. These methods employ a promiscuous labelling enzyme that is targeted to a specific cellular location through its genetic fusion to a protein of interest. Addition of a small molecule substrate then enables the promiscuous enzyme to covalently tag other proteins within its vicinity, allowing those proteins to be enriched and identified. Commonly used proximity labelling methods include APEX2 (based on an engineered ascorbate peroxidase) and BioID/TurboID (based on a mutant biotin ligase).
- Small open reading frame
-
(smORF). A compact DNA sequence with fewer than 100 codons that can be translated into one or more proteins.
- Spatial proteomics
-
In the Mitochondrial high Confidence Proteome (MitoCoP) manuscript, spatial proteomics is defined as a technique whereby tissues or subcellular fractions are isolated and then analysed using liquid chromatography–mass spectrometry proteomics. These data can then be used to establish sub-tissue or subcellular protein mapping.
- Subtractive proteomics
-
A technique that helps to assign a protein’s subcellular localization by quantifying its enrichment during purification (for example, quantifying the enrichment of a protein from crude to pure mitochondria).
- Tandem mass spectrometry
-
(MS/MS). A technique that relies on multiple mass analysers assembled in a single mass spectrometer. Following ionization and separation by the first mass analyser, often a quadrupole, ions are fragmented and then analysed in the second mass analyser. This allows for the detection of unique molecules with the same parent (unfragmented) mass.
- Top-down and bottom-up approaches
-
A top-down approach aims to uncover missing elements in a well-defined process (known unknowns), whereas a bottom-up approach aims to assign functions to poorly understood genes or proteins without a predefined notion of what those functions might be (unknown unknowns).
- Whole genome or exome sequencing
-
Techniques used to identify causal genetic variants in patients by sequencing the entire genome or only its protein-coding (exome) portion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baker, Z.N., Forny, P. & Pagliarini, D.J. Mitochondrial proteome research: the road ahead. Nat Rev Mol Cell Biol 25, 65–82 (2024). https://doi.org/10.1038/s41580-023-00650-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00650-7