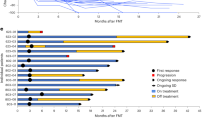
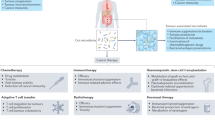
Abstract
Our understanding of how the microbiota affects the balance between response to and failure of cancer treatment by modulating the tumour microenvironment and systemic immune system has advanced rapidly in recent years. Microbiota-targeting interventions in patients with cancer are an area of intensive investigation. Promisingly, phase I–II clinical trials have shown that interventions such as faecal microbiota transplantation can overcome resistance to immune checkpoint blockade in patients with melanoma, improve therapeutic outcomes in treatment-naive patients and reduce therapy-induced immunotoxicities. Here, we synthesize the evidence showing that the microbiota is an important determinant of both cancer treatment efficacy and treatment-induced acute and long-term toxicity, and we discuss the complex and inter-related mechanisms involved. We also assess the potential of microbiota-targeting interventions, including bacterial engineering and phage therapy, to optimize the response to and recovery from cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
McGrail, D. J. et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 32, 661–672 (2021).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). Together with Routy et al. (2018) and Matson et al. (2018), this early study reports a link between the composition of the gut microbiota and clinical response to anti-PD1.
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). This landmark publication reports a link between the composition of the gut microbiota and clinical response to ICB (anti-CTLA4).
Stein-Thoeringer, C. K. et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023).
Smith, M. et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022).
Hu, Y. et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat. Commun. 13, 5313 (2022).
Blumenberg, V. et al. Antibiotic therapy and low gut microbiome diversity is associated with decreased response and high toxicity in BCP-ALL and DLBCL patients after treatment with CD19. CAR T-cells. Blood 136, 33–34 (2020).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023).
Daillere, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28, 2344–2352 (2022).
Blake, S. J. et al. The immunotoxicity, but not anti-tumor efficacy, of anti-CD40 and anti-CD137 immunotherapies is dependent on the gut microbiota. Cell Rep. Med. 2, 100464 (2021). This early study reports that the immunotoxicity, but not antitumour efficacy, of IAAs depends on the gut microbiota in mice.
Peled, J. U. et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 382, 822–834 (2020). This large clinical study identifies that microbiota diversity can predict rates of infection, GVHD and survival following HCT.
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Chua, L. L. et al. Reduced microbial diversity in adult survivors of childhood acute lymphoblastic leukemia and microbial associations with increased immune activation. Microbiome 5, 35 (2017).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021). Together with Baruch et al. (2021), this pioneering phase I clinical study shows that FMT from anti-PD1 responders can confer responsiveness to anti-PD1 in patients with melanoma who were previously unresponsive.
Zhao, Y. et al. Safety and efficacy of fecal microbiota transplantation for grade IV steroid refractory GI-GvHD patients: interim results from FMT2017002 trial. Front. Immunol. 12, 678476 (2021).
Malard, F. et al. Pooled allogenic fecal microbiotherapy MaaT013 for the treatment of steroid-refractory gastrointestinal acute graft-versus-host disease: results from the phase IIa HERACLES study and expanded access program. Blood 138, 262–262 (2021).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Vangay, P., Ward, T., Gerber, J. S. & Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17, 553–564 (2015).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020). This work highlights the presence of tumour-colonizing bacteria in several cancer types, a paradigm shift in the field.
Narunsky-Haziza, L. et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185, 3789–3806.e17 (2022).
Broecker, F. & Moelling, K. The roles of the virome in cancer. Microorganisms 9, 2538 (2021).
Wolf, Y. & Samuels, Y. Intratumor heterogeneity and antitumor immunity shape one another bidirectionally. Clin. Cancer Res. 28, 2994–3001 (2022).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Delaney, G., Jacob, S., Featherstone, C. & Barton, M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104, 1129–1137 (2005).
Yang, K. et al. Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation. J. Exp. Med. 218, e20201915 (2021).
Shiao, S. L. et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 39, 1202–1213.e6 (2021).
