Abstract
Maintenance of protein homeostasis and organelle integrity and function is critical for cellular homeostasis and cell viability. Autophagy is the principal mechanism that mediates the delivery of various cellular cargoes to lysosomes for degradation and recycling. A myriad of studies demonstrate important protective roles for autophagy against disease. However, in cancer, seemingly opposing roles of autophagy are observed in the prevention of early tumour development versus the maintenance and metabolic adaptation of established and metastasizing tumours. Recent studies have addressed not only the tumour cell intrinsic functions of autophagy, but also the roles of autophagy in the tumour microenvironment and associated immune cells. In addition, various autophagy-related pathways have been described, which are distinct from classical autophagy, that utilize parts of the autophagic machinery and can potentially contribute to malignant disease. Growing evidence on how autophagy and related processes affect cancer development and progression has helped guide efforts to design anticancer treatments based on inhibition or promotion of autophagy. In this Review, we discuss and dissect these different functions of autophagy and autophagy-related processes during tumour development, maintenance and progression. We outline recent findings regarding the role of these processes in both the tumour cells and the tumour microenvironment and describe advances in therapy aimed at autophagy processes in cancer.
Similar content being viewed by others
Introduction
Macroautophagy (herein referred to as autophagy) is a key homeostatic pathway that facilitates the degradation and recycling of cellular material1. The benefits of stimulating autophagy in disease have received increasing interest, for example, in the removal of protein aggregates contributing to neurodegeneration. In cancer, however, the role of autophagy appears to be more complex and depends on tumour stage, biology and the surrounding microenvironment.
During autophagy, a panel of autophagy-related (ATG) gene products orchestrates the formation of a double-membrane vesicle, known as the autophagosome, which encapsulates cellular cargo and fuses with lysosomes, resulting in the degradation of its contents through the activities of lysosomal hydrolases2 (Fig. 1). The ULK complex, which includes UNC-51-like kinase 1 (ULK1) and ULK2, FIP200, ATG13 and ATG101, initiates autophagosome formation and relays cues from cellular signalling hubs involved in nutrient and energy sensing, such as through mechanistic target of rapamycin complex 1 (mTORC1) signalling. Downstream of the ULK complex is the autophagy-specific VPS34 complex I (comprising VPS34, beclin-1, ATG14 and VPS15), which catalyses the production of phosphatidylinositol-3-phosphate (PI3P) on autophagic membranes. PI3P triggers the recruitment of the autophagy conjugation machinery, including the ATG16L1–ATG5–ATG12 complex, ATG3 and ATG7. These proteins facilitate the lipid conjugation of the ATG8 family members (consisting of the microtubule-associated protein 1A/1B-light chain 3 (LC3) and GABARAP subfamilies), which are important during cargo recruitment and autophagosome maturation3,4 (Fig. 1), as well as other processes that involve ATG8–lipid conjugation (see below and Supplementary Box 1). Although cargo recruitment can be non-selective, for example in nutrient-depleted cells where autophagosomes take up different cargoes to recycle crucial nutrients such as amino acids or lipids, autophagy is largely highly selective. This selectivity is facilitated by autophagy cargo receptors (ACRs) (Fig. 1 and Supplementary Box 2), which bind to specific cargoes that have been tagged for degradation via ubiquitin-dependent or ubiquitin-independent processes5. To add further to this complexity, recent studies have unravelled additional roles of ATG proteins beyond autophagosome formation, thereby expanding their functions and implications in disease6 (Box 1). Two additional lysosomal degradation processes exist that are related to (macro)autophagy but do not require the activities of ATG proteins. These include chaperone-mediated autophagy and microautophagy, in which cargo delivery to the lysosome relies on chaperone activity and invagination of the lysosomal membrane to encapsulate cellular material, respectively1.
Autophagy is initiated when nascent double membranes are formed from the endoplasmic reticulum and other sources forming the phagophore. The process is regulated by a complex containing the kinase UNC-51-like kinase 1 (ULK1), which works together with the class III PI3K kinase complex, containing beclin-1 and VPS34, to generate phosphatidylinositol-3-phosphate, thus facilitating expansion of the autophagosome membrane. ATG8 family members, including MAP1LC3 (microtubule-associated protein 1A/1B-light chain 3), commonly referred to as LC3, are converted to a lipidated form (LC3-II) by conjugation to phosphatidylethanolamine, via a complex containing ATG5, ATG12 and ATG16L1. They are then tethered in the phagophore membrane and regulate various steps of autophagosome biogenesis. During selective autophagy, lipidated ATG8 proteins also function in cargo selection, by associating with autophagy cargo receptors (ACRs; also known as selective autophagy receptors (SARs)) that recognize ubiquitylated cargo. The membranous structures grow to form an organelle termed an autophagosome, which ultimately fuses with lysosomes. Cargoes are then degraded by lysosomal hydrolases and the resulting constituent parts such as amino acids, lipids or sugars are transported into the cytosol for de novo biosynthesis or energy production. Autophagy serves to remove misfolded proteins and damaged organelles, which would otherwise lead to aberrant cellular functions, reactive oxygen species (ROS) imbalances, inflammation or defective antigen presentation, thus predisposing the cell to malignant transformation. In some cases, autophagy can facilitate tumour suppression by removing specific factors such as the ACR p62, elevated levels of which are found in many cancers and are thought to be tumour-promoting. Several cancer-associated factors, including the RAS oncoproteins and p53 tumour suppressor, have been shown to regulate autophagy and influence tumour initiation and development. For example, the nutrient-sensing mechanistic target of rapamycin complex 1 (mTORC1) is a repressor of autophagy, whereas the AMP-activated protein kinase (AMPK), which is activated in situations of energetic stress, is a promoter of autophagy. The regulation of autophagy by p53 is complex: at basal, unstimulated levels the tumour suppressor p53 has been reported to repress autophagy; however, when elevated and activated by cellular stress, p53 activates a panel of target genes (including those encoding damage-regulated autophagy modulator 1 (DRAM1) and AMPK through its subunit PRKAB1) that promote autophagy. Conversely, mutant RAS protein is considered to promote autophagy, but its inhibition can also promote autophagy, indicating that the control of autophagy by RAS is complex and probably context specific.
Early findings indicated a dual role of autophagy in cancer, and ongoing studies are contributing to our growing understanding of the underlying mechanisms through which autophagy influences cancer initiation and progression7. It is now widely accepted that autophagy suppresses tumour initiation, but evidence suggests that autophagy processes in established tumours are required to support uncontrolled cell growth and increased metabolic activities, leading to autophagy dependency for tumour maintenance. Moreover, autophagy has important functions within tumour cells themselves (intrinsic) and in the surrounding stroma (extrinsic), both of which have consequences for tumour growth and drug resistance. Overall, the effects of autophagy appear to depend on tumour stage, specific oncogenic mutations and cellular context.
In this Review, we discuss the current understanding and recent developments regarding the role of autophagy during cancer initiation, development and treatment. Also addressed in this Review are the role of autophagy in the tumour environment and recent findings investigating how autophagy in stromal cells can impact various aspects of tumour biology. Furthermore, we present growing evidence that ATG proteins are used for a number of alternative processes that are distinct from classical autophagy and have been broadly termed “autophagy-related” pathways. We discuss these additional functions of ATG proteins and their potential contribution to malignant disease progression. Finally, we describe and discuss the current therapeutic advances that are being investigated and developed to target autophagy to treat tumour development.
Suppression of tumour development
In line with the initial investigations of autophagy in yeast, it is generally accepted that this process functions as a mechanism to promote cell survival8. Seminal studies showed that autophagy was activated to degrade cellular components for the provision of nutrients during periods of nutrient deprivation, and this response was found to be conserved in higher eukaryotes9. It has also become clear that autophagy is highly adaptable to respond to and mitigate different forms of cellular stress including protein and organelle damage and redox imbalance. Autophagy not only contributes to nutrient availability and provides a means for metabolic adaptation, but is also a major homeostatic mechanism within cells that promotes cellular integrity, redox balance and proteostasis1 (Fig. 1). In light of these functions, it is not surprising that autophagy has roles that protect against cancer. In the following subsections we first briefly summarize work on the roles of autophagy as a tumour suppressor mechanism that has been discussed in greater detail elsewhere10,11,12,13,14, to provide essential background understanding for our more detailed discussion of recent developments in the field.
Evidence for autophagy in tumour suppression
The first indication for a tumour-suppressive role of autophagy came from studies of the BECN1 gene, which encodes beclin-1. Analysis of breast cancer cell lines and primary mammary tumour material revealed frequent allelic loss of BECN1 and that mice hemizygous for BECN1 are tumour-prone15,16,17. Subsequent studies have questioned these findings, suggesting that allelic loss of BECN1 may be a result of linkage to the BRCA1 tumour suppressor on chromosome 17q21 (ref. 18). Although the consequences of the loss of the region containing BECN1 remain to be conclusively dissected, it is established that autophagy genes are often perturbed in early tumorigenesis and that autophagy functions in tumour suppression19.
