Abstract
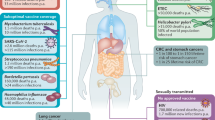
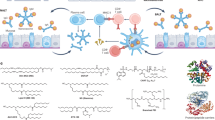
Mucosal pathogens, as exemplified by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), human immunodeficiency virus (HIV) and Mycobacterium tuberculosis, lead to substantial morbidity and mortality worldwide and pose serious threats to global health. Mucosal vaccination is crucial to combating mucosal pathogens because it enables the immune system to directly target and neutralize pathogens at their point of entry. Mucosal vaccines need to penetrate the mucus layer, reach the target tissue and activate robust immune responses in the mucosal tissues. Material-based strategies are necessary to meet these requirements. In this Review, we provide an overview of current mucosal vaccines, categorized by administration route, to highlight the importance of material design in overcoming the existing delivery challenges. We discuss the different classes of materials currently being used as vaccine carriers to induce antigen-specific mucosal immunity, including lipids, natural and synthetic polymers, inorganic materials and pathogen-inspired materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Miquel-Clopés, A., Bentley, E. G., Stewart, J. P. & Carding, S. R. Mucosal vaccines and technology. Clin. Exp. Immunol. 196, 205–214 (2019).
Knisely, J. M. et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities — workshop report. NPJ Vaccines 8, 53 (2023).
Mudgal, R., Nehul, S. & Tomar, S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum. Vaccin. Immunother. 16, 2921–2931 (2020).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e13 (2020).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2021).
Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 34, 575–608 (2016).
France, M. M. & Turner, J. R. The mucosal barrier at a glance. J. Cell Sci. 130, 307–314 (2017).
Huang, M., Zhang, M., Zhu, H., Du, X. & Wang, J. Mucosal vaccine delivery: a focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 12, 3456–3474 (2022).
Anggraeni, R., Ana, I. D. & Wihadmadyatami, H. Development of mucosal vaccine delivery: an overview on the mucosal vaccines and their adjuvants. Clin. Exp. Vaccin. Res. 11, 235 (2022).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
Holmgren, J., Czerkinsky, C., Lycke, N. & Svennerholm, A.-M. Mucosal immunity: implications for vaccine development. Immunobiology 184, 157–179 (1992).
Kraan, H. et al. Buccal and sublingual vaccine delivery. J. Control. Release 190, 580–592 (2014).
Allen, A. & Carroll, N. J. H. Adherent and soluble mucus in the stomach and duodenum. Dig. Dis. Sci. 30, 55S–62S (1985).
Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 (2001).
Corr, S. C., Gahan, C. C. G. M. & Hill, C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 52, 2–12 (2008).
Leal, J., Smyth, H. D. C. & Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 532, 555–572 (2017).
Cone, R. A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85 (2009).
Schuster, B. S., Suk, J. S., Woodworth, G. F. & Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 34, 3439–3446 (2013).
Li, M. et al. Mucosal vaccines: strategies and challenges. Immunol. Lett. 217, 116–125 (2020).
Skwarczynski, M. & Toth, I. Non-invasive mucosal vaccine delivery: advantages, challenges and the future. Expert Opin. Drug Deliv. 17, 435–437 (2020).
Bull, N. C. et al. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+ KLRG1− CD4+ T cells. Mucosal Immunol. 12, 555–564 (2019).
Dijkman, K. et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 25, 255–262 (2019).
Li, Y., Jin, L., Chen, T. & Pirozzi, C. J. The effects of secretory IgA in the mucosal immune system. Biomed. Res. Int. https://doi.org/10.1155/2020/2032057 (2020).
Kubagawa, H. et al. Analysis of paraprotein transport into the saliva by using anti-idiotype antibodies. J. Immunol. 138, 435–439 (1987).
Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 11, S45–S53 (2005).
Sasson, S. C., Gordon, C. L., Christo, S. N., Klenerman, P. & Mackay, L. K. Local heroes or villains: tissue-resident memory T cells in human health and disease. Cell. Mol. Immunol. 17, 113–122 (2020).
Kozlowski, P. A., Cu-Uvin, S., Neutra, M. R. & Flanigan, T. P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65, 1387–1394 (1997).
Eriksson, K. et al. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect. Immun. 66, 5889–5896 (1998).
Gockel, C. M., Bao, S. & Beagley, K. W. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol. Immunol. 37, 537–544 (2000).
Johansson, E. L., Wassén, L., Holmgren, J., Jertborn, M. & Rudin, A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69, 7481–7486 (2001).
Belyakov, I. M., Hammond, S. A., Ahlers, J. D., Glenn, G. M. & Berzofsky, J. A. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Invest. 113, 998–1007 (2004).
Moldoveanu, Z., Clements, M. L., Prince, S. J., Murphy, B. R. & Mestecky, J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 13, 1006–1012 (1995).
