Abstract
The recent epidemic of Zika virus (ZIKV) in the Americas has revealed the devastating consequences of ZIKV infection, particularly in pregnant women. Congenital Zika syndrome, characterized by malformations and microcephaly in neonates as well as developmental challenges in children, highlights the need for the development of a safe and effective vaccine. Multiple vaccine candidates have been developed and have shown promising results in both animal models and phase I clinical trials. However, important challenges remain for the clinical development of these vaccines. In this Progress article, we discuss recent preclinical studies and lessons learned from first-in-human clinical trials with ZIKV vaccines.
Similar content being viewed by others
Introduction
Zika virus (ZIKV), a flavivirus of the family Flaviviridae, was first isolated in 1947 in the Zika forest in Uganda1. ZIKV is an enveloped, positive-sense single-stranded RNA virus. Its 11 kb genome encodes a single polyprotein that is cleaved into individual proteins. Structural proteins capsid (C), precursor membrane (prM) — which is cleaved into the mature membrane protein (M) — and envelope (ENV) are assembled in virus particles (Fig. 1). The non-structural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 are involved in replication and control host cell processes to favour virus production. Until recently, infection with ZIKV was generally regarded as a self-limited, mild illness with rash, headache, myalgia and conjunctivitis, and few ZIKV infections were reported globally2. In 2007, ZIKV was recognized as the cause of an outbreak in Yap Island, Federated States of Micronesia3, followed in 2013 by an outbreak in French Polynesia4 before spreading to the Americas in 2015 (ref.5) via Easter Island, Chile6. As a result of the sudden rise in congenital abnormalities and occurrences of Guillain–Barré syndrome, the scientific community established a causal association between ZIKV infection and these neurological adverse outcomes7,8,9. This led the WHO to declare ZIKV and its suspected link to birth defects10,11 a Public Health Emergency of International Concern in February 2016 (ref.12).
A single positive-strand RNA copy is packaged in an enveloped virus particle that is assembled by the structural proteins (part a). The non-structural proteins are involved in viral replication and immune evasion. Structural proteins capsid (C), precursor membrane (prM) and envelope (ENV) and non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) are flanked by 5′ and 3′ UTRs (black boxes) (part b). The primary target of neutralizing antibodies is the envelope, which together with the membrane protein is properly folded to display binding epitopes. M protein, mature membrane protein.
Research on this virus then markedly increased13,14,15,16. Studies resolved structures of the virion and the proteins that contribute to pathogenicity17,18,19,20 and defined candidate entry receptors and cell tropism21,22. Neuroprogenitor cells have been described as a preferred target for ZIKV, leading to apoptosis of these cells and congenital Zika syndrome (CZS), including microcephaly and other brain malformations23,24. The tyrosine-protein kinase receptor UFO (AXL)25,26,27, which is highly expressed on human radial glial cells, astrocytes and microglia in the developing human cortex, has been hypothesized to account for the observed neurotropism and the related congenital malformations. However, the role of AXL as an entry receptor for ZIKV remains unknown28.
The close relationship between ZIKV and other well-studied flaviviruses, such as West Nile virus (WNV), Japanese encephalitis virus (JEV), dengue virus (DENV) and tick-borne encephalitis virus (TBEV), has facilitated ZIKV research and the development of vaccines29,30,31. Experience gained over 2 decades of research on these flaviviruses guided vaccine design and suggested that protection against ZIKV can be achieved by antibodies that bind ENV25. Currently, several vaccine candidates are under development (Table 1). These include DNA vaccines, purified inactivated viruses (PIVs), live attenuated viruses (LAVs), mRNA vaccines and viral vectored vaccines (modified vaccinia virus Ankara (MVA), measles virus (MV) and adenovirus (Ad) vectors). These efforts from multiple laboratories have led to the unprecedented pace of ZIKV vaccine development.
In this Progress article, we discuss recent advances in animal models and the results from the first-in-human phase I clinical trials of ZIKV vaccine candidates. In addition, we address potential challenges for the late-stage development of ZIKV vaccine candidates.