The impacts of autophagy perturbation on tumour formation are both tissue-specific and autophagy gene-specific. Early studies of the Becn1 gene in mice found that whole-body hemizygosity of Becn1 led to tumour formation in lung, liver and lymphatic tissue, but not in other organs and tissues17,20. In addition, deletion of Atg7 alone, without other genetic events, led to the formation of tumours only in the liver21. Subsequent work demonstrated that loss of autophagy in the liver results in cycles of tissue destruction and regeneration, which causes the emergence of hepatocyte-derived progenitor cells that drive early stages of liver tumour initiation22. In other tissues, the role of autophagy is only evident in combination with other genetic lesions. This raises the question as to whether autophagy is an active tumour-suppressive process or whether its complete loss simply results in a microenvironment that is tumour-promoting. Nevertheless, most studies argue for a direct role of autophagy in tumour suppression. Several studies have shown that autophagy itself can be regulated by tumour-suppressive pathways. In particular, the major tumour-suppressive transcription factor p53 has been shown to modulate autophagy in multiple ways (Fig. 1). At basal levels, cytoplasmic p53 can act as a repressor of autophagy23, but when activated by cellular stress such as DNA damage, p53 levels become elevated, resulting in activation of a myriad of genes involved in the promotion of autophagy including DRAM1 (encoding damage-regulated autophagy modulator 1 (DRAM1)) and PRKAB1 (encoding a regulatory subunit of AMP-activated protein kinase (AMPK))24,25. The relationship between p53 and autophagy is somewhat reciprocal, with studies showing that ATG7 represses p53 activation and that chaperone-mediated autophagy elicits the degradation of mutant p53 (refs. 26,27). Other studies describe a selection for cells harbouring inactivation of specific autophagy proteins during disease progression, thus supporting the theory of autophagy pathways as active tumour suppressors. The above-described studies on allelic loss of BECN1 in breast and ovarian cancers provide an example of this. Although they did not establish a definitive link between autophagy and tumour suppression in human cancer, further studies have reported allelic loss or decreased expression of BECN1 in other cancer types28,29. Moreover, recent findings have shown that other autophagy genes, or factors that regulate ATG proteins, are mutated or inactivated to evade the tumour-suppressive effects of autophagy as tumour development progresses. For example, several ATG genes — ATG2B, ATG5, ATG9B and ATG12 — have been reported to contain frameshift mutations in gastrointestinal and liver cancers, and ATG5 and ATG7 have also been shown to be down-regulated in melanoma30,31. Moreover, studies in mouse models found that deletion of the mitophagy receptors BNIP3 or BNIP3L (also known as NIX) in the context of otherwise functional autophagy promoted the development of breast and pancreatic cancer32,33. Effects observed following perturbation of autophagy need to be evaluated carefully to distinguish between effects stemming from total loss of autophagy and those caused by specific components or pathways.
Selective autophagy in tumour suppression
Recent work has implicated selective forms of autophagy in various diseases, including cancer. The multiple forms of selective autophagy have been reviewed extensively elsewhere34,35,36,37. Of these, two forms are particularly relevant to tumour suppression, both of which are involved in mitigating cellular stress caused by reactive oxygen species (ROS), which can cause damage to DNA resulting in mutagenesis and transformation.
Mitophagy, the selective removal of mitochondria, was one of the first forms of selective autophagy to be described and remains the best characterized. As the mechanisms to repair mitochondrial DNA and proteins are less complex and efficient than those active in the nucleus and cytoplasm, mitochondrial fidelity is preserved predominantly by autophagic degradation of damaged mitochondria and replacement by de novo biogenesis38. The importance of mitophagy in tumour suppression is evidenced by accumulation of damaged mitochondria in cells in which key autophagy genes are deleted, leading to accumulation of ROS and DNA damage39,40.
The second form of selective autophagy that is intrinsically connected to the balance of ROS is pexophagy, which mediates the selective removal of peroxisomes41,42. Although it is clear that fatty acid β-oxidation is important in cancer and that pexophagy has an important role in maintaining the balance of ROS42, in comparison with mitophagy, the involvement and importance of pexophagy in cancer are less well defined.
As detailed in Supplementary Box 2, several ACRs are known to function in selective autophagy. The first ACR to be identified was p62 (also known as SQSTM1). Aside from functioning as an ACR, p62 has multiple roles in cancer that are outlined below, including activation of the NF-κB and NRF2 pathways. Activation of either of these pathways is considered tumour-promoting, or, at least, tumour-supporting. Hence, maintaining appropriate levels of p62 through autophagy-mediated degradation is a key tumour-suppressive effect of autophagy. This is best exemplified by studies of liver cancer in mice, in which tumour development caused by deletion of key autophagy genes is reversed upon concomitant deletion of p62 (ref. 21) (see below).
Roles in tumour progression
Initial evidence supporting a role for autophagy in the maintenance of established cancers was based on the finding that some tumour tissues exhibit high levels of LC3 puncta and lipidated LC3 (LC3-II), indicative of accumulated autophagosomes43. However, these static tissue-based readouts strictly show only the levels of autophagosomes, hence they are largely unable to distinguish between induction of autophagy or impairment of autophagosome turnover. This inability to analyse autophagic flux in tissue remains a major limitation of studying autophagy in human cancer. Nevertheless, multiple preclinical studies have demonstrated that autophagy supports the growth and metabolism of advanced tumours downstream of the activation of various oncogenes and/or inactivation of tumour suppressors39,44 (Fig. 2).
Autophagy can support tumour growth and survival through various paths. For example, autophagy has important roles during metabolic adaptation of tumour cells (for example, through the clearance of dysfunctional mitochondria) and escaping immune detection (for example, through NBR1-mediated degradation of major histocompatibility complex class I (MHC-I)). During metastasis, opposing roles have been described for autophagy. Autophagy can support resistance to detachment-induced cell death (anoikis) in delaminating or circulating tumour cells and can promote adaptation to nutrient limitations. However, autophagy has also been shown to be required to maintain tumour dormancy (for example, through the autophagic degradation of the glycolysis mediator PFKFB3) and genomic stability, leading to an increase in polyploid tumour cells following inactivation of autophagy148. Thus, inhibition of autophagy can result in enhanced metastatic growth. Although the mechanisms underlying this tumour-suppressive activity of autophagy are largely unknown, they probably involve multiple autophagic targets, such as NBR1. In epithelial–mesenchymal transition, autophagy has both metastasis-promoting and metastasis-inhibitory effects (through degradation of the epithelial–mesenchymal transition master regulator TWIST1, not shown). ECM, extracellular matrix.
Autophagy promotes cancer following oncogenic activation
Studies using genetically engineered mouse models of cancer driven by oncogenic Ras revealed a requirement for functional autophagy pathways in tumour development. RAS genes are often mutated in certain cancers: for example, 90% of pancreatic ductal adenocarcinomas involve mutation of the KRAS gene45. In its activated state, RAS promotes tumour proliferation and survival and can alone drive tumour development. However, this causes increased demand on cellular energy and anabolic precursors, and, through self-digestion, autophagy serves to mitigate the limited availability of external nutrients and thus to sustain and promote tumour development. Studies have shown that this role leads to autophagy dependency in the progression of certain RAS-driven cancers, and such tumours progress only to a certain degree in the absence of autophagy. In some cases, because autophagy has tumour-suppressive effects in normal cells, the absence of autophagy may even enhance the early stages of tumour development, but in RAS-driven cancers, further progression to cancer was blocked in the absence of other genetic lesions46,47,48.
Progression to cancer is driven not only by the activation of oncogenic factors such as RAS that promote tumour development, but also by the loss of factors that restrict tumour development. These tumour suppressor genes can be activated by oncogenic factors such as RAS49, and they have also been studied in the context of autophagy in tumour development. Two important tumour suppressor genes in cancer are p53 (also known as Trp53 in mice, TP53 in humans) and Pten, the latter encoding phosphatase and tensin homologue (PTEN). Studies in mice have shown that deletion of either of these genes can alleviate this block of tumour development in the absence of autophagy, although this does not always lead to fully established cancers46,48,50,51,52. The progression of pancreatic cancer appears to depend on the p53 status, with total loss of p53 promoting tumour development46, whereas hemizygous deletion or the presence of mutant p53 alone did not48. Moreover, in the case of lung cancer, deletion of p53 in combination with mutant KRAS permits tumour development beyond the state reached with KRAS mutation alone, but only leads to benign tumours (termed oncocytomas) that contain excessive dysfunctional mitochondria52. The loss of a tumour suppressor does not, however, always circumvent autophagy dependency. Mouse models of lung tumours driven by loss of the AMPK activator and tumour suppressor LKB1 (also known as STK11) showed a decreased capacity to adapt to nutrient and energy depletion. In line with this deficiency, it was shown that some tumours depend on autophagy to maintain lipid and amino acid reserves, so much so that deletion of both LKB1 and ATG7 was synthetically lethal53. These different examples indicate that the role of autophagy in cancer can be dependent on the type of oncogenic lesion driving transformation. Further studies are therefore required in other tumour types and in additional models to ascertain where and when autophagy contributes to or inhibits tumour development. These studies are fundamental to target the pathway therapeutically in different cancer types.
Autophagy and tumour metabolism
A common function of autophagy in normal development and tumour progression is to mitigate cellular stress and thus maintain homeostasis and cell survival8,9. This homeostatic role ranges from the provision of nutrients during limited periods of exogenous nutrient deprivation, as occurs in poorly vascularized regions of developing tumours, to the balance of ROS, which if uncontrolled may lead to cell death.
One key difference between tumours and normal tissues lies in their metabolism. Tumours commonly rewire their metabolism to become more anabolic, including a switch from oxidative phosphorylation to glycolysis and the subsequent redirection of glycolytic intermediates into biosynthetic pathways such as the pentose phosphate pathway (required for nucleotide synthesis)54. In such contexts, despite a decreased requirement for ATP production, mitochondrial function is still required for certain anabolic reactions, and autophagy preserves mitochondrial integrity as evidenced by the fact that loss of autophagy leads to an accumulation of defective mitochondria in KRAS-driven cancers52. Furthermore, the deletion of Atg7 in BRAFV600E-driven lung cancer results in deficiency of glutamine, which is crucial to support mitochondrial respiration and survival of tumour cells driven by BRAFV600E (ref. 55). Interestingly, these effects of autophagy inhibition on primary tumour metabolism may result in metabolic and redox adaptations that favour metastatic outgrowth (Fig. 2). For example, mammary cancer cells with impaired mitophagy display enhanced metastatic capacity32. These phenotypes probably arise from the accumulation of damaged mitochondria in mitophagy-deficient cancer cells, resulting in increased ROS levels and consequently a shift from oxidative to glycolytic metabolism, which is proposed to favour both primary tumour growth and metastatic progression.