Marconescu, P. S., Smallshaw, J. E., Pop, L. M., Ruback, S. L. & Vitetta, E. S. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine 28, 5315–5322 (2010).
Challacombe, S. J., Rahman, D. & O’Hagan, D. T. Salivary, gut, vaginal and nasal antibody responses after oral immunization with biodegradable microparticles. Vaccine 15, 169–175 (1997).
Eng, N. F., Garlapati, S., Gerdts, V., Babiuk, L. A. & Mutwiri, G. K. PCEP enhances IgA mucosal immune responses in mice following different immunization routes with influenza virus antigens. J. Immune Based Ther. Vaccines 8, 4 (2010).
Yang, Z., Zhao, Q., Gao, Y. A. & Zhang, W. Combined oral and intravenous immunization stimulates strong IgA responses in both systemic and mucosal compartments. PLoS ONE 11, e0168037 (2016).
Kuo-Haller, P., Cu, Y., Blum, J., Appleton, J. A. & Saltzman, W. M. Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity. Mol. Pharm. 7, 1585–1595 (2010).
Henriques, P., Fortuna, A. & Doktorovová, S. Spray dried powders for nasal delivery: process and formulation considerations. Eur. J. Pharm. Biopharm. 176, 1–20 (2022).
Chavda, V. P., Baviskar, K. P., Vaghela, D. A., Raut, S. S. & Bedse, A. P. Nasal sprays for treating COVID-19: a scientific note. Pharmacol. Rep. 75, 249–265 (2023).
Luczo, J. M. et al. Intranasal powder live attenuated influenza vaccine is thermostable, immunogenic, and protective against homologous challenge in ferrets. NPJ Vaccines 6, 59 (2021).
Patil, H. P. et al. Adjuvantation of pulmonary-administered influenza vaccine with GPI-0100 primarily stimulates antibody production and memory B cell proliferation. Vaccines 5, 19 (2017).
Canelli, E. et al. Nano-adjuvanted dry powder vaccine for the mucosal immunization against airways pathogens. Front. Vet. Sci. 10, 1116722 (2023).
Rossi, I. et al. A respirable HPV-L2 dry-powder vaccine with GLA as amphiphilic lubricant and immune-adjuvant. J. Control. Rel. 340, 209–220 (2021).
Prausnitz, M. R., Mitragotri, S. & Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug. Discov. 3, 115–124 (2004).
Bachmann, M. F. & Jennings, G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Czerkinsky, C., Çuburu, N., Kweon, M.-N., Anjuere, F. & Holmgren, J. Sublingual vaccination. Hum. Vaccin. 7, 110–114 (2011).
Aran, K. et al. An oral microjet vaccination system elicits antibody production in rabbits. Sci. Transl. Med. 9, eaaf6413 (2017).
Jones, A. T. et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 10, 798 (2019).
Ma, Y. et al. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm. Res. 31, 2393–2403 (2014).
Mašek, J. et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles — important step towards effective mucosal vaccines. J. Control. Release 249, 183–195 (2017).
Ou, B., Yang, Y., Lv, H., Lin, X. & Zhang, M. Current progress and challenges in the study of adjuvants for oral vaccines. BioDrugs 37, 143–180 (2023).
Mokabari, K., Iriti, M. & Varoni, E. M. Mucoadhesive vaccine delivery systems for the oral mucosa. J. Dent. Res. 102, 709–718 (2023).
Coffey, J. W., Gaiha, G. Das & Traverso, G. Oral biologic delivery: advances toward oral subunit, DNA, and mRNA vaccines and the potential for mass vaccination during pandemics. Annu. Rev. Pharmacol. Toxicol. 61, 517–540 (2021).
Corthésy, B. & Bioley, G. Lipid-based particles: versatile delivery systems for mucosal vaccination against infection. Front. Immunol. 9, 431 (2018).
Schwendener, R. A. Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines 2, 152–182 (2014).
Wang, D. et al. Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response. Vaccine 28, 3134–3142 (2010).
Pang, Y. et al. Reduction of salmonella enteritidis number after infections by immunization of liposome-associated recombinant SefA. Avian Dis. 57, 627–633 (2013).
Gupta, P. N. & Vyas, S. P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf. B 82, 118–125 (2011).
Wang, N. et al. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 88, 194–206 (2014).
Ma, T., Wang, L., Yang, T., Ma, G. & Wang, S. M-cell targeted polymeric lipid nanoparticles containing a Toll-like receptor agonist to boost oral immunity. Int. J. Pharm. 473, 296–303 (2014).
He, H. et al. Adapting liposomes for oral drug delivery adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 9, 36–48 (2019).
Shukla, A., Mishra, V. & Kesharwani, P. Bilosomes in the context of oral immunization: development, challenges and opportunities. Drug. Discov. Today 21, 888–899 (2016).
Eliasson, D. G. et al. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine 29, 3951–3961 (2011).