CZS and developmental problems
With the rapid spread of ZIKV through the Americas, many detrimental effects on fetuses and neonates were observed following infection in pregnant women32,33. In Brazil, potential confounders, such as the insecticide pyriproxyfen and the tetanus, diphtheria and pertussis (Tdap) vaccine, did not correlate with the increased incidence of birth defects, whereas ZIKV infection confirmed by reverse transcription-PCR (RT-PCR) or antibody detection did correlate, suggesting that ZIKV was the causative agent of CZS8,34. Furthermore, animal studies have shown that ZIKV infection affects fetal development22,35. Moreover, severe developmental problems have been observed in follow-up studies of children born with microcephaly to women confirmed to have been infected with ZIKV during pregnancy36. Developmental problems are also likely to occur in children infected during pregnancy who are born without microcephaly, although detailed studies have not yet been completed37,38,39.
The confirmation of ZIKV as the causative agent for CZS, combined with the severe developmental problems of neonates born with CZS, emphasizes the urgent need for a preventive vaccine. Lessons learned from congenital rubella syndrome further support that an effective vaccine might drastically reduce the incidence of infection and prevent birth defects40. However, until a vaccine is available, education and other preventive measures need to be implemented to prevent CZS41, including the development of antiviral medications42,43.
Vaccines in clinical trials
Several vaccine candidates have undergone successful preclinical development. Neutralizing antibodies were induced for all vaccines tested in mice (Table 1). All vaccines were able to provide short-term protection in mice against challenge with ZIKV. To date, DNA vaccines, mRNA vaccines, PIV vaccines and Ad-based vaccines have also conferred protection in monkeys. There has also been rapid advancement of these candidates into phase I clinical trials44. To date, there are 13 open clinical trials testing a range of ZIKV vaccine concepts, including DNA vaccines, mRNA vaccines, PIV vaccines and viral-vector-based vaccines (Table 2).
DNA vaccines
DNA vaccines are plasmids encoding a transgene of interest under the control of a promoter. DNA vaccines can be developed and produced rapidly, and they can induce both humoral and cellular immune responses45. The first clinical assessment of the safety and immunogenicity of a ZIKV DNA vaccine, expressing the ZIKV precursor membrane and envelope (prM-ENV) genes, was led by GeneOne Life Science and Inovio Pharmaceuticals (clinical trial NCT02809443)46. A total of 40 participants were divided equally between two groups and received either a 1 mg or 2 mg dose of the GLS-5700 DNA vaccine by intradermal injection with electroporation at baseline, with boosts at week 4 and week 12 (Table 2). The vaccine was well tolerated with no severe adverse reactions related to the vaccine. ZIKV-specific antibody levels at week 14 were assessed by enzyme-linked immunosorbent assay (ELISA) and showed 100% seroconversion for binding antibodies in both dose groups, with a geometric mean titre (GMT) of 1,642 (347–7,760) for the 1 mg dose group and a GMT of 2,871 (705–11,688) for the 2 mg dose group. These results indicated that the vaccine-induced antibody responses were dose-dependent. Neutralizing antibody titres above the detection limit were detected in 60% and 63% of the 1 mg and 2 mg dose groups, respectively. Passive transfer of week 14 serum into interferon (alpha and beta) receptor (Ifnar) knockout mice followed by a lethal challenge of ZIKV47 resulted in 92% survival of mice, and this survival was independent of neutralizing antibody titre. This phase I clinical trial showed that the DNA vaccine was safe and well tolerated and that vaccine-induced antibodies were able to protect mice from a lethal challenge of ZIKV.
Clinical trials with DNA vaccines have also been conducted by the Vaccine Research Center (VRC) of the US National Institute of Allergy and Infectious Diseases (NIAID). The first DNA vaccine was designed to express ZIKV prM-ENV with a JEV envelope stem region; the JEV envelope stem was added to increase subvirus particle formation (vaccine VRC5288 and study VRC319; clinical trial NCT02840487). The second DNA vaccine expressed wild-type ZIKV prM-ENV (vaccine VRC5283 and study VRC320; clinical trial NCT02996461)48. In study VRC319, participants received 4 mg doses at 0 and 8 weeks, 0 and 12 weeks, 0, 4 and 8 weeks, or 0, 4, and 20 weeks by intramuscular injection (Table 2). In VRC320, participants received 4 mg doses at 0, 4 and 8 weeks through intramuscular injection or split-dose needle and syringe or needle-free injection with the Stratis device49. Only mild to moderate vaccine-associated adverse events were reported. Neutralizing antibody responses were highest at 4 weeks after final vaccination. In study VRC319, neutralizing antibody GMT titres were 120 (73–197), with detectable neutralizing antibodies in 89% of the participants. In study VRC320, neutralizing antibody responses were detected in 100% of participants of the split-dose, needle-free delivery group, with neutralizing antibody GMTs of 304 (215–430). Both trials showed that the DNA vaccines were well tolerated and immunogenic. The immunogenicity of the wild-type ZIKV prM-ENV DNA vaccine was higher than the immunogenicity observed with the DNA vaccine that included the JEV envelope stem. The VRC5283 vaccine recently advanced into a phase II efficacy trial in regions endemic for ZIKV transmission in South and Central America, the Caribbean and the United States (NCT03110770).