Beyond mitophagy, the accumulation of the ACR p62 in autophagy-deficient breast cancer cells prevents the proteasomal degradation of a critical glycolysis mediator, PFKFB3, which promotes proliferation and outgrowth of otherwise dormant metastatic tumour cells56. Excessive ROS concentrations in autophagy-deficient cells are frequently mitigated by the induction of NRF2-mediated antioxidant transcriptional programmes secondary to accumulation of p62 (ref. 57). Importantly, NRF2 induction has been implicated in the promotion of metastasis in diverse cancer models58,59. Together, these results show that autophagy deficiency can promote both glycolytic metabolism and NRF2-driven antioxidant programmes, which ultimately activate metabolic programmes that facilitate the dissemination of tumour cells.
Dual roles of autophagy in metastasis
Currently, the role of autophagy on cancer metastasis, the primary cause of mortality in cancer patients, remains controversial. Initial work provided evidence that autophagy promotes several biological pathways crucial for efficient metastasis including migration and invasion60,61,62, modulation of epithelial–mesenchymal transition63,64, resistance to detachment-induced cell death (anoikis)65, adaptation to nutrient deprivation and hypoxia66, and survival in foreign tissue microenvironments44 (Fig. 2). These pro-metastatic effects spurred interest in autophagy inhibition as a potential therapeutic strategy to prevent metastatic disease and late recurrent disease in various cancers44. Preclinical studies using mouse models indeed demonstrated reduced metastasis upon loss or inhibition of autophagy. For example, an in vivo model of hepatocellular carcinoma determined that autophagy promoted both anoikis resistance and metastatic dissemination67. These findings support the hypothesis that autophagy confers a survival advantage to tumour cells lacking contact to extracellular matrix as they disseminate to secondary organs65. Furthermore, early studies using the polyoma middle T oncogene-driven (PyMT) mammary tumour model demonstrated that the genetic deletion of Fip200 (also known as Rb1cc1), a critical regulator of autophagy induction, resulted in reduced primary tumour growth and a concomitant reduction in metastasis to the lung64. However, these initial studies did not examine the specific effects of autophagy on primary tumour versus metastatic phenotypes68.
By contrast, more recent work in multiple models demonstrates that autophagy may restrict key rate-limiting steps in the metastatic cascade (Fig. 2). Many cancers, such as melanoma and carcinomas of the breast and prostate, have been shown to disseminate tumour cells that remain dormant, and clinically undetectable, in the metastatic organ for extended periods of time. Ultimately, these cells undergo proliferative growth, resulting in macro-metastatic lesions that frequently result in the death of the patient. This process of outgrowth of disseminated tumour cells into lethal metastasis is termed ‘metastatic colonization’ and is considered to be a key rate-limiting step in metastatic progression69,70. In recent years, several studies have illuminated important roles for the autophagy pathway in controlling emergence from dormancy and more specifically in suppressing metastatic colonization and outgrowth. For example, transplanted D2.OR mammary cancer cells exhibit dormant behaviour and fail to progress into active metastasis in syngeneic hosts71. Knockdown of Atg3 in these cells causes them to exit dormancy, resulting in proliferative metastatic cells with increased cancer stem-like properties, indicating that autophagy inhibition gives rise to aggressive subpopulations in vivo56. Similarly, in dormant breast cancer models induced via doxorubicin treatment, stable autophagy inhibition by Atg5 knockdown resulted in both escape from dormancy and metastatic recurrence earlier than in autophagy-proficient control cells72. In this study, it is noteworthy that autophagy-deficient metastases exhibited higher frequencies of proliferating polyploid-like cells, suggesting that loss of autophagy may promote genomic instability; however, it remains uncertain how autophagy protects tumour cells from genomic instability or whether such events functionally contribute to metastatic recurrence in these models.
Finally, consistent with the original work on Fip200 in the PyMT model68, PyMT cells genetically deficient for either Atg12 or Atg5 displayed reduced primary tumour growth when orthotopically transplanted into mammary glands73. Yet, upon excision of primary tumours, autophagy-deficient tumours displayed profound increases in spontaneous metastatic recurrence compared to autophagy-competent counterparts. Follow-up experiments demonstrated that the conditional genetic deletion of Atg5 or Atg12 in tumour cells after their dissemination to the lungs resulted in a highly proliferative subpopulation capable of enhanced metastatic outgrowth73. Similar results were found upon Atg12 knockdown in experimental metastasis models based on 4T1 mammary cancer cells73. By contrast, stimulating autophagy by genetic depletion of Rubcn, an established negative regulator of autophagy, was sufficient to attenuate macro-metastatic outgrowth73. Remarkably, autophagy inhibition resulted in the expansion of tumour cell subpopulations exhibiting basal epithelial differentiation, marked by the upregulation of the transcription factor TP63 (p63) and keratin type I cytoskeletal 14 (also known as cytokeratin-14 (CK-14))73. Basal differentiation has been implicated in aggressive, pro-metastatic phenotypes in breast cancer74, yet how autophagy modulates these subpopulations during the metastatic cascade remains an important unanswered question. Overall, these studies implicate autophagy as a stage-specific suppressor of metastatic colonization.
The exact mechanisms through which autophagy inhibition enhances metastatic colonization and outgrowth remains an active area of investigation. In recent years, specific scrutiny has turned to the impaired turnover of ACRs, which mediate selective autophagy and function as multidomain signalling hubs (Supplementary Box 2). The accumulation of ACRs, most notably p62, promotes oncogenic progression and therapeutic resistance in autophagy-deficient cells via diverse, non-mutually exclusive signalling pathways7,75. The most well-characterized role for p62 as a signalling scaffold is its ability to potentiate pro-tumorigenic NF-κB signalling, which has been linked to increased primary tumour growth in the setting of autophagy deficiency64,76. Whether p62-mediated activation of NF-κB pathways similarly promote metastases remains unclear. In addition, p62 has been shown to suppress the degradation of the transcription factor TWIST1, a master regulator of EMT. Accordingly, p62 overexpression promotes mesenchymal differentiation and enhances metastatic tumour growth in vivo77. The accumulation of NBR1, an ACR closely related to p62, has similarly been implicated in metastasis. In mouse mammary cancer models, impaired autophagy results in the accumulation of NBR1, resulting in the development of aggressive subpopulations of tumour cells exhibiting pro-metastatic basal differentiation73. Functional studies support that increased levels of NBR1 are both necessary and sufficient for pulmonary metastatic colonization and the acquisition of these basal differentiation traits73. Overall, these studies implicate accumulation of the ACRs p62 and NBR1 in autophagy-deficient backgrounds as key mediators of the metastatic phenotype.
Roles in the tumour microenvironment
Although most studies of autophagy in cancer have focused on the genetic deletion of ATG genes in tumour cells, a key consideration when employing autophagy modulators in vivo is that such agents invariably regulate autophagy in tumour cells along with the surrounding and distant stromal cells throughout the host. Studies in model organisms have begun to illuminate the effects of systemic genetic autophagy inhibition in various host cells. One elegant, groundbreaking study investigated a role for host autophagy in promoting tumour growth using systemic Atg7 deletion in mice78. The resultant loss of autophagy throughout the animal led to a significantly greater regression of KRAS-driven tumours when compared to inhibiting autophagy only in tumour cells78,79. Importantly, these beneficial effects on tumour regression occurred more rapidly than the lethal metabolic and neurological deteriorations that developed upon conditional Atg7 deletion in adult mice. These results indicate the presence of an optimal therapeutic window for systemic autophagy inhibition as anticancer therapy. As most mice succumbed to neurodegenerative disease, it was proposed that the potential toxicity of autophagy inhibitors could be mitigated by developing agents unable to cross the blood–brain barrier78. In addition, in a model of systemic autophagy inhibition achieved via the inducible expression of a dominant-negative Atg4b mutant, acute autophagy inhibition in established Kras-driven pancreatic tumours resulted in profound tumour regression, implying that both host and tumour cell autophagy contributed to tumorigenesis80.
Autophagy supports host–tumour metabolic cooperation
Tumours are not independent entities but are connected to and develop in concert with host stromal and immune cells. Growing evidence shows that autophagy in host cells contributes to the anabolic rate of tumours. In transplantation models of pancreatic ductal adenocarcinoma (PDAC), autophagy in pancreatic stellate cells, a key constituent of the tumour stroma, is crucial to both generate and extracellularly secrete the nonessential amino acid alanine, which is then used by pancreatic tumour cells for growth and survival in adverse microenvironments81. Systemically, autophagy in one organ may support the growth of a tumour at a distant site. Although arginine is a non-essential amino acid, the enhanced anabolic state associated with tumour development creates a high demand for this amino acid that effectively renders tumour cells auxotrophic for this amino acid82. Whole-body or liver-specific deletion of autophagy results in the release of the arginine-degrading enzyme arginase I from the liver into the blood, which in turn causes decreased levels of circulating arginine and an inability to sustain the growth of a distant primary tumour in the lung79. This may be particularly relevant in tumours with reduced argininosuccinate synthase activity, which is required for de novo arginine synthesis83. This causes tumours to become auxotrophic for arginine and therefore potentially excellent targets for autophagy inhibition in the liver.
These results were further reinforced using a model of autophagy inhibition achieved via the inducible expression of a dominant-negative Atg4b mutant. In this model, acute, whole-body autophagy inhibition in established Kras-driven pancreatic tumours resulted in tumour regression80. Moreover, by inhibiting autophagy in various combinations of host and tumour cells, this study revealed that both host and tumour cell autophagy contributed to tumour growth. Studies in the Drosophila RasV12; scrib−/− tumour model demonstrated that these tumours develop non-cell autonomously and systemically induce autophagy throughout host tissues84,85. Autophagy in the host stromal cells thereby promotes the aggressive growth and invasion of RasV12; scrib−/− tumours throughout the fly. Similar to studies of adult systemic autophagy deletion in mice, the genetic loss of host autophagy in RasV12; scrib−/− tumour-bearing flies has stronger effects on inhibiting tumour growth and proliferation than the loss of autophagy only in the tumour compartment84. Notably, systemic autophagy inhibition achieved via transient Atg5 knockdown has recently been demonstrated to suppress the uptake of glucose and lactate into KrasG12D/+; p53−/− lung tumours in mice, which resulted in impaired tumour growth, adding a new example of how stromal cell autophagy may more broadly influence host–tumour metabolite transfer86.