Mohamedi, S. A., Heath, A. W. & Jennings, R. A comparison of oral and parenteral routes for therapeutic vaccination with HSV-2 ISCOMs in mice; cytokine profiles, antibody responses and protection. Antivir. Res. 49, 83–99 (2001).
Aguila, A. et al. Induction of protective and mucosal immunity against diphtheria by a immune stimulating complex (ISCOMS) based vaccine. Vaccine 24, 5201–5210 (2006).
Baudner, B. C. & O’Hagan, D. T. Bioadhesive delivery systems for mucosal vaccine delivery. J. Drug. Target. 18, 752–770 (2010).
Jabbal-Gill, I., Watts, P. & Smith, A. Chitosan-based delivery systems for mucosal vaccines. Expert. Opin. Drug. Deliv. 9, 1051–1067 (2012).
Surwase, S. S. et al. Engineered nanoparticles inside a microparticle oral system for enhanced mucosal and systemic immunity. ACS Appl. Mater. Interfaces 14, 11124–11143 (2022).
Van der Lubben, I. M., Verhoef, J. C., Borchard, G. & Junginger, H. E. Chitosan for mucosal vaccination. Adv. Drug. Deliv. Rev. 52, 139–144 (2001).
Hori, M., Onishi, H. & Machida, Y. Evaluation of Eudragit-coated chitosan microparticles as an oral immune delivery system. Int. J. Pharm. 297, 223–234 (2005).
Borges, O. et al. Uptake studies in rat Peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J. Control. Release 114, 348–358 (2006).
Borges, O. et al. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci. 32, 278–290 (2007).
De Smet, R., Allais, L. & Cuvelier, C. A. Recent advances in oral vaccine development. Hum. Vaccin. Immunother. 10, 1309–1318 (2014).
Kamaly, N., Yameen, B., Wu, J. & Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016).
Vroman, I. & Tighzert, L. Biodegradable polymers. Materials 2, 307–344 (2009).
Corthésy, B. et al. A pathogen-specific epitope inserted into recombinant secretory immunoglobulin A is immunogenic by the oral route. J. Biol. Chem. 271, 33670–33677 (1996).
Nochi, T. et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl Acad. Sci. USA 104, 10986–10981 (2007).
Tokuhara, D. et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl Acad. Sci. USA 107, 8794–8799 (2010).
Atwe, S. U., Ma, Y. & Gill, H. S. Pollen grains for oral vaccination. J. Control. Release 194, 45–52 (2014).
Pantazica, A. M. M., Cucos, L. M., Stavaru, C., Clarke, J. L. & Branza-Nichita, N. Challenges and prospects of plant-derived oral vaccines against hepatitis B and C viruses. Plants 10, 2037 (2021).
Chan, H. T. & Daniell, H. Plant-made oral vaccines against human infectious diseases — are we there yet? Plant Biotechnol. J. 13, 1056–1070 (2015).
Conway, M. A., Madrigal-Estebas, L., McClean, S., Brayden, D. J. & Mills, K. H. G. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine 19, 1940–1950 (2001).
Maloy, K. J., Donachie, A. M., O’Hagan, D. T. & Mowat, A. M. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology 81, 661–667 (1994).
Chen, S. C. et al. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J. Virol. 72, 5757–5761 (1998).
Kaneko, H. et al. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology 267, 8–16 (2000).
Zhu, Q. et al. Large intestine–targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 18, 1291–1296 (2012).
Tzeng, S. Y. et al. Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc. Natl Acad. Sci. USA 115, E5269–E5278 (2018).
Van De Weert, M., Hennink, W. E. & Jiskoot, W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 17, 1159–1167 (2000).
Mittal, G., Sahana, D. K., Bhardwaj, V. & Ravi Kumar, M. N. V. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 119, 77–85 (2007).
Butreddy, A., Gaddam, R. P., Kommineni, N., Dudhipala, N. & Voshavar, C. PLGA/PLA-based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int. J. Mol. Sci. 22, 8884 (2021).
Ghitman, J., Biru, E. I., Stan, R. & Iovu, H. Review of hybrid PLGA nanoparticles: future of smart drug delivery and theranostics medicine. Mater. Des. 193, 108805 (2020).
Operti, M. C. et al. PLGA-based nanomedicines manufacturing: technologies overview and challenges in industrial scale-up. Int. J. Pharm. 605, 120807 (2021).
Snook, J. D. et al. Peptide nanofiber–CaCO3 composite microparticles as adjuvant-free oral vaccine delivery vehicles. J. Mater. Chem. B 4, 1640–1649 (2016).
Barhate, G., Gautam, M., Gairola, S., Jadhav, S. & Pokharkar, V. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J. Pharm. Sci. 103, 3448–3456 (2014).
Sun, Z. et al. The potential of calcium phosphate nanoparticles as adjuvants and vaccine delivery vehicles. Front. Mater. https://doi.org/10.3389/fmats.2021.788373 (2021).