Uribe-Herranz, M. et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest. 130, 466–479 (2020). Together with Yang et al. (J. Exp. Med., 2021), this work highlights that the immune response to radiotherapy can be increased or decreased by the gut microbiota-produced metabolite, butyrate.
Deng, X. et al. Comparison of microbiota in patients treated by surgery or chemotherapy by 16S rRNA sequencing reveals potential biomarkers for colorectal cancer therapy. Front. Microbiol. 9, 1607 (2018).
Zhao, Z. et al. Metagenome association study of the gut microbiome revealed biomarkers linked to chemotherapy outcomes in locally advanced and advanced lung cancer. Thorac. Cancer 12, 66–78 (2021).
Terrisse, S. et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021).
Sims, T. T. et al. Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation. Commun. Biol. 4, 237 (2021).
Jang, B. S. et al. Gut microbiome composition is associated with a pathologic response after preoperative chemoradiation in patients with rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 107, 736–746 (2020).
Montassier, E. et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 42, 515–528 (2015).
Nam, Y. D., Kim, H. J., Seo, J. G., Kang, S. W. & Bae, J. W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 8, e82659 (2013).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Haslam, A. & Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2, e192535 (2019).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Costea, P. I. et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 35, 1069–1076 (2017).
Gupta, V. K., Paul, S. & Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 8, 1162 (2017).
Gharaibeh, R. Z. & Jobin, C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 68, 385–388 (2019).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Lee, K. A. et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 28, 535–544 (2022).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016).
Eng, L. et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: a population-based study. J. Clin. Oncol. 41, 3122–3134 (2023).
Jing, Y. et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother. Cancer 10, e003779 (2022).
Giordan, Q., Salleron, J., Vallance, C., Moriana, C. & Clement-Duchene, C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front. Immunol. 12, 716317 (2021).
Zhao, S. et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 130, 10–17 (2019).
Pinato, D. J. et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 5, 1774–1778 (2019).
Fessas, P. et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer 10, 583–592 (2021).
Serpas Higbie, V. et al. Antibiotic exposure does not impact immune checkpoint blockade response in MSI-H/dMMR metastatic colorectal cancer: a single-center experience. Oncologist 27, 952–957 (2022).
Vihinen, H. et al. Antibiotic treatment is an independent poor risk factor in NSCLC but not in melanoma patients who had received anti-PD-1/L1 monotherapy. Clin. Lung Cancer 24, 295–304 (2023).
Rashidi, A. et al. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome 9, 211 (2021).
Maier, L. et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 599, 120–124 (2021).
Kanjilal, S. et al. A decision algorithm to promote outpatient antimicrobial stewardship for uncomplicated urinary tract infection. Sci. Transl. Med. 12, eaay5067 (2020).
Wang, Z. et al. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J. Cell Mol. Med. 23, 3747–3756 (2019).
Alexander, J. L. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 14, 356–365 (2017).
Huang, B. et al. Chemotherapeutic drugs induce different gut microbiota disorder pattern and NOD/RIP2/NF-κB signaling pathway activation that lead to different degrees of intestinal injury. Microbiol. Spectr. 10, e0167722 (2022).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Reis Ferreira, M. et al. Microbiota- and radiotherapy-induced gastrointestinal side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin. Cancer Res. 25, 6487–6500 (2019).
Ramadan, M. et al. Alterations in skin microbiome mediated by radiotherapy and their potential roles in the prognosis of radiotherapy-induced dermatitis: a pilot study. Sci. Rep. 11, 5179 (2021).
Hong, B. Y. et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 7, 66 (2019).
Vesty, A. et al. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support. Care Cancer 28, 2683–2691 (2020).
Hamada, K. et al. Turicibacter and acidaminococcus predict immune-related adverse events and efficacy of immune checkpoint inhibitor. Front. Immunol. 14, 1164724 (2023).
Zhou, X. et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 18, 87 (2020).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Rashidi, A. et al. Analysis of antibiotic exposure and development of acute graft-vs-host disease following allogeneic hematopoietic cell transplantation. JAMA Netw. Open 6, e2317188 (2023).