Purified inactivated virus vaccines
Three ZIKV purified inactivated virus vaccine (ZPIV) phase I clinical trials (NCT02963909, NCT02952833 and NCT02937233) were reported as a combined interim analysis of the preliminary results for the identical group for each individual study50. These studies (Table 2) were conducted at Walter Reed Army Institute of Research (WRAIR), Silver Spring, Maryland, United States; Saint Louis University, Saint Louis, Missouri, United States; and Beth Israel Deaconess Medical Center (BIDMC), Boston, Massachusetts, United States. ZPIV contains a chromatographic column-purified, formalin-inactivated ZIKV strain (PRVABC59) that was grown in Vero cells. The interim analysis included the group from each study that received the two-dose regimen of 5 µg aluminium hydroxide adjuvanted ZPIV vaccine, administered intramuscularly on day 1 and day 29. Adverse events related to the vaccine were mild to moderate, with no serious adverse events reported. Neutralizing antibody titres were determined by microneutralization (MN50) assays at WRAIR for all three trials. A total of 95% of participants had peak neutralizing antibody titres with a GMT of 286 (170–481) after the second dose. Adoptive transfer studies with purified immunoglobulin G resulted in complete protection against ZIKV challenge in 41 out of 50 BALB/c mice and reduced viraemia in the mice that were infected. Results from these trials showed that the inactivated ZIKV vaccine was well tolerated and immunogenic and that vaccine-induced antibodies were protective in adoptive transfer studies in mice. The impact of different doses and immunization schedules will be determined in follow-up analyses of the completed studies.
Another phase I clinical trial with a ZIKV inactivated vaccine (TAK-426) led by Takeda Pharmaceutical Company Ltd is ongoing (NCT03343626). A dose escalation study in 240 healthy individuals will assess the safety and immunogenicity of this vaccine candidate.
mRNA vaccines
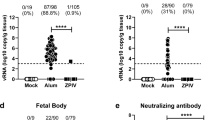
A newer class of vaccines, mRNA vaccines51, has also been developed against ZIKV. mRNA vaccines encode a gene of interest under the control of a promoter. As mRNA is directly translated into a protein after entering the cell cytoplasm, mRNA vaccines bypass the need to traverse the nuclear envelope to be expressed. This pathway could potentially lower the doses needed for mRNA vaccines while retaining the immunogenicity observed with DNA vaccines. ZIKV prM-ENV mRNA was encapsulated in a lipid nanoparticle for delivery and stability52, and immunization of both mice and monkeys with this vaccine induced high levels of neutralizing antibodies that protected against ZIKV infection53,54. In mouse pregnancy models, these mRNA vaccines prevented fetal demise, whereas fetal resorption was observed in nonimmunized infected pregnant mice. However, levels of ZIKV RNA could still be detected in the maternal spleen and brain as well as in the placenta and fetal head in immunized mice55. As these results were obtained in an immunocompromised mouse model, which supports increased viral replication, it remains to be determined whether viral replication would be observed in immunocompetent animal models. The first-in-human, phase I/II clinical trial led by Moderna Therapeutics is currently ongoing to assess the safety and immunogenicity of escalating doses of prM-ENV mRNA (NCT03014089) (Table 1). mRNA vaccines could be cost-effective, as a large number of doses can be produced efficiently. However, it remains to be determined whether the promising preclinical data translate into humans. Additionally, stability of mRNA vaccines needs to be taken into consideration.
