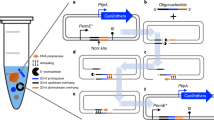
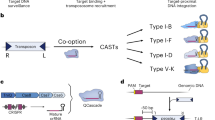
Abstract
Genetic manipulation of microorganisms has been crucial in understanding their biology, yet for many microbial species, robust tools for comprehensive genetic analysis were lacking until the advent of CRISPR–Cas-based gene editing techniques. In this Progress article, we discuss advances in CRISPR-based techniques for the genetic analysis of genetically intractable microorganisms, with an emphasis on mycobacteria, fungi and parasites. We discuss how CRISPR-based analyses in these organisms have enabled the discovery of novel gene functions, the investigation of genetic interaction networks and the identification of virulence factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Griffith, F. The significance of pneumococcal types. J. Hyg. 27, 113 (1928).
Avery, O. T., Macleod, C. M. & McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. J. Exp. Med. 79, 137–158 (1944).
Cohen, S. N., Chang, A. C., Boyer, H. W. & Helling, R. B. Construction of biologically functional bacterial plasmids in vitro. Proc. Natl Acad. Sci. USA 70, 3240–3244 (1973).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Giaever, G. et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 (2002).
Costanzo, M. et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 353, aaf1420 (2016).
van Opijnen, T. & Camilli, A. Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms. Nat. Rev. Microbiol. 11, 435–442 (2013).
Langridge, G. C. et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 19, 2308–2316 (2009).
Gallagher, L. A., Shendure, J. & Manoil, C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2, e00315–10 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Sander, J. D. & Joung, J. K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
DiCarlo, J. E. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343 (2013).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Bikard, D. et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 32, 1146–1150 (2014).
Citorik, R. J., Mimee, M. & Lu, T. K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 32, 1141–1145 (2014).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR–Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17, 5–15 (2015).
La Russa, M. F. & Qi, L. S. The new state of the art: Cas9 for gene activation and repression. Mol. Cell. Biol. 35, 3800–3809 (2015).
Kendall, S. L. & Frita, R. Construction of targeted mycobacterial mutants by homologous recombination. Methods Mol. Biol. 465, 297–310 (2009).
Choudhary, E., Thakur, P., Pareek, M. & Agarwal, N. Gene silencing by CRISPR interference in mycobacteria. Nat. Commun. 6, 6267 (2015).
da Silva Ferreira, M. E. et al. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5, 207–211 (2006).
Weld, R. J., Plummer, K. M., Carpenter, M. A. & Ridgway, H. J. Approaches to functional genomics in filamentous fungi. Cell Res. 16, 31–44 (2006).
Jiang, D. et al. Molecular tools for functional genomics in filamentous fungi: recent advances and new strategies. Biotechnol. Adv. 31, 1562–1574 (2013).
Peng, D., Kurup, S. P., Yao, P. Y., Minning, T. A. & Tarleton, R. L. CRISPR-Cas9-mediated single-gene and gene family disruption in Trypanosoma cruzi. mBio 6, e02097–14 (2014).
Xu, D., Brandán, C. P., Basombrío, M. A. & Tarleton, R. L. Evaluation of high efficiency gene knockout strategies for Trypanosoma cruzi. BMC Microbiol. 9, 90 (2009).
Meissner, M., Breinich, M. S., Gilson, P. R. & Crabb, B. S. Molecular genetic tools in Toxoplasma and Plasmodium: achievements and future needs. Curr. Opin. Microbiol. 10, 349–356 (2007).
Donald, R. G. & Roos, D. S. Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in. Toxoplasma gondii. Mol. Biochem. Parasitol. 63, 243–253 (1994).
Fuller, K. K., Chen, S., Loros, J. J. & Dunlap, J. C. Development of the CRISPR/Cas9 system for targeted gene disruption in. Aspergillus fumigatus. Eukaryot. Cell 14, 1073–1080 (2015).
Enkler, L., Richer, D., Marchand, A. L., Ferrandon, D. & Jossinet, F. Genome engineering in the yeast pathogen Candida glabrata using the CRISPR-Cas9 system. Sci. Rep 6, 35766 (2016).
Liu, Q. et al. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol. Biofuels 10, 1 (2017).
Liu, R., Chen, L., Jiang, Y., Zhou, Z. & Zou, G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 1, 15007 (2015).
Peters, J. M. et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165, 1493–1506 (2016).
Singh, A. K. et al. Investigating essential gene function in Mycobacterium tuberculosis using an efficient CRISPR interference system. Nucleic Acids Res. 44, e143 (2016).
Rock, J. M. et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2, 16274 (2017).
Vyas, V. K., Barrasa, M. I. & Fink, G. R. A. Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci. Adv. 1, e1500248 (2015).
Min, K., Ichikawa, Y., Woolford, C. A. & Mitchell, A. P. Candida albicans gene deletion with a transient CRISPR-Cas9 system. mSphere 1, e00130–16 (2016).