Burgos da Silva, M. et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood 140, 2385–2397 (2022).
Vossen, J. M. et al. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS ONE 9, e105706 (2014).
Fredricks, D. N. The gut microbiota and graft-versus-host disease. J. Clin. Invest. 129, 1808–1817 (2019).
Khuat, L. T. et al. Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation. Sci. Transl. Med. 12, eaay7713 (2020).
Holler, E. et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transpl. 20, 640–645 (2014).
Simms-Waldrip, T. R. et al. Antibiotic-induced depletion of anti-inflammatory Clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol. Blood Marrow Transpl. 23, 820–829 (2017).
Shono, Y. et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 8, 339ra371 (2016).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184, 5338–5356 e5321 (2021). This work identifies that secretion of STING agonists such as cyclic di-AMP by microbiota species induces type I interferons in the TME, which can remodel the myeloid compartment to a proinflammatory phenotype to enhance responses to ICB.
Griffin, M. E. et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 373, 1040–1046 (2021).
Heimesaat, M. M. et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010).
Frank, M. et al. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 194, 1983–1995 (2015).
Yonekura, S. et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 12, 1128–1151 (2022).
Choi, Y. et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci. Immunol. 8, eabo2003 (2023).
Younginger, B. S. et al. Enrichment of oral-derived bacteria in inflamed colorectal tumors and distinct associations of Fusobacterium in the mesenchymal subtype. Cell Rep. Med. 4, 100920 (2023).
Jiang, S. S. et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797.e9 (2023).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 33, 988–1000.e7 (2021).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e8 (2022). Together with Bender et al. (2023), this work identifies that tryptophan metabolite signalling through AHR can enhance or inhibit responses to ICB depending on the context.
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Wang, F., Yin, Q., Chen, L. & Davis, M. M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl Acad. Sci. USA 115, 157–161 (2018).
Sun, S. et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 117, 27509–27515 (2020).
Renga, G. et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J. Immunother. Cancer 10, e003725 (2022).
Chen, Y. et al. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα–CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 41, 1 (2022).
Mathewson, N. D. et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016).
Fujiwara, H. et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 9, 3674 (2018).
Docampo, M. D. et al. Alloreactive T cells deficient of the short-chain fatty acid receptor GPR109A induce less graft-versus-host disease. Blood 139, 2392–2405 (2022).
Guo, H. et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370, eaay9097 (2020).
Xiao, H. W. et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 8, 69 (2020).
Bessell, C. A. et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight 5, e135597 (2020).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020). This work reports that epitopes encoded by a bacteriophage in the microbiota can prime cross-reactive T cells against tumour neoantigens, markedly increasing responses to ICB and chemotherapies in both mice and humans.
Naghavian, R. et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature 617, 807–817 (2023).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Hu, Z. I. et al. Immune checkpoint inhibitors unleash pathogenic immune responses against the microbiota. Proc. Natl Acad. Sci. USA 119, e2200348119 (2022).
Pedersen, T. K. et al. The CD4+ T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in Crohn’s disease. Immunity 55, 1909–1923.e6 (2022).
Hone Lopez, S. et al. Immune checkpoint inhibitor treatment induces colitis with heavy infiltration of CD8+ T cells and an infiltration pattern that resembles ulcerative colitis. Virchows. Arch. 479, 1119–1129 (2021).
Park, J. S. et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance. Nature 617, 377–385 (2023).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
Feliu, V. et al. Distant antimetastatic effect of enterotropic colon cancer-derived α4β7+CD8+ T cells. Sci. Immunol. 8, eadg8841 (2023).
Hassan-Zahraee, M. et al. Anti-MAdCAM antibody increases β7+ T cells and CCR9 gene expression in the peripheral blood of patients with Crohn’s disease. J. Crohns Colitis 12, 77–86 (2018).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019). This work shows how a microbial consortium can be administered as a probiotic to enhance CD8+ T cell-mediated control of tumours in mice.