Cao, P., Han, F. Y., Grøndahl, L., Xu, Z. P. & Li, L. Enhanced oral vaccine efficacy of polysaccharide-coated calcium phosphate nanoparticles. ACS Omega 5, 18185–18197 (2020).
Huang, X. et al. Characterization of calcium phosphate nanoparticles based on a pegylated chelator for gene delivery. ACS Appl. Mater. Interfaces 9, 10435–10445 (2017).
Wei, X. et al. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 19, 1914–1921 (2019).
Wang, T. et al. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: effect of silica architecture on immunological properties. Int. J. Pharm. 436, 351–358 (2012).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT02868073 (2018).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT01335347 (2013).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04563702 (2023).
Liebowitz, D. et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 20, 435–444 (2020).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT02918006 (2022).
Barrett, J. C., Acar, H., Mellas, M. J. & Tirrell, M. V. in Peptide Applications in Biomedicine, Biotechnology and Bioengineering (ed. Koutsopoulos, S.) 287–326 (Woodhead, 2018).
Langel, S. N. et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 14, eabn6868 (2022).
Raya Tonetti, F. et al. Immunomodulatory properties of bacterium-like particles obtained from immunobiotic lactobacilli: prospects for their use as mucosal adjuvants. Front Immunol. 11, 15 (2020).
Villena, J. et al. Lactiplantibacillus plantarum as a potential adjuvant and delivery system for the development of SARS-Cov-2 oral vaccines. Microorganisms 9, 683 (2021).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04334980 (2022).
Acevedo, R. et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 5, 21 (2014).
Manus, J.-M. Brève: Covid-19 — proposition pour un vaccin oral monodose. Rev. Francoph. Lab. 2021, 13 (2021).
Ramvikas, M., Arumugam, M., Chakrabarti, S. R. & Jaganathan, K. S. in Micro and Nanotechnology in Vaccine Development, 279–301 (Elsevier, 2017).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915 (2022).
King, R. G. et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines 9, 881 (2021).
Ganesan, S. et al. Intranasal nanoemulsion adjuvanted S-2P vaccine demonstrates protection in hamsters and induces systemic, cell-mediated and mucosal immunity in mice. PLoS ONE 17, e0272594 (2022).
Wang, Q. et al. Intranasal booster using an Omicron vaccine confers broad mucosal and systemic immunity against SARS-CoV-2 variants. Signal. Transduct. Target. Ther. 8, 167 (2023).
Wang, Z. et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 6, 791–805 (2022).
Wang, Z. et al. Homologous sequential immunization using salmonella oral administration followed by an intranasal boost with ferritin-based nanoparticles enhanced the humoral immune response against H1N1 influenza virus. Microbiol. Spectr. 11, e00102–e00123 (2023).
Stauft, C. B. et al. Intranasal or airborne transmission-mediated delivery of an attenuated SARS-CoV-2 protects Syrian hamsters against new variants. Nat. Commun. 14, 3393 (2023).
Hartwell, B. L. et al. Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity. Sci. Transl. Med. 14, eabn1413 (2022).
Wasan, E. K. et al. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine 37, 1503–1515 (2019).
Rose, F. et al. A strong adjuvant based on glycol-chitosan-coated lipid–polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522. J. Controlled Rel. 271, 88–97 (2018).
Dhakal, S. et al. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front. Immunol. 9, 934 (2018).
Nguyen, K. G., Mantooth, S. M., Vrabel, M. R. & Zaharoff, D. A. Intranasal delivery of thermostable subunit vaccine for cross-reactive mucosal and systemic antibody responses against SARS-CoV-2. Front. Immunol. 13, 858904 (2022).
Pavot, V. et al. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials 75, 327–339 (2016).
Nochi, T. et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 9, 572–578 (2010).
Fukuyama, Y. et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 8, 1144–1153 (2015).
Shi, W. et al. Novel intranasal pertussis vaccine based on bacterium-like particles as a mucosal adjuvant. Immunol. Lett. 198, 26–32 (2018).
Deng, S. et al. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters. Nat. Commun. 14, 2081 (2023).
Afkhami, S. et al. Intranasal multivalent adenoviral-vectored vaccine protects against replicating and dormant M.tb in conventional and humanized mice. NPJ Vaccines 8, 25 (2023).
Freitag, T. L. et al. Intranasal administration of adenoviral vaccines expressing SARS-CoV-2 spike protein improves vaccine immunity in mouse models. Vaccine 41, 3233–3246 (2023).
Moser, M. J. et al. Intranasal single-replication influenza vector induces cross-reactive serum and mucosal antibodies against SARS-CoV-2 variants. Vaccines 11, 1063 (2023).
Zhang, Y. et al. Comparison of the immunogenicity of nasal‐spray rVSV vector, adenovirus vector, and inactivated COVID‐19‐based vaccines in rodent models. J. Med. Virol. 95, e28806 (2023).