Grahl, N., Demers, E. G., Crocker, A. W. & Hogan, D. A. Use of RNA-protein complexes for genome editing in non-albicans Candida species. mSphere 2, e00218–17 (2017).
Ren, B. & Gupta, N. Taming parasites by tailoring them. Front. Cell. Infect. Microbiol. 7, 292 (2017).
Sidik, S. M. et al. A genome-wide CRISPR screen in Toxoplasma identifies essential Apicomplexan genes. Cell 166, 1423–1435.e12 (2016).
Ghorbal, M. et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 32, 819–821 (2014).
Wagner, J. C., Platt, R. J., Goldfless, S. J., Zhang, F. & Niles, J. C. Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nat. Methods 11, 915–918 (2014).
Vinayak, S. et al. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523, 477–480 (2015).
Zhang, W.-W. & Matlashewski, G. CRISPR-Cas9-mediated genome editing in Leishmania donovani. mBio 6, e00861 (2015).
Babu, M. et al. Quantitative genome-wide genetic interaction screens reveal global epistatic relationships of protein complexes in Escherichia coli. PLoS Genet. 10, e1004120 (2014).
Butler, G. et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459, 657–662 (2009).
Ohtani, N., Tomita, M. & Itaya, M. An extreme thermophile. Thermus thermophilus, is a polyploid bacterium. J. Bacteriol. 192, 5499–5505 (2010).
Soppa, J. Polyploidy in archaea and bacteria: about desiccation resistance, giant cell size, long-term survival, enforcement by a eukaryotic host and additional aspects. J. Mol. Microbiol. Biotechnol. 24, 409–419 (2014).
Dave, K. et al. in Genetic Transformation Systems in Fungi Vol. 2 (eds van den Berg, M. A. & Maruthachalam, K.) 141–153 (Springer, Cham, 2014).
Nødvig, C. S., Nielsen, J. B., Kogle, M. E. & Mortensen, U. H. A. CRISPR-Cas9 system for genetic engineering of filamentous fungi. PLoS ONE 10, e0133085 (2015).
Striepen, B. & Soldati, D. In Toxoplasma gondii 1st edn (eds Weiss, L. M. & Kim, K.) 391–418 (Academic Press, 2007).
Kangussu-Marcolino, M. M., Cunha, A. P., Avila, A. R., Herman, J.-P. & DaRocha, W. D. Conditional removal of selectable markers in Trypanosoma cruzi using a site-specific recombination tool: proof of concept. Mol. Biochem. Parasitol. 198, 71–74 (2014).
Shapiro, R. S. et al. A CRISPR-Cas9-based gene drive platform for genetic interaction analysis in Candida albicans. Nat. Microbiol. 3, 73–82 (2018).
Norris, A. D., Gracida, X. & Calarco, J. A. CRISPR-mediated genetic interaction profiling identifies RNA binding proteins controlling metazoan fitness. eLife 6, e28129 (2017).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Huang, H. et al. CRISPR/Cas9-based efficient genome editing in Clostridium ljungdahlii, an autotrophic gas-fermenting bacterium. ACS Synth. Biol. 5, 1355–1361 (2016).
Wang, Y. et al. Bacterial genome editing with CRISPR-Cas9: deletion, integration, single nucleotide modification, and desirable ‘clean’ mutant selection in Clostridium beijerinckii as an example. ACS Synth. Biol. 5, 721–732 (2016).
Nagaraju, S., Davies, N. K., Walker, D. J. F., Köpke, M. & Simpson, S. D. Genome editing of Clostridium autoethanogenum using CRISPR/Cas9. Biotechnol. Biofuels 9, 219 (2016).
Cobb, R. E., Wang, Y. & Zhao, H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 4, 723–728 (2015).
Zhang, M. M. et al. CRISPR–Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat. Chem. Biol. 13, 607–609 (2017).
Pohl, C., Kiel, J. A. K. W., Driessen, A. J. M., Bovenberg, R. A. L. & Nygård, Y. CRISPR/Cas9 based genome editing of Penicillium chrysogenum. ACS Synth. Biol. 5, 754–764 (2016).
Weninger, A., Hatzl, A.-M., Schmid, C., Vogl, T. & Glieder, A. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris. J. Biotechnol. 235, 139–149 (2016).
Schwartz, C., Shabbir-Hussain, M., Frogue, K., Blenner, M. & Wheeldon, I. Standardized markerless gene integration for pathway engineering in Yarrowia lipolytica. ACS Synth. Biol. 6, 402–409 (2017).
Mohr, S. E., Smith, J. A., Shamu, C. E., Neumüller, R. A. & Perrimon, N. RNAi screening comes of age: improved techniques and complementary approaches. Nat. Rev. Mol. Cell Biol. 15, 591–600 (2014).