Koyama, M. et al. Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity. Immunity 56, 1876–1893.e8 (2023).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021). This work shows that increased dietary fibre intake leads to better responses to anti-PD1 in mice and humans.
Pires da Silva, I. et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 22, 836–847 (2021).
Fasanello, M. K., Robillard, K. T., Boland, P. M., Bain, A. J. & Kanehira, K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep. J. 7, e00360 (2020).
Wang, Y. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 24, 1804–1808 (2018).
Wang, Y. et al. Effect of fecal transplantation on patients’ reported outcome after immune checkpoint inhibitor colitis. J. Clin. Oncol. 41, 2645–2645 (2023).
Routy, B. et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat. Med. 29, 2121–2132 (2023). This work determines that FMT improves frontline anti-PD1 efficacy in ICB-naive patients.
van Lier, Y. F. et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 12, eaaz8926 (2020).
Duvallet, C. et al. Framework for rational donor selection in fecal microbiota transplant clinical trials. PLoS ONE 14, e0222881 (2019).
Derosa, L. et al. Friendly-user score assessing gut dysbiosis and resistance to immune checkpoint inhibitors (ICI). J. Clin. Oncol. 41, 103 (2023).
Schmidt, T. S. B. et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat. Med. 28, 1902–1912 (2022).
Ianiro, G. et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat. Med. 28, 1913–1923 (2022).
Lee, K. A., Shaw, H. M., Bataille, V., Nathan, P. & Spector, T. D. Role of the gut microbiome for cancer patients receiving immunotherapy: dietary and treatment implications. Eur. J. Cancer 138, 149–155 (2020).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2022).
Panigrahi, P. et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412 (2017).
Ebrahimi, H. et al. Effect of CBM588 in combination with cabozantinib plus nivolumab for patients (pts) with metastatic renal cell carcinoma (mRCC): a randomized clinical trial. J. Clin. Oncol. 41, LBA104 (2023).
Dizman, N. et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat. Med. 28, 704–712 (2022). This phase I clinical study shows that a commercial probiotic, CBM588, can increase responses to ICB in patients with metastatic renal cell carcinoma.
Tomita, Y. et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol. Res. 8, 1236–1242 (2020).
Tomita, Y. et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology 11, 2081010 (2022).
Lee, S. H. et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 6, 277–288 (2021).
Olivia, I.G. et al. MCGRAW trial: evaluation of the safety and efficacy of an oral microbiome intervention (SER-401) in combination with nivolumab in first line metastatic melanoma patients. J. ImmunoTher. Cancer 10, A638 (2022).
Spreafico, A. et al. First-in-class microbial ecosystem therapeutic 4 (MET4) in combination with immune checkpoint inhibitors in patients with advanced solid tumors (MET4-IO trial). Ann. Oncol. 34, 520–530 (2023).
Kaleko, M. et al. Development of SYN-004, an oral β-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 41, 58–67 (2016).
Cubillos-Ruiz, A. et al. An engineered live biotherapeutic for the prevention of antibiotic-induced dysbiosis. Nat. Biomed. Eng. 6, 910–921 (2022).
de Gunzburg, J. et al. Protection of the human gut microbiome from antibiotics. J. Infect. Dis. 217, 628–636 (2018).
Yang, B., Fang, D., Lv, Q., Wang, Z. & Liu, Y. Targeted therapeutic strategies in the battle against pathogenic bacteria. Front. Pharmacol. 12, 673239 (2021).
Gencay, Y. E. et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01759-y (2023).
Bhatt, A. P. et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl Acad. Sci. USA 117, 7374–7381 (2020).
Vinderola, G., Sanders, M. E. & Salminen, S. The concept of postbiotics. Foods 11, 1077 (2022).
Babjuk, M. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur. Urol. 81, 75–94 (2022).
Zhao, M. et al. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc. Natl Acad. Sci. USA 104, 10170–10174 (2007).
Toso, J. F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20, 142–152 (2002).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12, eaax0876 (2020).
Leventhal, D. S. et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 11, 2739 (2020).