Viral-vector-based vaccines
Viral-vector-based vaccines are another promising approach to immunize against various pathogens. These vaccines induce high humoral and cellular immune responses that have been shown to lead to protection against infection in preclinical models56,57. An MV ZIKV vaccine developed by Themis Bioscience GmbH is currently in a phase I clinical trial. The MV Schwarz vaccine strain58 was engineered to express ZIKV prM-ENV (MV-ZIKA) and was tested for immunogenicity in mice and monkeys59. The ongoing clinical trial is assessing the safety and immunogenicity of a high or low dose when given as single or two-dose regimens (NCT02996890). An Ad serotype 26 (Ad26) ZIKV-based vaccine (Ad26.ZIKV.001) expressing the identical antigen as the rhesus Ad serotype 52 (RhAd52) preclinical vaccine candidate, sponsored by Janssen Vaccines and Prevention B.V., is currently also in a phase I clinical trial (NCT03356561). This study aims to test the safety and immunogenicity of two different doses of the vaccine in a double-blind, placebo-controlled clinical trial at two sites in Kansas and Massachusetts, United States. Experience that has already been gained with the Ad26 vaccine vector in clinical trials for other pathogens60,61,62,63 may facilitate the advancement of this vaccine candidate.
Viral-vector-based vaccines have shown promising results in preclinical models for ZIKV, and certain vectors benefit from prior experience in clinical trials for other pathogens61,64. Development of additional vectors with minimal to no pre-existing immunity is also in progress65.
Protection in preclinical models
At the height of the ZIKV epidemic in Brazil, multiple laboratories started to develop vaccine candidates and animal models to assess vaccine efficacy. For example, ZIKV infection in wild-type BALB/c mice and rhesus monkeys largely recapitulated the magnitude and duration of ZIKV viraemia in humans, exhibiting 7 to 10 days of viraemia with minimal clinical symptoms. By contrast, ZIKV infection in immunodeficient mice, such as type I or I/II interferon (A129 or AG129)-knockout mice or signal transducer and activator of transcription 2 (Stat2)-knockout mice, were shown to exhibit prolonged viraemia and have been used to study neurological disease in adult and fetal mice.
The protective efficacy of a ZPIV and a DNA vaccine was first demonstrated in mice66. Moreover, ZPIV, a DNA vaccine and a RhAd52-vector-based vaccine expressing a modified M-ENV ZIKV antigen were shown to block ZIKV infection in rhesus monkeys67. Furthermore, the RhAd52-based vaccine was found to provide durable complete protection in rhesus monkeys against ZIKV a year after immunization with remarkably stable neutralizing antibody titres68.
Studies from a number of groups have reported the protective efficacy of inactivated virus vaccines, DNA vaccines, viral-vector-based vaccines and mRNA vaccines53,54,55,67,69,70,71,72,73,74,75 (Table 1). DNA vaccines expressing variations of the prM-ENV antigen were quickly developed and tested successfully for efficacy in both mice and monkeys69. mRNA vaccines expressing wild-type or modified prM-ENV antigens, leading to the generation of subviral particles, were able to protect mice with a single dose as low as 10 µg (ref.53) or 50 µg for monkeys54. Several live attenuated vaccines have also been developed based on the yellow fever virus YF17D model or the JEV vaccine SA14-14-2 backbone or attenuated through systematic deletions in the 3′ UTR region in the ZIKV genome73,74,75. All live attenuated ZIKV vaccines have proved immunogenic and protective in mice and monkeys. Finally, MVA and MV vectors have been engineered to express the NS1 or prM-ENV proteins of ZIKV, respectively. Protection in preclinical models with these candidates has also been reported72.
The consistent finding from these studies is that protection against ZIKV infection is predominantly antibody-mediated. Several assays are available to measure vaccine-induced antibodies. The observation that protection is antibody-mediated is concordant with the antibody-based protection observed for WNV, JEV and DENV76,77,78. Data suggest that titres of neutralizing antibodies of ~100, as measured by MN50 assays, are protective against ZIKV68. The plaque-reduction neutralization test (PRNT) and a ZIKV reporter viral particle (RVP) assay are other methods that are commonly used to measure neutralizing antibodies54,69. Titres between the assays vary, with the RVP assay reportedly being more sensitive and yielding approximately tenfold higher titres69. CD4+ and CD8+ T cell responses may not be required if levels of neutralizing antibodies exceed this protective threshold66. However, CD8+ T cells induced by ZIKV or DENV infection have been shown to be able to reduce ZIKV viral burden in mice79,80, and further research is needed to determine the impact on short-term and long-term protection.