Matsuda, K. et al. A replication-competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Invest. 131, e140794 (2021).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT01443936 (2019).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT01806909 (2019).
Matsuda, K. et al. Prolonged evolution of the memory B cell response induced by a replicating adenovirus-influenza H5 vaccine. Sci. Immunol. 4, eaau2710 (2019).
Lund, F. E. & Randall, T. D. Scent of a vaccine. Science 373, 397–399 (2021).
van Doremalen, N. et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 13, eabh0755 (2021).
Hassan, A. O. et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 36, 109452 (2021).
Hellfritzsch, M. & Scherließ, R. Mucosal vaccination via the respiratory tract. Pharmaceutics 11, 375 (2019).
Sudduth, E. R., Trautmann-Rodriguez, M., Gill, N., Bomb, K. & Fromen, C. A. Aerosol pulmonary immune engineering. Adv. Drug Deliv. Rev. 199, 114831 (2023).
Wang, H., Qin, L., Zhang, X., Guan, J. & Mao, S. Mechanisms and challenges of nanocarriers as non-viral vectors of therapeutic genes for enhanced pulmonary delivery. J. Control. Rel. 352, 970–993 (2022).
Tang, W., Zhang, Y. & Zhu, G. Pulmonary delivery of mucosal nanovaccines. Nanoscale 14, 263–276 (2022).
Ebensen, T. et al. Pulmonary application of novel antigen-loaded chitosan nano-particles co-administered with the mucosal adjuvant C-Di-AMP resulted in enhanced immune stimulation and dose sparing capacity. Pharmaceutics 15, 1238 (2023).
Kimoto, T. et al. Induction of systemic, mucosal, and cellular immunity against SARS‐CoV‐2 in mice vaccinated by trans‐airway with a S1 protein combined with a pulmonary surfactant‐derived adjuvant SF‐10. Influenza Other Respir. Viruses 17, e13119 (2023).
Sun, S. et al. Respiratory mucosal vaccination of peptide-poloxamine-DNA nanoparticles provides complete protection against lethal SARS-CoV-2 challenge. Biomaterials 292, 121907 (2023).
Thomas, C., Rawat, A., Hope-Weeks, L. & Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 8, 405–415 (2011).
Fromen, C. A. et al. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc. Natl Acad. Sci. USA 112, 488 (2015).
Li, A. V. et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 5, 204ra130 (2013).
Rakhra, K. et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci. Immunol. 6, eabd8003 (2021).
Choudhary, P. et al. Intrauterine immunizations trigger antigen-specific mucosal and systemic immunity in pigs and passive protection in suckling piglets. Vaccine 39, 6322–6332 (2021).
Castro, I. M. et al. Recombinant herpesvirus vectors: durable immune responses and durable protection against simian immunodeficiency virus SIVmac239 acquisition. J. Virol. 95, 10–1128 (2021).
Klatt, N. R. et al. Effects of persistent modulation of intestinal microbiota on SIV/HIV vaccination in rhesus macaques. NPJ Vaccines 6, 34 (2021).
Peter, C. M. et al. Immunogenicity of an inactivated vaccine for intravaginal application against bovine alphaherpesvirus type 5 (BoHV-5). Mol. Immunol. 155, 69–78 (2023).
Russi, R. C. et al. Heterologous prime-boost vaccination based on Polymorphic protein D protects against intravaginal Chlamydia trachomatis infection in mice. Sci. Rep. 12, 6664 (2022).
Labuda, J. C. et al. Circulating immunity protects the female reproductive tract from Chlamydia infection. Proc. Natl Acad. Sci. USA 118, e2104407118 (2021).
Chebloune, Y. et al. A single lentivector DNA based immunization contains a late heterologous SIVmac251 mucosal challenge infection. Vaccine 38, 3729–3739 (2020).
Kato, S. et al. CD8 T cells show protection against highly pathogenic simian immunodeficiency virus (SIV) after vaccination with SIV gene-expressing BCG prime and vaccinia virus/Sendai virus vector boosts. J. Virol. 95, e01718–e01720 (2021).
Ji, Z., Xie, Z., Zhang, Z., Gong, T. & Sun, X. Engineering intravaginal vaccines to overcome mucosal and epithelial barriers. Biomaterials 128, 8–18 (2017).
Stary, G. et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348, aaa8205 (2015).
Martelli, P. et al. Immune B cell responsiveness to single-dose intradermal vaccination against Mycoplasma hyopneumoniae. Res. Vet. Sci. 141, 66–75 (2021).
Chandrasekar, S. S. et al. Systemic neutralizing antibodies and local immune responses are critical for the control of SARS-CoV-2. Viruses 14, 1262 (2022).
Mishra, D., Dubey, V., Asthana, A., Saraf, D. & Jain, N. Elastic liposomes mediated transcutaneous immunization against Hepatitis B. Vaccine 24, 4847–4855 (2006).