Luke, J. J. et al. Phase I study of SYNB1891, an engineered E. coli Nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin. Cancer Res. 29, 2435–2444 (2023).
Chen, Y. E. et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. Science 380, 203–210 (2023). This work shows that engineered bacteria expressing tumour antigens can elicit antigen-specific immune responses against cancers following administration to the skin.
Thomas, R. et al. Gut microbial composition difference between pediatric ALL survivors and siblings. Pediatr. Hematol. Oncol. 37, 475–488 (2020).
Morel, S. et al. Biomarkers of cardiometabolic complications in survivors of childhood acute lymphoblastic leukemia. Sci. Rep. 10, 21507 (2020).
Fromentin, S. et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 28, 303–314 (2022).
Held, C. et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J. Am. Heart Assoc. 6, e005077 (2017).
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334 (2001).
Agus, A., Clement, K. & Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182 (2021).
Meadows, A. T. et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 27, 2356–2362 (2009).
Mody, R. et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood 111, 5515–5523 (2008).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Thomas, A. M. et al. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat. Rev. Clin. Oncol. 20, 583–603 (2023).
Ott, S. J. et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 152, 799–811.e7 (2017).
Mitra, A. et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 11, 1999 (2020).
Lev-Sagie, A. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504 (2019).
Bolte, L. A. et al. Association of a mediterranean diet with outcomes for patients treated with immune checkpoint blockade for advanced melanoma. JAMA Oncol. 9, 705–709 (2023).
Mao, Y. Q. et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat. Metab. 5, 96–110 (2023).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 6, e145207 (2021).
Dai, X. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 81, 2317–2331.e6 (2021).
McGough, C., Baldwin, C., Frost, G. & Andreyev, H. J. Role of nutritional intervention in patients treated with radiotherapy for pelvic malignancy. Br. J. Cancer 90, 2278–2287 (2004).
Stein-Thoeringer, C. K. et al. Lactose drives enterococcus expansion to promote graft-versus-host disease. Science 366, 1143–1149 (2019).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Lichtenstein, A. H. et al. Perspective: design and conduct of human nutrition randomized controlled trials. Adv. Nutr. 12, 4–20 (2021).
Cougnoux, A. et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Alam, A. et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 40, 153–167.e11 (2022).
Oh, B. et al. Emerging evidence of the gut microbiome in chemotherapy: a clinical review. Front. Oncol. 11, 706331 (2021).
Kajihara, K. K. et al. Potent killing of Pseudomonas aeruginosa by an antibody-antibiotic conjugate. mBio 12, e0020221 (2021).
Cullin, N., Azevedo Antunes, C., Straussman, R., Stein-Thoeringer, C. K. & Elinav, E. Microbiome and cancer. Cancer Cell 39, 1317–1341 (2021).
Prorok-Hamon, M. et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63, 761–770 (2014).
Yu, L. C. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J. Biomed. Sci. 25, 79 (2018).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC — mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Bertocchi, A. et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39, 708–724.e11 (2021).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Fletcher, A. A., Kelly, M. S., Eckhoff, A. M. & Allen, P. J. Revisiting the intrinsic mycobiome in pancreatic cancer. Nature 620, E1–E6 (2023).
Poore, G. D. et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020).
Gihawi, A., Cooper, C. S. & Brewer, D. S. Caution regarding the specificities of pan-cancer microbial structure. Microb. Genom. 9, mgen0001088 (2023).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Watson, A. S. et al. Association of immune-related adverse events, hospitalization, and therapy resumption with survival among patients with metastatic melanoma receiving single-agent or combination immunotherapy. JAMA Netw. Open. 5, e2245596 (2022).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
Mayes, P. A., Hance, K. W. & Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug. Discov. 17, 509–527 (2018).
Johnson, P. et al. Clinical and biological effects of an agonist anti-CD40 antibody: a cancer research UK phase I study. Clin. Cancer Res. 21, 1321–1328 (2015).