Protection in pregnancy
ZIKV infection in pregnant women is distinct from infection in non-pregnant women and men81,82,83. For example, more-extended periods of viraemia have been observed in pregnant women and fetuses84, and ZIKV RNA was detected throughout the mother and the fetus in animal models85,86,87. Immune responses of pregnant monkeys and mice infected with ZIKV appear similar to those of non-pregnant infected animals86,88. The ability of ZIKV to cross the fetal–placental barrier and cause damage to the fetus emphasizes the need for a vaccine and highlights the primary goal of vaccination, that is, to prevent CZS. Therefore, it will be important to measure the efficacy of vaccines to prevent fetal malformations. There are a number of aspects to this research that will need to be considered. For example, is sterilizing immunity required for efficacy or is reducing viral replication sufficient? Additionally, do immune correlates established in non-pregnant animals translate to pregnant animals?
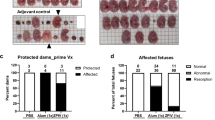
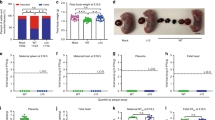
The development of immunodeficient mouse pregnancy models for ZIKV infection has led to important advances owing to the increased viral replication and impact on the central nervous system88,89, and the first prevention of fetal malformations and demise was observed using mRNA and live attenuated ZIKV vaccines55,75,90. However, even though a statistically significant impact on fetal demise was observed, the protection was not sterilizing. ZIKV RNA was still detected in the maternal brain and spleen as well as in the placenta and fetal heads in the majority of animals. Interestingly, pups born to mothers vaccinated with a LAV were protected against lethal intracranial challenge with ZIKV75. Further research is needed to assess the impact of low-level viraemia in fetal and maternal compartments. In addition, with the increasing knowledge on the long-term impact on children born without microcephaly but with confirmed ZIKV infection during pregnancy in humans, it is too early to tell if sterilizing immunity is required to prevent all long-term sequelae91. As a result of the differences between rodents and primates92, a monkey pregnancy model would be preferred93, and initial progress has been reported86,94. In both rhesus and pigtail monkeys, efficient transmission of ZIKV from mother to child during pregnancy has been observed. ZIKV viraemia could be detected in various anatomical compartments in the mothers and fetuses, including the brain and placenta86,94. In addition, anomalies to the brains of the fetuses were detected, ranging from white matter hypoplasia to pathology to the optic nerve and eyes. Similarly, as seen in humans, detrimental effects were more evident when infection occurred in early pregnancy95,96.
It is important to consider that the primary goal of a ZIKV vaccine is to prevent CZS. To realize this goal, vaccines considered for clinical development should ideally be assessed for protective efficacy in preclinical pregnancy models.
Challenges for clinical trials
With the current reduction in ZIKV transmission97,98, a phase III clinical efficacy trial could prove challenging to execute. Further development of animal pregnancy models that can effectively assess protective efficacy against CZS may therefore be important. In addition, ongoing discussions on alternative paths to licensure are being explored. Human challenge clinical trials have been conducted for other diseases, such as typhoid fever99 and influenza100; however, human challenge studies for ZIKV have raised ethical discussions101. Invocation of the FDA Animal Efficacy Rule (also known as the Animal Rule) could also be considered if strong correlations of protection in preclinical models are deemed likely to translate to humans, as outlined below102. Recently, a vaccine against anthrax was the first vaccine that was approved based on the Animal Rule102,103. According to the FDA, the Animal Rule can be pursued only if human efficacy studies cannot be performed, for example, because the conduct of such trials is unethical or because field trials after an accidental or deliberate exposure are not feasible104. For the Animal Rule to apply, the results of well-controlled animal studies need to demonstrate that clinical benefits in humans would likely be observed with the same study products. For vaccines, a clear immune correlate would facilitate the use of the Animal Rule. For ZIKV, the correlate of protection in nonhuman primates appears to be neutralizing antibody titres68.
Conclusions and perspectives
In summary, several ZIKV vaccine candidates have been shown to be safe, well tolerated and immunogenic in humans. In the majority of trial participants, neutralizing antibody titres were induced that were comparable to titres shown to be protective in preclinical models. In addition, development of animal models to test vaccine efficacy in the prevention of CZS is underway.
The remarkable speed with which ZIKV vaccines have been developed has led to a rapid increase in our understanding of this virus. Nevertheless, important challenges remain for conducting clinical efficacy trials and vaccine licensure. Because of CZS and the potential lifelong impact on children born to mothers infected with ZIKV, a vaccine is urgently needed. The rubella vaccine highlights that prevention of congenital defects can be achieved, and similar success may be possible for ZIKV.