Mishra, D. et al. Systemic and mucosal immune response induced by transcutaneous immunization using Hepatitis B surface antigen-loaded modified liposomes. Eur. J. Pharm. Sci. 33, 425–433 (2008).
Glenn, G. M. et al. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med 6, 1403–1406 (2000).
Güereña-Burgueño, F. et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70, 1874–1880 (2002).
Alving, C. R., Peachman, K. K., Matyas, G. R., Rao, M. & Beck, Z. Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 19, 279–292 (2020).
Hammerschmidt, S. I. et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 121, 3051–3061 (2011).
Saadeddin, A., Torres-Molina, F., Cárcel-Trullols, J., Araico, A. & Peris, J. E. Pharmacokinetics of the time-dependent elimination of all-trans-retinoic acid in rats. AAPS Pharm. Sci. 6, 1–9 (2004).
Lim, S. J., Lee, M. K. & Kim, C. K. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. J. Control. Release 100, 53–61 (2004).
Christensen, D. et al. A liposome-based adjuvant containing two delivery systems with the ability to induce mucosal immunoglobulin a following a parenteral immunization. ACS Nano 13, 1116–1126 (2019).
Xia, Y. et al. Bridging systemic immunity with gastrointestinal immune responses via oil-in-polymer capsules. Adv. Mater. 30, e1801067 (2018).
Du, Y. et al. Exploiting the lymph-node-amplifying effect for potent systemic and gastrointestinal immune responses via polymer/lipid nanoparticles. ACS Nano 13, 13809–13817 (2019).
Shakya, A. K., Chowdhury, M. Y. E., Tao, W. & Gill, H. S. Mucosal vaccine delivery: current state and a pediatric perspective. J. Control. Release 240, 394–413 (2016).
Hameed, S. A., Paul, S., Dellosa, G. K. Y., Jaraquemada, D. & Bello, M. B. Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. NPJ Vaccines 7, 71 (2022).
Suberi, A. et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci. Transl. Med. 15, eabq0603 (2023).
Li, B. et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 41, 1410–1415 (2023).
Hajam, I. A., Senevirathne, A., Hewawaduge, C., Kim, J. & Lee, J. H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 51, 37 (2020).
Ball, R. L., Bajaj, P. & Whitehead, K. A. Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci. Rep. https://doi.org/10.1038/s41598-018-20632-6 (2018).
Chandrasekar, S. S., Kingstad-Bakke, B., Wu, C.-W., Suresh, M. & Talaat, A. M. A novel mucosal adjuvant system for immunization against avian coronavirus causing infectious bronchitis. J. Virol. 94, e01016–e01020 (2020).
Vander Straeten, A. et al. A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01774-z (2023).
Ai, L. et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 9, 9 (2023).
Thomas, S. N. & Schudel, A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 7, 65–74 (2015).
Schudel, A., Francis, D. M. & Thomas, S. N. Material design for lymph node drug delivery. Nat. Rev. Mater. 4, 415–428 (2019).
Pebody, R. et al. Approaches to use the WHO respiratory syncytial virus surveillance platform to estimate disease burden. Influenza Other Respir. Viruses 14, 615–621 (2020).
Stein, R. T. et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr. Pulmonol. 52, 556–569 (2017).
Corbett, E. L. et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021 (2003).
WHO Coronavirus Disease (COVID-19) Dashboard. Bangladesh Physiother. J. https://doi.org/10.46945/bpj.10.1.03.01 (2020).
O’Brien, K. L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902 (2009).
Kandeil, W. et al. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev. Vaccines 19, 621–638 (2020).
Watt, J. P. et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374, 903–911 (2009).
Khalil, I. A. et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 18, 1229–1240 (2018).
Salih, B. Helicobacter pylori infection in developing countries: the burden for how long? Saudi J. Gastroenterol. 15, 201–207 (2009).
Troeger, C. et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18, 1211–1228 (2018).
Stanaway, J. D. et al. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 19, 1312–1324 (2019).
Lessa, F. C. et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 (2015).
Pandey, A. & Galvani, A. P. The global burden of HIV and prospects for control. Lancet HIV 6, e809–e811 (2019).
Kirkcaldy, R. D., Weston, E., Segurado, A. C. & Hughes, G. Epidemiology of gonorrhoea: a global perspective. Sex. Health 16, 401–411 (2019).
Formana, D. et al. Global burden of human papillomavirus and related diseases. Vaccine https://doi.org/10.1016/j.vaccine.2012.07.055 (2012).
Looker, K. J. et al. The global and regional burden of genital ulcer disease due to herpes simplex virus: a natural history modelling study. BMJ Glob. Health 5, e001875 (2020).
Kojima, N. & Klausner, J. D. An update on the global epidemiology of syphilis. Curr. Epidemiol. Rep. 5, 24–38 (2018).