Taken together, these studies demonstrate important roles for autophagy in different host cells in providing key metabolites, most importantly amino acids, that are employed by proliferating tumour cells to sustain the core metabolic functions of the proliferating tumour. These studies also show that although systemic therapeutic targeting of autophagy may have unwanted side effects in normal tissues such as neurons, autophagy inhibition in the host improves the therapeutic response against the tumour compared to tumour cell-specific targeting of autophagy (Fig. 3).
Studies of autophagy inhibition in host stromal cells, including cancer-associated fibroblasts (CAFs), have illuminated three principal non-cell-autonomous functions through which host cell autophagy impacts the tumour microenvironment. First, autophagy facilitates the production of diverse metabolites such as amino acids, which are released by stromal cells and subsequently used by the tumour cell compartment for growth and proliferation (centre). This metabolic exchange is particularly crucial for tumour cells as these often switch to a largely anabolic state and require high levels of essential amino acids, most notably alanine and asparagine, and non-essential amino acids (NEAA). Second, autophagy supports secretion of pro-inflammatory cytokines from CAFs, including IL-6, IL-8 and IL-1β. These promote tumorigenesis by directly facilitating tumour cell proliferation and modulating innate and adaptive immune cells to create a tumour-permissive immune microenvironment (left). In addition to cytokine secretion, autophagy-related processes, such as microtubule-associated protein 1A/1B-light chain 3 (LC3)-dependent extracellular vesicle (EV) loading and secretion (LDELS) and conjugation of ATG8 to single membrane (CASM), may promote biogenesis and secretion of EVs from both tumour cells and associated stromal cells. How such ATG-dependent EV subpopulations communicate with stromal elements to influence the tumour microenvironment remains unclear. Third, autophagy promotes procollagen proteostasis, which is necessary for type I collagen deposition and creates a stiff, desmoplastic extracellular matrix (ECM) that promotes neo-angiogenesis and primary tumour growth (right).
Autophagy supports the function of cancer-associated fibroblasts
Additional roles for stromal cell autophagy have been implicated in tumorigenesis, including, most notably, the control of protein secretion. These new roles for stromal autophagy have largely been illuminated through studies in cancer-associated fibroblasts (CAFs), the fibroblasts residing within most solid tumours that modulate tumour cell proliferation and behaviour through diverse mechanisms87. CAFs secrete a spectrum of growth and angiogenic factors, inflammatory cytokines, extracellular matrix components and proteases. In head and neck cancer, increased autophagy in fibroblasts correlated with poor patient outcome87. Accordingly, inhibiting fibroblast autophagy was associated with reduced tumour progression in in vitro co-culture models owing to the attenuated secretion of multiple pro-tumorigenic factors, including IL-6, IL-8 and basic fibroblast growth factor (FGF)88.
Autophagy in CAFs has also been implicated in key secretory events required for the desmoplastic stromal response (Fig. 3). Tumour desmoplasia refers to the fibrotic and inflammatory microenvironment associated with poor prognosis in different human solid tumours. Histologically, desmoplasia is marked by evidence of fibroblast activation and type I collagen deposition along with increased tissue stiffness and inflammation89. Autophagy in pancreatic stellate cells, the cells that give rise to the desmoplastic fibrotic stroma commonly observed in PDACs, has been shown to promote the secretion of both extracellular matrix components and inflammatory cytokines from CAFs90. Recent work further provides important mechanistic insight into how fibroblast autophagy promotes this desmoplastic response: in both autochthonous and orthotopic transplant mammary tumour models driven by the PyMT oncogene, the genetic loss of autophagy in CAFs is sufficient to profoundly attenuate primary tumour growth and improve survival of the tumour-bearing host91. Furthermore, the genetic loss of autophagy in fibroblasts causes specific defects in procollagen proteostasis, resulting in impaired type I collagen secretion both in vitro and in vivo91,92. Atomic force microscopic analysis confirmed that these reductions in type I collagen deposition in stroma derived from autophagy-deficient fibroblasts results in reduced tissue stiffness, a biophysical promoter of cancer progression93. In addition to these effects on type I collagen secretion and tissue stiffness, autophagy deficiency in fibroblasts results in reduced secretion of multiple pro-inflammatory cytokines and neo-angiogenesis factors, thereby supporting a role for fibroblast autophagy in directing multiple secretory events that orchestrate the tumour desmoplastic response91. Overall, these studies point to the critical role of stromal autophagy in primary tumour progression and illuminate important mechanisms that may contribute to the potentially beneficial impact of autophagy inhibition in all constituent parts of the tumour for anticancer therapy.
Secretory autophagy
The studies above illustrating the importance of autophagy in the host stroma have coincided with a growing appreciation in the field that autophagy controls extracellular secretion. In addition to its role in lysosomal degradation, the core autophagy machinery has now been implicated in both conventional and unconventional secretory pathways (Fig. 3). Most of the mechanistic work to understand autophagy-dependent secretion has focused on the unconventional secretion of proteins lacking an N-terminal signal peptide using diverse mechanisms collectively termed secretory autophagy94,95. In contrast to proteins that utilize the canonical endoplasmic reticulum–Golgi pathway, these so-called leaderless proteins follow multiple divergent mechanisms that bypass the Golgi on their way to the plasma membrane for secretion outside the cell. ATG proteins were first implicated in the unconventional secretion of acyl-CoA-binding protein (Acb1) in yeast96,97. Multiple targets of secretory autophagy have now been identified in mammals, including IL-1β and IL-18, the high mobility group protein B1 (HMGB1), the integral membrane protein CFTR, cathepsins and insulin-degrading enzymes95. Among these targets, analysis of IL-1β, an important mediator of the inflammatory response, has yielded mechanistic insights. A seminal study demonstrated that mature IL-1β is incorporated into autophagosomes, but subsequently trafficked to the plasma membrane for secretion rather than degraded by lysosomal fusion98. Follow-up studies proposed that IL-1β is incorporated into the space between the outer and inner membrane of double-membrane autophagosome intermediates99. Recent work has suggested that this vesicular structure may in part correspond to the endoplasmic reticulum–Golgi intermediate compartment, and that IL-1β is transported into this compartment through the protein channel TMED10 (ref. 100). During inflammasome activation, IL-1β is released through gasdermin D pores at the plasma membrane, suggesting that autophagy-independent pathways are probably the principal mode of IL-1β secretion in physiological settings101,102. IL-1β directs pleotropic functions in the tumour microenvironment, including effects on inflammation and angiogenesis that promote tumour progression and metastasis103. Hence, clarifying the relative contribution between secretory autophagy and gasdermin D-mediated IL-1β secretion remains an important topic for future study.
More recently, research has implicated autophagy regulators in the unconventional secretion of proteins via small extracellular vesicles (EVs), also known as exosomes (Fig. 3). The ATG8 conjugation machinery was shown to mediate the cargo loading of multiple RNA-binding proteins into EVs through a process termed LC3‐dependent EV loading and secretion (LDELS)104. LDELS also requires LC3-dependent activation of neutral sphingomyelinase (nSMase-2, also known as SMPD3), which has been proposed to mediate intraluminal budding at the multivesicular body during EV biogenesis104. Although the precise roles of LDELS in cancer still remain unknown, it is noteworthy that the ATG8 family protein GABARAPL1 facilitates both cargo loading and the biogenesis of pro-angiogenic EVs in hypoxic tumour cells105. In addition to LDELS, ATG8 family proteins have been implicated in the release of extracellular DNA and histones independently of EVs, although the genetic role of ATG proteins involved in such processes remains obscure106. Recent work has revealed another secretory autophagy pathway activated upon lysosomal inhibition such as treatment with hydroxychloroquine (HCQ), an agent used to inhibit autophagy during anticancer therapy by increasing lysosomal pH11. Several independent studies have demonstrated that pharmacological lysosome inhibition elicits robust extracellular release of both LC3-II and autophagic cargo via EVs and EV-associated secretory intermediates107,108,109. Specifically, lysosomal blockade promotes the extracellular secretion of ACRs, including p62, that are released as EV-associated nanoparticles in a fraction of extracellular vesicles termed extracellular vesicles and particles (EVPs)107. This pathway, termed secretory autophagy during lysosome inhibition (SALI), requires multiple ATG proteins for the progressive steps in autophagosome formation as well as RAB27A, which mediates the release of vesicles outside the cells. Importantly, the ACRs secreted via SALI are detected in vivo in EVPs isolated from blood plasma following HCQ treatment. Accordingly, measuring the autophagy-dependent EVP secretome in human plasma may be a powerful biomarker for non-invasively monitoring the efficacy of next-generation lysosomal inhibitors in cancer treatment110. Overall, these studies highlight potential connections between autophagy regulators and endolysosomal acidification in the control of unconventional secretion mediated by EVs and EVPs. Increasing evidence shows that EVPs facilitate intracellular communication between tumour, stromal and immune cells in the tumour microenvironment and support pre-metastatic niches that favour metastatic growth111. An important unanswered question is how autophagic control of specific EVP cargoes influences cancer progression and the response to therapy.
Despite an abundance of genetic evidence supporting a functional role for ATG proteins in modulating the secretion of cytokines and growth factors in diverse cancer models, our understanding of the cell biological mechanisms through which the autophagy machinery governs conventional secretion is still rudimentary. As detailed above, studies of cancer fibroblasts have revealed a genetic role for autophagy in the secretion of IL-6, IL-8 and other inflammatory cytokines that promote tumorigenesis88,90,91 (Fig. 3). Moreover, multiple ATG players have been implicated in the efficient production and secretion of pro-tumorigenic factors during oncogene-induced senescence and RAS-driven cancer cell invasion in 3D culture models62,112. Nevertheless, to date, it remains uncertain whether autophagy pathways play any direct role in mediating the extracellular release of pro-tumorigenic mediators. Overall, delineating the functions of autophagy-dependent secretion, not only in cancer but also in other disease pathologies, remains an important area for future study.