References
Dick, G. W., Kitchen, S. F. & Haddow, A. J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520 (1952).
Petersen, L. R., Baden, L. R., Jamieson, D. J., Powers, A. M. & Honein, M. A. Zika virus. N. Engl. J. Med. 374, 1552–1563 (2016).
Duffy, M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 360, 2536–2543 (2009).
Oehler, E. et al. Zika virus infection complicated by Guillain-Barré syndrome — case report, French Polynesia, December 2013. Euro Surveill. 19, 20720 (2014).
Kindhauser, M. K., Allen, T., Frank, V., Santhana, R. S. & Dye, C. Zika: the origin and spread of a mosquito-borne virus. Bull. World Health Organ. 94, 675–686C (2016).
Tognarelli, J. et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch. Virol. 161, 665–668 (2016).
Carteaux, G. et al. Zika virus associated with meningoencephalitis. N. Engl. J. Med. 374, 1595–1596 (2016).
de Araújo, T. V. B. et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect. Dis. 18, 328–336 (2018).
de Oliveira, C. S. & da Costa Vasconcelos, P. F. Microcephaly and Zika virus. J. Pediatr. (Rio J.) 92, 103–105 (2016).
Martines, R. B. et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses — Brazil, 2015. MMWR Morb. Mortal. Wkly Rep. 65, 159–160 (2016).
de Oliveira, W. K. et al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 390, 861–870 (2017).
Valladeau, J. et al. The monoclonal antibody DCGM4 recognizes Langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur. J. Immunol. 29, 2695–2704 (1999).
Sirohi, D. & Kuhn, R. J. Zika virus structure, maturation, and receptors. J. Infect. Dis. 216, S935–S944 (2017).
Faye, O. et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 8, e2636 (2014).
Cunha, M. S. et al. First complete genome sequence of Zika Virus (flaviviridae, flavivirus) from an autochthonous transmission in Brazil. Genome Announc. 4, e00032-16 (2016).
Aid, M. et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell 169, 610–620.e14 (2017).
Kostyuchenko, V. A. et al. Structure of the thermally stable Zika virus. Nature 533, 425–428 (2016).
Song, H., Qi, J., Haywood, J., Shi, Y. & Gao, G. F. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat. Struct. Mol. Biol. 23, 456–458 (2016).
Sirohi, D. et al. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352, 467–470 (2016).
Osuna, C. E. et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 22, 1448–1455 (2016).
Chen, J. et al. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat. Microbiol. 3, 302–309 (2018).
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016).
Tang, H. et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18, 587–590 (2016).
Lin, M.-Y. et al. Zika virus infects intermediate progenitor cells and post-mitotic committed neurons in human fetal brain tissues. Sci. Rep. 7, 14883 (2017).
Nowakowski, T. J. et al. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 18, 591–596 (2016).
Retallack, H. et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl Acad. Sci. USA 113, 14408–14413 (2016).
Meertens, L. et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 18, 324–333 (2017).
Hastings, A. K. et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 19, 558–568 (2017).
Putnak, R. et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in vero cells: immunogenicity and protection in mice and rhesus monkeys. J. Infect. Dis. 174, 1176–1184 (1996).
Monath, T. P. et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J. Infect. Dis. 188, 1213–1230 (2003).
Davis, B. S. et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75, 4040–4047 (2001).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016).
Guillemette-Artur, P., Besnard, M., Eyrolle-Guignot, D., Jouannic, J. M. & Garel, C. Prenatal brain MRI of fetuses with Zika virus infection. Pediatr. Radiol. 46, 1032–1039 (2016).
Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016).
Martinot, A. J. et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 173, 1111–1122.e10 (2018).
Satterfield-Nash, A. et al. Health and development at age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak — Brazil, 2017. MMWR Morb. Mortal. Wkly Rep. 66, 1347–1351 (2017).
Durkin, M. Systematic review of neuromotor impairments in infancy following congenital Zika virus infection. Dev. Med. Child Neurol. 59, 13–13 (2017).
Ventura, C. V., Maia, M., Bravo-Filho, V., Gois, A. L. & Belfort, R. Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 387, 228 (2016).
Schuler-Faccini, L. et al. Possible association between Zika virus infection and microcephaly — Brazil, 2015. MMWR Morb. Mortal. Wkly Rep. 65, 59–62 (2016).