Averhoff, F. M., Glass, N. & Holtzman, D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin. Infect. Dis. https://doi.org/10.1093/cid/cis361 (2012).
Thrift, A. P., El-Serag, H. B. & Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 14, 122–132 (2017).
Treuting, P. M., Dintzis, S. M. & Montine, K. S. (eds) Comparative Anatomy and Histology: A Mouse, Rat, and Human Atlas 2nd edn (Academic, 2017).
Jotwani, R. et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J. Immunol. 167, 4693–4700 (2001).
Reinartz, S. M. et al. Dendritic cell subsets in oral mucosa of allergic and healthy subjects. PLoS ONE 11, e0154409 (2016).
Williams, D. W. et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 184, 4090–4104.e15 (2021).
Hovav, A. H. Dendritic cells of the oral mucosa. Mucosal Immunol. 7, 27–37 (2014).
Squier, C. & Brogden, K. A. Human Oral Mucosa: Development, Structure and Function. https://doi.org/10.1002/9781118710470 (2013).
Jahan, N., Archie, S. R., Al Shoyaib, A., Kabir, N. & Cheung, K. Recent approaches for solid dose vaccine delivery. Sci. Pharm. 87, 27 (2019).
Helke, K. L. Book review: comparative anatomy and histology: a mouse, rat, and human atlas. Vet. Pathol. https://doi.org/10.1177/0300985818795862 (2018).
Houston, S. A. et al. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9, 468–478 (2016).
Esterházy, D. et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130 (2019).
Tyler, C. J. et al. Inherent immune cell variation within colonic segments presents challenges for clinical trial design. J. Crohns Colitis 14, 1364–1377 (2020).
Gallo, O., Locatello, L. G., Mazzoni, A., Novelli, L. & Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 14, 305–316 (2021).
Sánchez Fernández, J. M. et al. Preliminary study of the lymphatic drainage system of the nose and paranasal sinuses and its role in detection of sentinel metastatic nodes. Acta Otolaryngol. 125, 566–570 (2005).
Yeaman, G. R., Collins, J. E., Fanger, M. W. & Wira, C. R. CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology 102, 434–440 (2001).
Gudisa, R., Goyal, K., Gupta, P. & Singh, M. P. Localized and systemic immune response in human reproductive tract. Front. Cell. Infect. Microbiol. 11, 649839 (2021).
Geppert, B., Lönnerfors, C., Bollino, M., Arechvo, A. & Persson, J. A study on uterine lymphatic anatomy for standardization of pelvic sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 145, 256–261 (2017).
Patton, D. L. et al. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 183, 967–973 (2000).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04816019 (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04679909 (2023).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04751682 (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04809389 (2021).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04619628 (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04839042 (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04798001 (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04954287 (2023).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04871737 (2023).
Registro Publico Cubano de Ensayos Clinicos. rpcec.sld.cu/en/trials/RPCEC00000345-En (2022).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT05067933 (2023).
US National Library of Medicine. clinicaltrials.gov/ct2/show/NCT04732468 (2021).
Author information
Authors and Affiliations
Contributions
B.E., A.S., U.v.A., R.L. and A.J. conceptualized the manuscript. B.E. A.S., I.S. and Z.C. contributed to literature review, manuscript writing and figure composition. A.H.L., M.K., F.T. and D.M.F. contributed to literature review and manuscript writing. B.I. and G.L. contributed to literature review. J.H. contributed to figure visualization. B.E., A.S., U.v.A., R.L. and A.J. edited and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.L. receives licensing fees (to patents on which he was an inventor) from, invested in, consults (or was on Scientific Advisory Boards or Boards of Directors) for, lectured (and received a fee), or conducts sponsored research at MIT for which he was not paid for the following entities: 611 Therapeutics; Abpro International; Acorda (formerly Civitas Therapeutics); Alfred University; Aleph Farms; Alivio Therapeutics; Alkermes; Allevi; Allurion; Alnylam Pharmaceuticals, Inc; Amberstone Bioscience; Amgen; aMoon; Apotex; Arcadia Biosciences, Inc; Arsenal Medical; Artificial Cell Technology, Inc; Avalon-Globocare; Bai Biosciences; BASF