Autophagy and tumour immunity
Based on its degradative and trafficking functions, it is unsurprising that important immunomodulatory roles for autophagy have been described, including the degradation and presentation of externally derived antigens on MHC-II, as well as cross-presentation of these antigens on MHC-I113,114. In light of the surging interest in the role of tumour-associated immunity in both tumour development and anticancer therapy, particularly immune checkpoint blockade therapy, a large number of recent studies have investigated these immunomodulatory roles of autophagy. A recent review has covered the topic comprehensively, so we refer the reader there for further information115 and restrict our discussion here to a few studies of particular interest. In a study in PDAC, the authors discovered an unexpected role for autophagy in the evasion of immune attacks by targeting MHC-I in cancer cells for autophagic degradation via selective mechanisms involving NBR1 (ref. 116).This process must be intricately controlled, as total loss of MHC-I would lead to an immune attack by natural killer (NK) cells. Encouragingly, blocking autophagy led to the restoration of MHC-I, which reversed the immune evasion seen in PDAC and led to a synergistic enhancement of immune checkpoint blockade therapy116.
Additional genome-wide screening studies have showed that autophagy is important for modulating host immune responses that regulate tumour development117. Furthermore, it was reported that autophagy in the liver represses antitumour T cell responses by stimulation of regulatory T cells. In the lung, enhanced autophagy caused by loss of LKB1 was associated with decreased antigen processing and presentation, thereby compromising immune checkpoint blockade therapy118,119. This seems in contrast to previous reports showing a positive role for autophagy in antigen presentation, whereby, as highlighted above, autophagy mediates degradation of cargoes to produce antigens, which are subsequently presented on the cell surface for recognition by immune cells120,121. Despite these conflicting results, the authors were able to show that inhibition of autophagy by targeting ULK1 restored antigen presentation and synergized with blockade of PD1 (ref. 119). In addition to antigen presentation, autophagy controls immune trafficking into tumours via altering chemokine and cytokine expression in the tumour microenvironment. One of the first examples of increased immune trafficking in response to autophagy inhibition was observed upon FIP200 deletion in PyMT mammary tumours, which led to elevated production of CXCL9 and CXCL10, chemokines that promote the recruitment of antitumour CD8+ cytotoxic T cells into tumours68. Similarly, the genetic or pharmacological ablation of autophagy in B16-F10 melanoma cells results in the increased expression and secretion of CCL5, which enhances NK cell infiltration into tumours122. Because cytotoxic T cells and NK cells play important roles in antitumour immunity and the efficacy of immune checkpoint blockade115, further understanding how tumour cell autophagy influences the infiltration and function of these cytotoxic immune cell populations remains an important area of active investigation.
ATG proteins in alternative pathways
In addition to autophagy, several ATG proteins play critical roles in alternative cellular pathways6. As a result, genetic modulation of ATG regulators affects not only canonical degradative autophagy but also additional processes. Below, we discuss the current state of knowledge and speculate how such processes may be important in cancer.
LC3-associated processes in tumour development
The observation that some phagocytic vesicles are decorated with LC3 led to the identification of a non-classical role of ATG proteins beyond autophagosome formation123. Subsequent studies further expanded this process of LC3-associated phagocytosis (LAP) and identified LAP-like LC3 conjugation on endosomes124, LC3-associated endocytosis (LANDO)125 and LDELS (mentioned above)104. These processes share the conjugation of ATG8 proteins on single membranes, recently referred to as CASM126. CASM processes can be distinguished by the requirement of specific ATG complexes127 (Supplementary Box 1).
During LAP, LC3 conjugation requires the activities of a distinct VPS34 complex (containing VPS34, UVRAG, beclin-1 and VPS15) and Rubicon (encoded by RUBCN), an inhibitor of autophagosome formation123. LAP enhances lysosomal recruitment to phagosomes and phagosome content degradation, thereby suppressing pro-inflammatory signals by facilitating the clearance of phagocytosed substrates. Inhibiting LAP by RUBCN deletion in myeloid cells was shown to enhance type I interferon signalling in tumour-associated macrophages, resulting in T cell-mediated suppression of tumour growth128. Interestingly, elevated expression of Rubicon, required for LAP but not canonical autophagy128,129,130, is observed in a number of cancers, including stomach, liver and breast, and is associated with poor prognosis in patients131. Whether LAP has exclusively tumour-promoting activities across various types and stages of cancer remains to be further studied. It is possible that, similar to canonical autophagy, LAP-mediated suppression of immune cells may have opposing effects during tumour initiation and maintenance.
In addition to LAP, LC3 lipidation on other endocytic compartments has also been observed. These processes are collectively referred to as LAP-like LC3 lipidation, and their relevance in cancer is beginning to emerge. LAP-like LC3 lipidation can be induced by lysosomotropic agents, including high doses of HCQ, and ionophores124 (Fig. 4). Given that HCQ is used as an agent to inhibit autophagy during anticancer therapy, it will be interesting to investigate the contribution of LAP-like LC3 lipidation to the antitumour activity of HCQ. In addition, the process of entosis (cell-in-cell invasion) has been shown to induce LC3 lipidation on the entotic vacuole surrounding the internalized cell, akin to LAP132. This LC3 lipidation promotes the death and lysosomal digestion of the internalized cell and may provide macromolecules to support host cell growth133. Entosis can therefore be pro-tumorigenic by supporting tumour evolution and killing of neighbouring normal cells, thus providing another role of LC3-associated processes during tumour growth134.
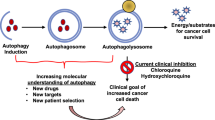
The autophagy pathway is a major contributor to tumour cell survival and as a result is considered a target for cancer therapy. To date, only a small number of autophagy modulators have been described, with the majority of studies focused on inhibition of the lysosomal degradation stage of autophagy, using agents such as hydroxychloroquine (HCQ) or the lysosomal autophagy inhibitor Lys05. Additional inhibitors targeting other stages of the process such as autophagosomal membrane elongation and closure as well as lysosomal fusion are currently largely lacking. Inhibitors against UNC-51-like kinase 1 (ULK1) and VPS34, which act on the initiation stage of autophagy, are showing promise in preclinical studies149,150,151,152. Alternatively, autophagy can be targeted in cancer backgrounds that are particularly dependent on autophagy owing to oncogenic activation of signalling pathways. For example, the RAS–mitogen-associated kinase (MAPK) pathway is activated in a large proportion of cancers through overexpression or mutation of receptor tyrosine kinases (RTKs) and/or mutation of the downstream effectors RAS and RAF153. Inhibitors of this pathway were designed to promote cell death in cases in which the pathway was activated. It was found that inhibition of RAS signalling pathway components causes activation of the kinase LKB1, resulting in the activation of AMP-activated protein kinase (AMPK) and leading to activation of autophagy, which in turn represses cell death and promotes cell survival154,155,156. This has motivated interest in combining autophagy inhibitors with RAS–MAPK pathway inhibitors. Given the opposing roles of autophagy in cancer, a few studies have also indicated that promotion of autophagy may be beneficial for cancer therapy. For example, combination of the tricyclic antidepressant imipramine with the purinergic receptor inhibitor ticlopidine was found to cause excessive autophagy and cell death dependent on autophagy157. The combination showed promising results in preclinical models of glioma. ACR, autophagy cargo receptor; ERK, extracellular signal-regulated kinase; LC3-II, lipidated microtubule-associated protein 1A/1B-light chain 3; MEK, mitogen-activated protein kinase kinase; ULK1i, inhibitor against ULK1; VPS34i, inhibitor against VPS34.
LC3-associated processes can also perform non-degradative roles. LANDO was found to regulate the recycling of cell surface receptors, and inhibition of LANDO in myeloid cells prevented the recycling of receptors involved in the uptake of Aβ amyloid (associated with Alzheimer disease pathogenesis), including CD36, TLR4 and TREM2 (ref. 125). Thus, LANDO inhibition results in increased extracellular levels of Aβ amyloids and an inflammatory response in mouse brains. Interestingly, the expression of TREM2 was recently shown to correlate with poor cancer prognosis135. Whether LANDO-mediated recycling of TREM2 or other receptors can regulate tumour growth and response to immune therapy remains to be investigated.
Autophagic membranes as signalling platforms
Accumulating evidence suggests that tissue and tumour cells derived from autophagy-deficient mice show reduced oncogenic signalling through pathways such as the AKT–PI3K and mitogen-associated kinase (MAPK)–extracellular signal-regulated kinase (ERK) signalling pathways78,136,137. This could simply be a result of the tumour-promoting roles of autophagy discussed throughout this Review, but direct interactions between autophagy players and growth factor signalling have also been reported. Autophagy proteins such as LC3B can co-localize with the receptor tyrosine kinase (RTK) MET (also known as HGFR) and phosphorylated ERK during hepatocyte growth factor stimulation, and the LC3 lipidation machinery is required for optimal MET activation and downstream signalling138,139. The ULK1 complex component ATG13, however, is dispensable for MET activation, indicating that autophagy proteins associate with signalling hubs, termed autophagy-related endomembranes, which are distinct from canonical autophagosomes139. Similarly, epithelial growth factor (EGF)-induced ERK signalling also appears to rely on core ATG players, including ATG5 and ATG7, and phosphorylated ERK colocalizes with LC3 and the ATG16L1–ATG5–ATG12 complex, but not with ULK1 or VPS34 (ref. 137). These results suggest that during the activation of some RTKs, autophagy-related membranes may be used for efficient signalling. It remains unclear, however, whether these signalling hubs are located on double or single membranes within cells and more detailed analyses (for example, using electron microscopy) are required to distinguish their nature.