Plotkin, S. A. Rubella eradication. Vaccine 19, 3311–3319 (2001).
van Wouwe, J. P. et al. Women’s reproductive health knowledge, attitudes and practices in relation to the Zika virus outbreak in northeast Brazil. PLoS ONE 13, e0190024 (2018).
Saiz, J.-C. & Martín-Acebes, M. A. The race to find antivirals for Zika virus. Antimicrob. Agents Chemother. 61, e00411-17 (2017).
Shiryaev, S. A. et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 7, 15771 (2017).
Barrett, A. D. T. Zika vaccine candidates progress through nonclinical development and enter clinical trials. NPJ Vaccines 1, 16023 (2016).
Ferraro, B. et al. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. 53, 296–302 (2011).
Tebas, P. et al. Safety and immunogenicity of an anti–Zika virus DNA vaccine — preliminary report. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1708120 (2017).
Aliota, M. T. et al. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 10, e0004682 (2016).
Gaudinski, M. R. et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet 391, 552–562 (2018).
Yousafzai, M. T. et al. Feasibility of conducting intradermal vaccination campaign with inactivated poliovirus vaccine using Tropis intradermal needle free injection system, Karachi, Pakistan. Heliyon 3, e00395 (2017).
Modjarrad, K. et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 391, 563–571 (2018).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Reichmuth, A. M., Oberli, M. A., Jaklenec, A., Langer, R. & Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 7, 319–334 (2016).
Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell 168, 1114–1125.e10 (2017).
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251 (2017).
Richner, J. M. et al. Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170, 273–283.e12 (2017).
Ura, T., Okuda, K. & Shimada, M. Developments in viral vector-based vaccines. Vaccines 2, 624–641 (2014).
Lauer, K. B., Borrow, R., Blanchard, T. J. & Papasian, C. J. Multivalent and multipathogen viral vector vaccines. Clin. Vaccine Immunol. 24, e00298-16 (2017).
Combredet, C. et al. A molecularly cloned schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 77, 11546–11554 (2003).
ZIKAVAX. ZIKAVAX. http://www.zikavax.eu/ (2018).
Barouch, D. H. et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 env vaccine in healthy adults (IPCAVD 001). J. Infect. Dis. 207, 248–256 (2013).
Baden, L. R. et al. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention. Ann. Intern. Med. 164, 313 (2016).
Milligan, I. D. et al. Safety and immunogenicity of novel adenovirus type 26– and modified vaccinia Ankara–vectored Ebola vaccines. JAMA 315, 1610 (2016).
Winslow, R. L. et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus Ankara–vectored Ebola vaccines at 1 year. JAMA 317, 1075 (2017).
Ledgerwood, J. E. et al. Chimpanzee adenovirus vector Ebola vaccine — preliminary report. N. Engl. J. Med. 373, 776 (2015).
Abbink, P. et al. Rapid cloning of novel rhesus adenoviral vaccine vectors. J. Virol. 92, e01924-17 (2018).
Larocca, R. A. et al. Vaccine protection against Zika virus from Brazil. Nature 536, 474–478 (2016).
Abbink, P. et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353, 1129–1132 (2016).
Abbink, P. et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci. Transl Med. 9, eaao4163 (2017).
Dowd, K. A. et al. Rapid development of a DNA vaccine for Zika virus. Science 354, 237–240 (2016).
Xu, K. et al. Recombinant chimpanzee adenovirus vaccine AdC7-M/E protects against Zika virus infection and testis damage. J. Virol. 92, e01722-17 (2018).
Larocca, R. A. et al. Adenovirus serotype 5 vaccine vectors trigger IL-27-dependent inhibitory CD4 + T cell responses that impair CD8 + T cell function. Sci. Immunol. 1, eaaf7643 (2016).
Brault, A. C. et al. A Zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Sci. Rep. 7, 14769 (2017).
Shan, C. et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 23, 763–767 (2017).
Kwek, S. S. et al. A systematic approach to the development of a safe live attenuated Zika vaccine. Nat. Commun. 9, 1031 (2018).
Li, X.-F. et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 9, 673 (2018).
Austin, S. K. & Dowd, K. A. B cell response and mechanisms of antibody protection to West Nile virus. Viruses 6, 1015–1036 (2014).
Xu, M. et al. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines 2, 2 (2017).
Larena, M., Prow, N. A., Hall, R. A., Petrovsky, N. & Lobigs, M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8+ T cells and pre-exposure neutralizing antibody. J. Virol. 87, 4395–4402 (2013).