Shabalina, S. A. & Koonin, E. V. Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. 23, 578–587 (2008).
Rusk, N. Microbiology: Prokaryotic RNAi. Nat. Methods 9, 220–221 (2012).
van der Oost, J., Swarts, D. C. & Jore, M. M. Prokaryotic Argonautes — variations on the RNA interference theme. Microb. Cell Fact. 1, 158–159 (2014).
Kolev, N. G., Tschudi, C. & Ullu, E. RNA interference in protozoan parasites: achievements and challenges. Eukaryot. Cell 10, 1156–1163 (2011).
Baum, J. et al. Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res. 37, 3788–3798 (2009).
van Opijnen, T., Bodi, K. L. & Camilli, A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6, 767–772 (2009).
Li, T. et al. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 39, 6315–6325 (2011).
Straimer, J. et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nat. Methods 9, 993–998 (2012).
Ji, W. et al. Specific gene repression by CRISPRi system transferred through bacterial conjugation. ACS Synth. Biol. 3, 929–931 (2014).
Yosef, I., Manor, M., Kiro, R. & Qimron, U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl Acad. Sci. USA 112, 7267–7272 (2015).
Soares Medeiros, L. C. et al. Rapid, selection-free, high-efficiency genome editing in protozoan parasites using CRISPR-Cas9 ribonucleoproteins. mBio 8, e01788–17 (2017).
Pyne, M. E., Bruder, M. R., Moo-Young, M., Chung, D. A. & Chou, C. P. Harnessing heterologous and endogenous CRISPR-Cas machineries for efficient markerless genome editing in Clostridium. Sci. Rep. 6, 25666 (2016).
Li, Y. et al. Harnessing Type I and Type III CRISPR-Cas systems for genome editing. Nucleic Acids Res. 44, e34 (2016).
Schaefer, K. A. et al. Unexpected mutations after CRISPR–Cas9 editing in vivo. Nat. Methods 14, 547–548 (2017).
Pawluk, A., Davidson, A. R. & Maxwell, K. L. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2018).
Gaudelli, N. M. et al. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Kuscu, C. et al. CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat. Methods 14, 710–712 (2017).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Luesch, H. et al. A genome-wide overexpression screen in yeast for small-molecule target identification. Chem. Biol. 12, 55–63 (2005).
Kitagawa, M. et al. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12, 291–299 (2005).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247.e17 (2016).
Cohen, N. R. et al. A role for the bacterial GATC methylome in antibiotic stress survival. Nat. Genet. 48, 581–586 (2016).
Rai, L. S., Singha, R., Brahma, P. & Sanyal, K. Epigenetic determinants of phenotypic plasticity in. Candida albicans. Fungal Biol. Rev. 32, 10–19 (2018).
Robert McMaster, W., Morrison, C. J. & Kobor, M. S. Epigenetics: a new model for intracellular parasite–host cell regulation. Trends Parasitol. 32, 515–521 (2016).
Adamson, B. et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167, 1867–1882.e21 (2016).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Kanjee, U. et al. CRISPR/Cas9 knockouts reveal genetic interaction between strain-transcendent erythrocyte determinants of Plasmodium falciparum invasion. Proc. Natl Acad. Sci. USA 114, E9356–E9365 (2017).
Friedland, A. E. et al. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10, 741–743 (2013).
Hwang, W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Li, J.-F. et al. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31, 688–691 (2013).
Acknowledgements
Work in the authors’ laboratory was supported by the Defense Threat Reduction Agency grant HDTRA1-15-1-0051, the Paul G. Allen Frontiers Group, the Wyss Institute for Biologically Inspired Engineering and the Broad Institute of MIT and Harvard. A.C. acknowledges support from the Burroughs Wellcome Fund Career Award for Medical Scientists.
Author information
Authors and Affiliations
Contributions
R.S.S. researched data for the article and wrote the article. J.J.C., A.C. and R.S.S. made substantial contributions to discussions of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shapiro, R.S., Chavez, A. & Collins, J.J. CRISPR-based genomic tools for the manipulation of genetically intractable microorganisms. Nat Rev Microbiol 16, 333–339 (2018). https://doi.org/10.1038/s41579-018-0002-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0002-7
This article is cited by
-
Microbial technologies for heavy metal remediation: effect of process conditions and current practices
Clean Technologies and Environmental Policy (2023)
-
A Simple Allelic Exchange Method for Efficient Seamless Knockout of Up to 34-kbp-Long Gene Cassettes in Pseudomonas
Applied Biochemistry and Biotechnology (2023)
-
Deciphering the recent trends in pesticide bioremediation using genome editing and multi-omics approaches: a review
World Journal of Microbiology and Biotechnology (2023)
-
High-content CRISPR screening
Nature Reviews Methods Primers (2022)
-
Antibiotic residues in environment: antimicrobial resistance development, ecological risks, and bioremediation
Environmental Science and Pollution Research (2022)