Growth factor-mediated signalling can also be regulated by ATG players through additional mechanisms. For example, EGFR signalling can be controlled by autophagy-mediated degradation of a pool of perturbed early endosomes that are enlarged and marked by galectin-8 (ref. 140). In the absence of autophagy, EGFR can accumulate on early endosomes, disrupting their endocytic recycling and compromising signalling. As another example, the ATG8 family member LC3C directly binds MET, resulting in its autophagic degradation and thereby negatively regulating MET signalling141. Altogether, these findings suggest a complex interplay between autophagic machinery and oncogenic signalling pathways and warrant further investigation to carefully dissect their role during tumour initiation and/or during later stages of cancer development.
Autophagy-independent roles of ATG proteins in tumorigenesis
The existence of non-autophagy-related activities of ATG proteins that can influence tumorigenesis is important to consider when targeting autophagy in cancer. For example, chemical inhibition of VPS34 lipid kinase activity or genetic ablation of its binding partner beclin-1 are commonly used to suppress autophagy. VPS34, however, is required to generate PI3P on various membranes, including endosomes142. Therefore, the phenotypes observed during VPS34 suppression can result from inhibiting autophagy, inhibiting endocytosis, or both.
As mentioned above, autophagy proteins have documented roles in the secretion of EVs143. This is likely to occur through both autophagy-dependent and autophagy-independent mechanisms. The formation of a non-canonical conjugate between ATG12 and ATG3 (ATG12–ATG3) was shown to be dispensable for LC3 lipidation. By contrast, ATG12–ATG3 can bind to Alix, a component of the endosomal sorting complexes required for transport (ESCRT) complex, to regulate late endosome trafficking and EV secretion144. ATG5 and ATG16L1, but not ATG7, are required for EV secretion through a lipidation-independent recruitment of LC3 that stimulates the de-acidification of multivesicular bodies145. Intriguingly, EV secretion in this model enhances breast cancer cell migration and metastasis, suggesting an autophagy-independent role of ATG proteins in cancer.
FIP200 was shown to suppress the activity of TBK1, a central regulator of both innate immune response and autophagic cargo binding. This regulation of TBK1 activity by FIP200 may occur through autophagy-dependent and autophagy-independent functions of FIP200 (ref. 146). An initial study showed that FIP200 and autophagy facilitate mammary gland tumorigenesis by regulating cancer cell growth and T cell infiltration68. Recent findings from the same group demonstrated that the tumour-supporting function of FIP200 can also be attributed to its autophagy-independent activities147. By expressing an autophagy-deficient mutant of FIP200, the authors showed that whereas autophagy-dependent activities of FIP200 are required during tumour growth and metastasis, its autophagy-independent roles suppress antitumour immune responses potentially by regulating TBK1 activity147.
Multiple additional autophagy-independent activities have been ascribed to ATG proteins with various implications in immune response, vesicular trafficking, cell death and p53 regulation6 (Box 1). Whether and how these functions can impact tumour development remain to be dissected in future studies.
Conclusions and perspectives
Over the last 15 to 20 years, studies delineating the role of autophagy in cancer and its potential as a target for therapy have gathered momentum (Fig. 4 and Box 2). First, from a scientific perspective, it is critical to fully re-evaluate observations based on mice lacking individual ATG genes, such as Atg5 or Atg7, as to whether the resultant cancer phenotypes are directly connected to autophagy or instead involve other processes, including those related to CASM. Second, as detailed above in the sections on autophagy in tumour suppression and tumour progression, it has been known for some time that autophagy has dual roles in cancer. Thus, we need a clearer understanding of how tumours overcome the growth-suppressive effects of autophagy in order to progress, but also to retain or perhaps reinstate autophagy for the survival and maintenance of established tumours. To this end, it is essential to select models that allow us to inhibit or activate autophagy in various tissues and at the different stages of tumour development. From a clinical perspective, it is clear that we need more potent and specific autophagy-targeting drugs. These can be designed to target (i) the turnover stage of autophagy by targeting lysosome activity, (ii) autophagy initiation by targeting factors such as VPS34 or ULK1, or (iii) the promotion of excessive autophagy (Fig. 4). In addition, it is important to consider the genetic background or mutational signatures of individual tumours — for example, by combining autophagy inhibition with RAS–MAPK pathway inhibitors in KRAS-driven cancers, or with therapeutics targeting immune checkpoints. Finally, it will be important to identify strategies to modulate autophagy in cancer that avoid unwanted side effects of autophagy inhibition on metastatic recurrence or potentially neurodegeneration. To achieve this, it is critical to better define the role of autophagy in different cancers and at different stages (for example, primary tumour vs. metastasis) to elucidate how different tumours depend on autophagy — in other words, how effective targeting autophagy will be in individual patients. In addition, understanding the different outcomes resulting from complete genetic inhibition of autophagy, as employed by most studies, and partial autophagy inhibition, as expected from its chemical targeting, may be beneficial when considering deleterious outcomes expected during anticancer treatment. To date, most approaches rely on evaluation of steady-state levels of autophagosomes or LC3-II, which fail to distinguish between autophagosome maturation arrest and enhanced induction of autophagy. Despite the rapid progress made to date in understanding how autophagy influences cancer, only when these issues have been resolved can we successfully leverage both existing and forthcoming novel strategies to inhibit autophagy for the benefit of cancer patients. Encouragingly, however, as detailed above, these approaches could be used in combination with classical chemotherapy, with novel agents that enhance autophagic dependency in tumours or tumour-supporting stroma, or with strategies to engage the antitumour immune response.
References
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Nishimura, T. & Tooze, S. A. Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov. 6, 32 (2020).
Zhao, Y. G., Codogno, P. & Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 22, 733–750 (2021).
Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 21, 439–458 (2020).
Grumati, P. & Dikic, I. Ubiquitin signaling and autophagy. J. Biol. Chem. 293, 5404–5413 (2018).
Galluzzi, L. & Green, D. R. Autophagy-independent functions of the autophagy machinery. Cell 177, 1682–1699 (2019).
Kenific, C. M. & Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 25, 37–45 (2015).
Tsukada, M. & Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333, 169–174 (1993).
Lum, J. J., DeBerardinis, R. J. & Thompson, C. B. Autophagy in metazoans: cell survival in the land of plenty. Nat. Rev. Mol. Cell Biol. 6, 439–448 (2005).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Amaravadi, R. K., Kimmelman, A. C. & Debnath, J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 9, 1167–1181 (2019).
Russell, R. C. & Guan, K. L. The multifaceted role of autophagy in cancer. EMBO J. 41, e110031 (2022).
Amaravadi, R., Kimmelman, A. C. & White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 30, 1913–1930 (2016).
Cassidy, L. D. & Narita, M. Autophagy at the intersection of aging, senescence, and cancer. Mol. Oncol. 16, 3259–3275 (2022).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999).
Aita, V. M. et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59, 59–65 (1999).
Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl Acad. Sci. USA 100, 15077–15082 (2003).
Laddha, S. V., Ganesan, S., Chan, C. S. & White, E. Mutational landscape of the essential autophagy gene BECN1 in human cancers. Mol. Cancer Res. 12, 485–490 (2014).
Lebovitz, C. B., Bortnik, S. B. & Gorski, S. M. Here, there be dragons: charting autophagy-related alterations in human tumors. Clin. Cancer Res. 18, 1214–1226 (2012).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Takamura, A. et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795–800 (2011).
Barthet, V. J. A. et al. Autophagy suppresses the formation of hepatocyte-derived cancer-initiating ductular progenitor cells in the liver. Sci. Adv. https://doi.org/10.1126/sciadv.abf9141 (2021).
Tasdemir, E. et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687 (2008).
Crighton, D. et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126, 121–134 (2006).
Kenzelmann Broz, D. et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 27, 1016–1031 (2013).
Yang, Y. et al. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes Dev. 34, 688–700 (2020).
Vakifahmetoglu-Norberg, H. et al. Chaperone-mediated autophagy degrades mutant p53. Genes Dev. 27, 1718–1730 (2013).
Minamoto, T. et al. Loss of beclin 1 expression in ovarian cancer: a potential biomarker for predicting unfavorable outcomes. Oncol. Lett. 15, 1170–1176 (2018).
Huang, X., Bai, H. M., Chen, L., Li, B. & Lu, Y. C. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J. Clin. Neurosci. 17, 1515–1519 (2010).
Frangez, Z. et al. ATG5 and ATG7 expression levels are reduced in cutaneous melanoma and regulated by NRF1. Front. Oncol. 11, 721624 (2021).
Kang, M. R. et al. Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. J. Pathol. 217, 702–706 (2009).
Chourasia, A. H. et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep. 16, 1145–1163 (2015).
Humpton, T. J. et al. Oncogenic KRAS induces NIX-mediated mitophagy to promote pancreatic cancer. Cancer Disco. 9, 1268–1287 (2019).
Gubas, A. & Dikic, I. A guide to the regulation of selective autophagy receptors. FEBS J. 289, 75–89 (2022).
Kirkin, V. & Rogov, V. V. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76, 268–285 (2019).
Mancias, J. D. & Kimmelman, A. C. Mechanisms of selective autophagy in normal physiology and cancer. J. Mol. Biol. 428, 1659–1680 (2016).
Vargas, J. N. S., Hamasaki, M., Kawabata, T., Youle, R. J. & Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-022-00542-2 (2022).
Pickles, S., Vigie, P. & Youle, R. J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28, R170–R185 (2018).
Mathew, R. & White, E. Autophagy, stress, and cancer metabolism: what doesn’t kill you makes you stronger. Cold Spring Harb. Symp. Quant. Biol. 76, 389–396 (2011).
Poole, L. P. & Macleod, K. F. Mitophagy in tumorigenesis and metastasis. Cell Mol. Life Sci. 78, 3817–3851 (2021).
Li, J. & Wang, W. Mechanisms and functions of pexophagy in mammalian cells. Cells https://doi.org/10.3390/cells10051094 (2021).
Zhang, J. et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat. Cell Biol. 17, 1259–1269 (2015).
Fujii, S. et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 99, 1813–1819 (2008).