Vonderheide, R. H. et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
Segal, N. H. et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 23, 1929–1936 (2017).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Acknowledgements
This work was supported by Ideas grants 1180799 and 2018793 awarded to D.J.L. and S.J.B. from the Australian National Health and Medical Research Council, the Australian Medical Research Future Fund (MRF2007441), the Leukaemia Foundation of Australia and a Tour De Cure Pioneering Research Grant (RSP-264–18/19). Y.W. is supported by a Melanoma Research Alliance grant (no.937368), the Rosetrees Trust (no. MYIA\100002), a research grant from Pfizer and the Lemelbaum family.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.J.B. and D.J.L. are co-inventors on International Patent Application No. PCT/AU2020/051278 related to this area of research. D.J.L. has received funding from GSK for research not related to this Review. Y.W. receives a research grant from Pfizer for research not related to this Review. B.B. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks B. Routy; M. van den Brink, who co-reviewed with C. Nguyen; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Chimeric antigen receptor T cells
-
(CAR T cells). A form of cell-based immunotherapy generated from donor T cells that are transduced with a construct that, at a minimum, expresses a B cell receptor against a leukaemic target such as CD19, CD20 or BCMA, coupled with a co-stimulatory receptor such as CD137 or CD28.
- Cytokine release syndrome
-
(CRS). A form of systemic inflammatory response syndrome characterized by increased serum cytokine levels that can be triggered by various factors including infection and immunotherapies.
- Dysbiosis
-
Disruption of the normal, homeostatic composition and function of a microbiota within its ecological niche.
- Faecal microbiota transplantation
-
(FMT). The transfer of faecal material from a donor to a recipient by lower or upper gastrointestinal tract endoscopy, enema or consumption of capsules of freeze-dried material. FMT describes both a clinical intervention and a tool for research purposes, such as xenotransplantation (FMT from human to mouse).
- Graft-versus-host disease
-
(GVHD). A life-threatening condition that arises following haematopoietic cell transplantation (HCT) whereby donor immune cells in the graft attack host cells, often in the skin, liver and gastrointestinal tract.
- Haematopoietic cell transplantation
-
(HCT). The transplantation of multipotent haematopoietic stem cells, usually derived from the bone marrow, peripheral blood or umbilical cord blood, which replicate and differentiate inside the recipient to produce additional normal blood cells.
- Immune-agonist antibodies
-
(IAAs). Antibodies against co-activating or co-stimulatory receptors, such as members of the tumour necrosis factor receptor superfamily that includes CD40 and CD137, that induce immune-activating responses upon receptor binding.
- Immune checkpoint blockade
-
(ICB). Inhibition of the interactions between immune checkpoint molecules and their ligands, such as between PD1 and PDL1 or between CTLA4 and CD80 or CD86, which blocks checkpoint-induced immune exhaustion.
- Immune effector cell-associated neurotoxicity syndrome
-
(ICANS). A clinical and neuropsychiatric syndrome that can occur in the days to weeks following administration of certain types of immunotherapy, particularly immune effector cell therapies such as chimeric antigen receptor T cells (CAR T cells) and T cell-engaging therapies.
- Immune-related adverse events
-
(irAEs). Autoimmune or inflammatory-like conditions that arise during immunotherapy, such as colitis, dermatitis and hepatitis.
- Postbiotics
-
A preparation of inanimate microorganisms and/or their components that confers a health benefit to the host.
- Prebiotics
-
Substrates that are selectively used by host microorganisms conferring a health benefit.
- Probiotics
-
Live microorganisms that, when administered in adequate amounts, confer a health benefit to the host.
- Short-chain fatty acids
-
(SCFAs). A group of immunomodulatory metabolites produced by various families of anaerobic bacteria, such as Clostridium, Bifidobacterium and Lactobacillus spp., through anaerobic fermentation of dietary fibre in the colon.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blake, S.J., Wolf, Y., Boursi, B. et al. Role of the microbiota in response to and recovery from cancer therapy. Nat Rev Immunol 24, 308–325 (2024). https://doi.org/10.1038/s41577-023-00951-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00951-0