Corporation; Bayer; Balzan Foundation; Bexson Biomedical; Bilayer Therapeutics; Biogen; BioInnovation Institute (Novo Nordisk Founden); BioTE Medical; Blackrock; Blackstone (formerly Clarus); Boston Children’s Hospital; CBC Group Investment Mgmt Group; Celanese; Celero; Cellink/BICO; Cellomics Technology LLC; Cellular Biomedical; CE&N/ACS; Charles River Laboratories, Inc.; Clontech Laboratories; Combined Therapeutics (CTx); Conference Forum; Cornell University; Crispr Therapeutics Ag; Crown Bioscience, Inc.; Daré Biosciences (formerly Microchips Biotech, Juniper Pharmaceuticals and Columbia Laboratories); Daros, Inc.; DeepBiome; Dewpoint Therapeutics; Dispendix; Eagle Pharmaceuticals; Earli; Edigene Biotechnology, Inc.; Editas Medicine, Inc.; ELC (Estee Lauder Companies); Eli Lilly; Eisai, Inc.; Entrega; EpiBone; Establishment Labs, SA.; Everlywell; Evox Therapeutics, Ltd.; Fate; Flagship Pioneering; Frequency Therapeutics, Inc.; GeneLeap Biotech; Genemedicine Co Lmtd; GenScript USA, Inc; Geneo Medicine; GENUV; Glaxosmithkline LLC; Glycobia; Glympse Bio; Goldman Sachs; Greenlight Biosciences; HCR (HealthCare Royalty Partners); HKF DNA Technologies; Hopewell Therapeutics; Horizon Discovery Group Plc; Humacyte, Inc.; IBEX Pharmaceuticals, Inc.; Immunai; ImmuneXcite, Inc.; Institute of Immunology Co. Ltd; Integrated DNA Technologies, Inc.; InVivo Therapeutics; IxBio; J.R. Simplot Company; Jnana Therapeutics; Kala Pharmaceuticals; Kallyope, Inc.; Kendall Capital; Kensa; Kodikaz Therapeutics; KAST (Korean Academy of Science and Technology); Ksq Therapeutics, Inc.; Kunlun Capital; Landsdowne Labs; LikeMinds; Lonza; Luminopia, Inc.; Luye (Shandong luye); Lyndra Therapeutics; Lyra Therapeutics (formerly 480 Biomedical); Maurice Marie Janot Award 2020; McGovern Institute; Medikinetics Co., Ltd.; Merck; MGH Ragon Institute; Micelle; Moderna Therapeutics; Momenta; Muse Biotechnologies, Inc.; Mylan; N2Tech; Nanobiosym; Nanobiotix; Neochromosone; Neoteny 4 LLP; NextRNA; Newbridge Ventures LLC; Noveome Biotherapeutics, Inc.; Novo Nordisk; Ohio State University; Olivo (acquired by Shiseido); Ovid Therapeutics; Particles for Humanity; Pfizer, Inc.; Pioneer Hi-Bred International, Inc.; Placon Therapeutics; Polaris Partners; Pontifical Academy of Sciences; Portal Instruments; Preceres LLC (acquired by Monsanto); PrognomIQ, Inc.; Pulmatrix; PureTech; Quris; ReLive; Rensselaer Polytechnic Institute/Department of Chemical and Biological Engineering; Reprocell USA, Inc. (formerly Stemgent); Replay Bio; Rubius Therapeutics; Satellite Bio; SBEF (Seoul Bio Economy Forum); Secant Medical, Inc.; Seer, Inc.; Selecta Biosciences; Senses LLC; Setsuro Tech Inc.; Seventh Sense Biosystems, Inc.; Shenzhen Rice Life Technology, Ltd; Shire Ag; Sigilon; Sigma Aldrich Co. LLC; SiO2 Materials Science; Ske S.R.L.; Soil Culture Solutions LLC (Dba Soilcea); Souffle Therapeutics; SQZ Biotechnologies; StemBioSys, Inc.; SuonoBio; T2 Biosystems; Taconic Biosciences, Inc. (formerly Taconic Farms); Taiwania Capital (Bio-Asia Taiwan Symposium); TARA; Tarveda Therapeutics; Teal Bio; Terasaki Institute; Tesio Pharmaceuticals; Third Rock Ventures; Tiba Biotech LLC; TissiuM (formerly Gecko); Transgenic, Inc.; Translate Bio (formerly Rana Therapeutics, Inc.); Trilink Biotechnologies, Inc.; Unilever (Living Proof); University of Bergen, Norway (Falch Lecture Honorarium); VasoRX; Verseau Therapeutics, Inc.; Virex Health; Vitakey; Vivtex Corporation; Westlake University; Whitehead Institute; Wiki Foods; Xenter; YourBio (formerly 7th Sense Biosystems); Yz Biosciences (Guangzhou), Inc.; Zenomics; ZWI Therapeutics. A.J. receives licensing fees (to patents on which she was an inventor) from, invested in, consults (or was on Scientific Advisory Boards or Boards of Directors) for, lectured (and received a fee), or conducts sponsored research at MIT for which she was not paid for the following entities: Moderna Therapeutics; OmniPulse Biosciences; Particles for Humanity; SiO2 Materials Science; Takeda (formerly Shire Ag); VitaKey. All the other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Dennis Christensen, who co-reviewed with Signe Tandrup Schmidt; Ed Lavelle; and Amirali Popat for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eshaghi, B., Schudel, A., Sadeghi, I. et al. The role of engineered materials in mucosal vaccination strategies. Nat Rev Mater 9, 29–45 (2024). https://doi.org/10.1038/s41578-023-00625-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-023-00625-2