Liu, J. & Debnath, J. The evolving, multifaceted roles of autophagy in cancer. Adv. Cancer Res. 130, 1–53 (2016).
Ying, H. et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 30, 355–385 (2016).
Rosenfeldt, M. T. et al. p53 Status determines the role of autophagy in pancreatic tumour development. Nature 504, 296–300 (2013).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Yang, A. et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 4, 905–913 (2014).
Lin, A. W. & Lowe, S. W. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl Acad. Sci. USA 98, 5025–5030 (2001).
Rao, S. et al. A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 5, 3056 (2014).
Rosenfeldt, M. T. et al. PTEN deficiency permits the formation of pancreatic cancer in the absence of autophagy. Cell Death Differ. 24, 1303–1304 (2017).
Guo, J. Y. et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 27, 1447–1461 (2013).
Bhatt, V. et al. Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesis. Genes Dev. 33, 150–165 (2019).
Pavlova, N. N., Zhu, J. & Thompson, C. B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 34, 355–377 (2022).
Strohecker, A. M. et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 3, 1272–1285 (2013).
La Belle Flynn, A. et al. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat. Commun. 10, 3668 (2019).
Inami, Y. et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J. Cell Biol. 193, 275–284 (2011).
Lignitto, L. et al. Nrf2 Activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell 178, 316–329.e18 (2019).
Zhou, X. L., Zhu, C. Y., Wu, Z. G., Guo, X. & Zou, W. The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2 and enhance breast cancer cell growth and metastasis. Oncogene 38, 4028–4046 (2019).
Kenific, C. M. et al. NBR1 enables autophagy-dependent focal adhesion turnover. J. Cell Biol. 212, 577–590 (2016).
Sharifi, M. N. et al. Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep. 15, 1660–1672 (2016).
Lock, R., Kenific, C. M., Leidal, A. M., Salas, E. & Debnath, J. Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov. 4, 466–479 (2014).
Marsh, T., Tolani, B. & Debnath, J. The pleiotropic functions of autophagy in metastasis. J. Cell Sci. https://doi.org/10.1242/jcs.247056 (2021).
Wei, H., Wang, C., Croce, C. M. & Guan, J. L. p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 28, 1204–1216 (2014).
Fung, C., Lock, R., Gao, S., Salas, E. & Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806 (2008).
Rabinowitz, J. D. & White, E. Autophagy and metabolism. Science 330, 1344–1348 (2010).
Peng, Y. F. et al. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9, 2056–2068 (2013).
Wei, H. et al. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 25, 1510–1527 (2011).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Morris, V. L., Tuck, A. B., Wilson, S. M., Percy, D. & Chambers, A. F. Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin. Exp. Metastasis 11, 103–112 (1993).
Aqbi, H. F. et al. Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget 9, 22113–22122 (2018).
Marsh, T. et al. Autophagic degradation of NBR1 restricts metastatic outgrowth during mammary tumor progression. Dev. Cell 52, 591–604 (2020).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Komatsu, M. Potential role of p62 in tumor development. Autophagy 7, 1088–1090 (2011).
Mathew, R. et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062–1075 (2009).
Qiang, L. et al. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl Acad. Sci. USA 111, 9241–9246 (2014).
Karsli-Uzunbas, G. et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 4, 914–927 (2014).
Poillet-Perez, L. et al. Autophagy maintains tumour growth through circulating arginine. Nature 563, 569–573 (2018).
Yang, A. et al. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 8, 276–287 (2018).
Sousa, C. M. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Delage, B. et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 126, 2762–2772 (2010).
Phillips, M. M., Sheaff, M. T. & Szlosarek, P. W. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res. Treat. 45, 251–262 (2013).
Katheder, N. S. et al. Microenvironmental autophagy promotes tumour growth. Nature 541, 417–420 (2017).
Khezri, R. et al. Host autophagy mediates organ wasting and nutrient mobilization for tumor growth. EMBO J. 40, e107336 (2021).
Khayati, K. et al. Transient systemic autophagy inhibition is selectively and irreversibly deleterious to lung cancer. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-22-1039 (2022).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
New, J. et al. Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 77, 6679–6691 (2017).
LeBleu, V. S. & Kalluri, R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Model Mech. https://doi.org/10.1242/dmm.029447 (2018).
Endo, S. et al. Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology 152, 1492–1506.e24 (2017).
Rudnick, J. A. et al. Autophagy in stromal fibroblasts promotes tumor desmoplasia and mammary tumorigenesis. Genes Dev. 35, 963–975 (2021).
Forrester, A. et al. A selective ER-phagy exerts procollagen quality control via a calnexin-FAM134B complex. EMBO J. 38, e99847 (2019).
Piersma, B., Hayward, M. K. & Weaver, V. M. Fibrosis and cancer: a strained relationship. Biochim. Biophys. Acta Rev. Cancer 1873, 188356 (2020).
Deretic, V., Jiang, S. & Dupont, N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 22, 397–406 (2012).
Ponpuak, M. et al. Secretory autophagy. Curr. Opin. Cell Biol. 35, 106–116 (2015).
Duran, J. M., Anjard, C., Stefan, C., Loomis, W. F. & Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188, 527–536 (2010).
Manjithaya, R., Anjard, C., Loomis, W. F. & Subramani, S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 188, 537–546 (2010).
Dupont, N. et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 (2011).
Zhang, M., Kenny, S. J., Ge, L., Xu, K. & Schekman, R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife https://doi.org/10.7554/eLife.11205 (2015).
Zhang, M. et al. A translocation pathway for vesicle-mediated unconventional protein secretion. Cell 181, 637–652.e15 (2020).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Rébé, C. & Ghiringhelli, F. Interleukin-1β and cancer. Cancers https://doi.org/10.3390/cancers12071791 (2020).
Leidal, A. M. et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187–199 (2020).
Keulers, T. G. et al. Secretion of pro-angiogenic extracellular vesicles during hypoxia is dependent on the autophagy-related protein GABARAPL1. J. Extracell. Vesicles 10, e12166 (2021).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445.e18 (2019).
Solvik, T. A. et al. Secretory autophagy maintains proteostasis upon lysosome inhibition. J. Cell Biol. https://doi.org/10.1083/jcb.202110151 (2022).
Sagini, K. et al. Drug-induced lysosomal impairment is associated with the release of extracellular vesicles carrying autophagy markers. Int. J. Mol. Sci. https://doi.org/10.3390/ijms222312922 (2021).
Xu, J. et al. Chloroquine treatment induces secretion of autophagy-related proteins and inclusion of Atg8-family proteins in distinct extracellular vesicle populations. Autophagy 18, 2547–2560 (2022).
Mizushima, N. & Murphy, L. O. Autophagy assays for biological discovery and therapeutic development. Trends Biochem. Sci. 45, 1080–1093 (2020).
Clancy, J. W. & D’Souza-Schorey, C. Tumor-derived extracellular vesicles: multifunctional entities in the tumor microenvironment. Annu. Rev. Pathol. 18, 205–229 (2023).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Gerada, C. & Ryan, K. M. Autophagy, the innate immune response and cancer. Mol. Oncol. 14, 1913–1929 (2020).
Munz, C. Autophagy proteins in antigen processing for presentation on MHC molecules. Immunol. Rev. 272, 17–27 (2016).
Xia, H., Green, D. R. & Zou, W. Autophagy in tumour immunity and therapy. Nat. Rev. Cancer 21, 281–297 (2021).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
Lawson, K. A. et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature 586, 120–126 (2020).
Poillet-Perez, L. et al. Autophagy promotes growth of tumors with high mutational burden by inhibiting a T-cell immune response. Nat. Cancer 1, 923–934 (2020).
Deng, J. et al. ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1 mutant lung cancer. Nat. Cancer 2, 503–514 (2021).
Paludan, C. et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307, 593–596 (2005).
Chemali, M., Radtke, K., Desjardins, M. & English, L. Alternative pathways for MHC class I presentation: a new function for autophagy. Cell Mol. Life Sci. 68, 1533–1541 (2011).
Mgrditchian, T. et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl Acad. Sci. USA 114, E9271–E9279 (2017).
Sanjuan, M. A. et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007).
Jacquin, E. et al. Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy 13, 854–867 (2017).
Heckmann, B. L. et al. LC3-Associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178, 536–551.e14 (2019).
Durgan, J. et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 81, 2031–2040.e8 (2021).
Martinez, J. et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 17, 893–906 (2015).
Cunha, L. D. et al. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell 175, 429–441.e16 (2018).
Matsunaga, K. et al. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 190, 511–521 (2010).
Zhong, Y. et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 (2009).
Asare, P. F. et al. LC3-associated phagocytosis (LAP): a potentially influential mediator of efferocytosis-related tumor progression and aggressiveness. Front. Oncol. 10, 1298 (2020).
Florey, O., Kim, S. E., Sandoval, C. P., Haynes, C. M. & Overholtzer, M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 13, 1335–1343 (2011).
Krajcovic, M., Krishna, S., Akkari, L., Joyce, J. A. & Overholtzer, M. mTOR regulates phagosome and entotic vacuole fission. Mol. Biol. Cell 24, 3736–3745 (2013).
Fais, S. & Overholtzer, M. Cell-in-cell phenomena in cancer. Nat. Rev. Cancer 18, 758–766 (2018).
Molgora, M. et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 182, 886–900.e17 (2020).
Fraser, J., Cabodevilla, A. G., Simpson, J. & Gammoh, N. Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking. Essays Biochem. 61, 597–607 (2017).
Martinez-Lopez, N., Athonvarangkul, D., Mishall, P., Sahu, S. & Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 4, 2799 (2013).
Lampada, A. et al. mTORC1-independent autophagy regulates receptor tyrosine kinase phosphorylation in colorectal cancer cells via an mTORC2-mediated mechanism. Cell Death Differ. 24, 1045–1062 (2017).
Barrow-McGee, R. et al. Beta 1-integrin–c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat. Commun. 7, 11942 (2016).