Wen, J. et al. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2, 17036 (2017).
Elong Ngono, A. et al. Mapping and role of the CD8 + T cell response during primary Zika virus infection in mice. Cell Host Microbe 21, 35–46 (2017).
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151 (2016).
El Costa, H. et al. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 6, 35296 (2016).
Osuna, C. E. & Whitney, J. B. Nonhuman primate models of Zika virus infection, immunity, and therapeutic development. J. Infect. Dis. 216, S928–S934 (2017).
Bhatnagar, J. et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg. Infect. Dis. 23, 405–414 (2017).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016).
Nguyen, S. M. et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 13, e1006378 (2017).
Li, C. et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126 (2016).
Vermillion, M. S. et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat. Commun. 8, 14575 (2017).
Mysorekar, I. U., Phimister, E. G. & Diamond, M. S. Modeling Zika virus infection in pregnancy. N. Engl. J. Med. 375, 481–484 (2016).
Shan, C. et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 8, 676 (2017).
van der Linden, V. et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb. Mortal. Wkly Rep. 65, 1343–1348 (2016).
Malassine, A., Frendo, J. L. & Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9, 531–539 (2003).
Grigsby, P. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin. Reprod. Med. 34, 011–016 (2016).
Adams Waldorf, K. M. et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 22, 1256–1259 (2016).
Lissauer, D., Smit, E. & Kilby, M. D. Zika virus and pregnancy. BJOG 123, 1258–1263 (2016).
Rather, I. A., Lone, J. B., Bajpai, V. K. & Park, Y.-H. Zika virus infection during pregnancy and congenital abnormalities. Front. Microbiol. 8, 581 (2017).
Ulmer, J. B., Deck, R. R., Dewitt, C. M., Donnhly, J. I. & Liu, M. A. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology 89, 59–67 (1996).
van Ballegooijen, M., Bogaards, J. A., Weverling, G. J., Boerlijst, M. C. & Goudsmit, J. AIDS vaccines that allow HIV-1 to infect and escape immunologic control: a mathematic analysis of mass vaccination. J. Acquir. Immune Defic. Syndr. 34, 214–220 (2003).
Feasey, N. A. & Levine, M. M. Typhoid vaccine development with a human challenge model. Lancet 390, 2419–2421 (2017).
Memoli, M. J. et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin. Infect. Dis. 60, 693–702 (2015).
Shah, S. K. et al. Ethical considerations for Zika virus human challenge trials. NIH https://www.niaid.nih.gov/sites/default/files/EthicsZikaHumanChallengeStudiesReport2017.pdf (2017).
US Food and Drug Administration. Product development under the animal rule. Guidance for industry. FDA https://www.fda.gov/downloads/drugs/guidances/ucm399217.pdf (2015).
Beasley, D. W. C., Brasel, T. L. & Comer, J. E. First vaccine approval under the FDA Animal Rule. NPJ Vaccines 1, 16013 (2016).
Hokey, D. A. TB vaccines: the (human) challenge ahead. Mycobact. Dis. 4, e128 (2014).
Author information
Authors and Affiliations
Contributions
P.A., K.E.S. and D.H.B. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests statement
P.A. and D.H.B. are co-inventors on ZIKV vaccine patents that have been licensed to Janssen Vaccines & Prevention B.V.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Microbiology thanks J. Miner, P.-Y. Shi and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Related link
ClinicalTrials.gov: https://clinicaltrials.gov/
Rights and permissions
About this article
Cite this article
Abbink, P., Stephenson, K.E. & Barouch, D.H. Zika virus vaccines. Nat Rev Microbiol 16, 594–600 (2018). https://doi.org/10.1038/s41579-018-0039-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0039-7
This article is cited by
-
Gain-of-function genetic screening identifies the antiviral function of TMEM120A via STING activation
Nature Communications (2022)
-
Protective Zika vaccines engineered to eliminate enhancement of dengue infection via immunodominance switch
Nature Immunology (2021)
-
A review on Zika virus outbreak, epidemiology, transmission and infection dynamics
Journal of Biological Research-Thessaloniki (2020)
-
The mosquito electrocuting trap as an exposure-free method for measuring human-biting rates by Aedes mosquito vectors
Parasites & Vectors (2020)
-
The vaccinia virus based Sementis Copenhagen Vector vaccine against Zika and chikungunya is immunogenic in non-human primates
npj Vaccines (2020)