Fraser, J. et al. Targeting of early endosomes by autophagy facilitates EGFR recycling and signalling. EMBO Rep. 20, e47734 (2019).
Bell, E. S. et al. LC3C-mediated autophagy selectively regulates the met RTK and HGF-stimulated migration and invasion. Cell Rep. 29, 4053–4068.e6 (2019).
Nascimbeni, A. C., Codogno, P. & Morel, E. Local detection of PtdIns3P at autophagosome biogenesis membrane platforms. Autophagy https://doi.org/10.1080/15548627.2017.1341465 (2017).
Leidal, A. M. & Debnath, J. Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB Bioadv 3, 377–386 (2021).
Murrow, L., Malhotra, R. & Debnath, J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 17, 300–310 (2015).
Guo, H. et al. Atg5 disassociates the V1V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev. Cell 43, 716–730.e7 (2017).
Schlütermann, D. et al. FIP200 controls the TBK1 activation threshold at SQSTM1/p62-positive condensates. Sci. Rep. 11, 13863 (2021).
Okamoto, T. et al. FIP200 Suppresses immune checkpoint therapy responses in breast cancers by limiting AZI2/TBK1/IRF signaling independent of its canonical autophagy function. Cancer Res. 80, 3580–3592 (2020).
Mathew, R. et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 21, 1367–1381 (2007).
Kocak, M. et al. Targeting autophagy in disease: established and new strategies. Autophagy 18, 473–495 (2022).
Karmacharya, U. & Jung, J. W. Small molecule inhibitors for Unc-51-like autophagy-activating kinase targeting autophagy in cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24020953 (2023).
Ianniciello, A. et al. ULK1 inhibition promotes oxidative stress-induced differentiation and sensitizes leukemic stem cells to targeted therapy. Sci. Transl. Med. 13, eabd5016 (2021).
Noman, M. Z. et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci. Adv. 6, eaax7881 (2020).
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Kinsey, C. G. et al. Protective autophagy elicited by RAF–>MEK–>ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 25, 620–627 (2019).
Bryant, K. L. et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628–640 (2019).
Lee, C. S. et al. MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Proc. Natl Acad. Sci. USA 116, 4508–4517 (2019).
Shchors, K., Massaras, A. & Hanahan, D. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell 28, 456–471 (2015).
Mathiassen, S. G., De Zio, D. & Cecconi, F. Autophagy and the cell cycle: a complex landscape. Front. Oncol. 7, 51 (2017).
Lee, I. H. et al. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336, 225–228 (2012).
Long, J. S. et al. ATG7 is a haploinsufficient repressor of tumor progression and promoter of metastasis. Proc. Natl Acad. Sci. USA 119, e2113465119 (2022).
Joshi, A. et al. Nuclear ULK1 promotes cell death in response to oxidative stress through PARP1. Cell Death Differ. 23, 216–230 (2016).
Sorbara, M. T. et al. The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity 39, 858–873 (2013).
Gammoh, N. The multifaceted functions of ATG16L1 in autophagy and related processes. J. Cell. Sci. https://doi.org/10.1242/jcs.249227 (2020).
Li, Q. X. et al. The Thr300Ala variant of ATG16L1 is associated with decreased risk of brain metastasis in patients with non-small cell lung cancer. Autophagy 13, 1053–1063 (2017).
Solomon, V. R. & Lee, H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 625, 220–233 (2009).
Chude, C. I. & Amaravadi, R. K. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18061279 (2017).
Eng, C. H. et al. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc. Natl Acad. Sci. USA 113, 182–187 (2016).
Maycotte, P. et al. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8, 200–212 (2012).
McAfee, Q. et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl Acad. Sci. USA 109, 8253–8258 (2012).
Moore, A. R., Rosenberg, S. C., McCormick, F. & Malek, S. RAS-targeted therapies. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-021-00220-6 (2021).
Roskoski, R. Jr. Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacol. Res. 135, 239–258 (2018).
Egan, D. F. et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 59, 285–297 (2015).
Zachari, M., Longo, M. & Ganley, I. G. Aberrant autophagosome formation occurs upon small molecule inhibition of ULK1 kinase activity. Life Sci. Alliance 3, e202000815 (2020).
Towers, C. G. et al. Cancer cells upregulate NRF2 signaling to adapt to autophagy inhibition. Dev. Cell 50, 690–703.e6 (2019).
Komatsu, M. et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12, 213–223 (2010).
Towers, C. G. et al. Mitochondrial-derived vesicles compensate for loss of LC3-mediated mitophagy. Dev. Cell 56, 2029–2042.e5 (2021).
Guo, J. Y. et al. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 30, 1704–1717 (2016).
Nishida, Y. et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 (2009).
Tsuboyama, K. et al. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 354, 1036–1041 (2016).
Acknowledgements
Work in the Tumour Cell Death and Autophagy Laboratory (K.R.) is supported by Cancer Research UK (A17196 and A22903). J.D. receives support from the US National Institutes of Health (CA201849, CA126792, CA213775 and AG057462), the Congressionally Directed Medical Research Program (W81XWH-22-2-0007), the Samuel Waxman Cancer Research Foundation and a Mark Foundation for Cancer Research (Endeavor Award). N.G. is supported by a Cancer Research UK fellowship (C52370/A21586). We would also like to acknowledge all researchers whose studies we have been unable to cite and discuss due to the wide nature of the field.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors have no financial or other conflicts of interest in relation to this review. J.D. would, however, like to disclose that he is on the Scientific Advisory Board of Vescor Therapeutics.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Tor Eric Rusten, Jessie Yanxiang Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Autochthonous
-
The transplantation of cells into the origin tissue from where they were derived.
- Cathepsins
-
Enzymes that cause protein breakdown, principally within lysosomes.
- Cross-presentation
-
The ability of antigen-presenting cells to present extracellular antigens, which are normally presented on MHC-II, on MHC-I; MHC-I and MHC-II are cell membrane proteins that typically present internal or externally derived antigens, respectively, to immune cells.
- Damage-regulated autophagy modulator 1
-
(DRAM1). A p53-inducible, inflammation-inducible lysosomal membrane protein linked to autophagy and mechanistic target of rapamycin complex 1 (mTORC1) activation.
- Detachment-induced cell death
-
(Anoikis) A form of programmed cell death by which cells die following detachment from the extracellular matrix.
- Doxorubicin
-
A chemotherapeutic drug that intercalates with DNA and is used to treat a variety of cancers.
- Endoplasmic reticulum–Golgi intermediate compartment
-
An organelle that mediates traffic from the endoplasmic reticulum to the Golgi complex.
- Endosomal sorting complexes required for transport
-
(ESCRT). A complex important in membrane remodelling during scission and budding and crucial for endosomal sorting.
- Entosis
-
A form of cell death whereby one cell inserts itself into the cytoplasm of a neighbouring cell.
- Epithelial–mesenchymal transition
-
A change in cell shape and structure from a polarized epithelial cell to one with mesenchymal characteristics, thus gaining migratory and invasive properties.
- Gasdermin D
-
A caspase substrate involved in the release of inflammatory cytokines in a form of cell death termed pyroptosis.
- High mobility group protein B1
-
(HMGB1). A chromatin protein that facilitates transcription factor function and alters chromatin structure. Release of HMGB1 from dying cells is engaged by immune cells, leading to inflammation.
- Immune checkpoint blockade
-
(ICB). Cancer therapies that are designed to interfere with the immune checkpoints that tumour cells establish to evade attack by cytotoxic T cells.
- Ionophores
-
Compounds that bind specific ions and facilitate their transport across membranes.
- Lysosomotropic agents
-
Weak bases that can accumulate in lysosomes and disrupt lysosomal function.
- Multivesicular body
-
A cellular organelle involved in trafficking of material to lysosomes and the recycling of factors via the endocytic pathway.
- Natural killer (NK) cells
-
Specialized cytotoxic lymphocytes that function in the innate immune response, particularly in the removal of cells lacking surface expression of MHC-I.
- Neutral sphingomyelinase
-
A hydrolase involved in the breakdown of sphingomyelin into the smaller lipids phosphocholine and ceramide.
- NF-κB pathway
-
A pathway leading to the activation of the NF-κB family of transcription factors, which control inflammatory responses and cell viability.
- NRF2 pathway
-
An antioxidant defence pathway driven by the transcription factor NRF2.
- Orthotopic
-
The grafting or implantation of cells or tissue into their natural site of origin.
- Pancreatic stellate cells
-
Fibroblast-like cells in the pancreas that generate matrix components that can lead to fibrosis.
- Phosphatase and tensin homologue
-
(PTEN). A tumour suppressor that dephosphorylates phosphorylated lipids involved in cellular signalling downstream of RAS.
- Phosphatidylinositol 3-phosphate
-
(PI3P). A phospholipid found in membranes that acts as a critical signalling molecule.
- Receptor tyrosine kinases
-
A family of tyrosine kinases resident on the cell membrane and involved in cell-to-cell communication.
- Senescence
-
A viable cell state induced by ageing or oncogene activation in which cells are permanently cell-cycle arrested.
- Type I interferon signalling
-
A cellular response pathway triggered as a defence mechanism to pathogen infection.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Debnath, J., Gammoh, N. & Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol 24, 560–575 (2023). https://doi.org/10.1038/s41580-023-00585-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00585-z
This article is cited by
-
Crosstalk between m6A modification and autophagy in cancer
Cell & Bioscience (2024)
-
Mechanism study of oleanolic acid derivative, K73-03, inducing cell apoptosis in hepatocellular carcinoma
Cancer Cell International (2024)
-
Crosstalk between cancer-associated fibroblasts and regulated cell death in tumors: insights into apoptosis, autophagy, ferroptosis, and pyroptosis
Cell Death Discovery (2024)
-
USP24-i-101 targeting of USP24 activates autophagy to inhibit drug resistance acquired during cancer therapy
Cell Death & Differentiation (2024)
-
Ferroptosis: a new hunter of hepatocellular carcinoma
Cell Death Discovery (2024)