Abstract
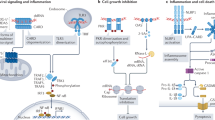
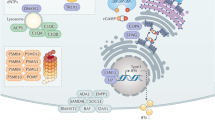
Recent progress in human and mouse genetics has transformed our understanding of the molecular mechanisms by which recognition of self double-stranded RNA (self-dsRNA) causes immunopathology. Novel mouse models recapitulate loss-of-function mutations in the RNA editing enzyme ADAR1 that are found in patients with Aicardi–Goutières syndrome (AGS) — a monogenic inflammatory disease associated with increased levels of type I interferon. Extensive analyses of the genotype–phenotype relationships in these mice have now firmly established a causal relationship between increased intracellular concentrations of endogenous immunostimulatory dsRNA and type I interferon-driven immunopathology. Activation of the dsRNA-specific immune sensor MDA5 perpetuates the overproduction of type I interferons, and chronic engagement of the interferon-inducible innate immune receptors PKR and ZBP1 by dsRNA drives immunopathology by activating an integrated stress response or by inducing excessive cell death. Biochemical and genetic data support a role for the p150 isoform of ADAR1 in the cytosol in suppressing the spontaneous, pathological response to self-dsRNA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weber, F., Wagner, V., Rasmussen, S. B., Hartmann, R. & Paludan, S. R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80, 5059–5064 (2006).
Son, K. N., Liang, Z. & Lipton, H. L. Double-stranded RNA is detected by immunofluorescence analysis in RNA and DNA virus infections, including those by negative-stranded RNA viruses. J. Virol. 89, 9383–9392 (2015).
Hur, S. Double-stranded RNA sensors and modulators in innate immunity. Annu. Rev. Immunol. 37, 349–375 (2019).
Rice, G. I. et al. Mutations in ADAR1 cause Aicardi–Goutières syndrome associated with a type I interferon signature. Nat. Genet. 44, 1243–1248 (2012). This study provides clinical evidence that the detection of self-dsRNA by the innate immune system may be causal to human immunopathology.
Chen, Y. G. & Hur, S. Cellular origins of dsRNA, their recognition and consequences. Nat. Rev. Mol. Cell Biol. 23, 286–301 (2022).
Bartok, E. & Hartmann, G. Immune sensing mechanisms that discriminate self from altered self and foreign nucleic acids. Immunity 53, 54–77 (2020).
Aicardi, J. & Goutieres, F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 15, 49–54 (1984).
Crow, Y. J. & Stetson, D. B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 22, 471–483 (2022).
Livingston, J. H. et al. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J. Med. Genet. 51, 76–82 (2014).
Crow, Y. J. et al. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics 45, 386–393 (2014).
La Piana, R. et al. Bilateral striatal necrosis in two subjects with Aicardi–Goutières syndrome due to mutations in ADAR1 (AGS6). Am. J. Med. Genet. A 164A, 815–819 (2014).
Rice, G. I. et al. Genetic, phenotypic, and interferon biomarker status in ADAR1-related neurological disease. Neuropediatrics 48, 166–184 (2017).
Miyamura, Y. et al. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria. Am. J. Hum. Genet. 73, 693–699 (2003).
Hayashi, M. & Suzuki, T. Dyschromatosis symmetrica hereditaria. J. Dermatol. 40, 336–343 (2013).
Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 17, 83–96 (2016).
Walkley, C. R. & Li, J. B. Rewriting the transcriptome: adenosine-to-inosine RNA editing by ADARs. Genome Biol. 18, 205 (2017).
Gallo, A., Vukic, D., Michalik, D., O’Connell, M. A. & Keegan, L. P. ADAR RNA editing in human disease; more to it than meets the I. Hum. Genet. 136, 1265–1278 (2017).
Eisenberg, E. & Levanon, E. Y. A-to-I RNA editing—immune protector and transcriptome diversifier. Nat. Rev. Genet. 19, 473–490 (2018).
Jain, M., Jantsch, M. F. & Licht, K. The editor’s I on disease development. Trends Genet 35, 903–913 (2019).
Samuel, C. E. Adenosine deaminase acting on RNA (ADAR1), a suppressor of double-stranded RNA-triggered innate immune responses. J. Biol. Chem. 294, 1710–1720 (2019).
Bass, B. L. & Weintraub, H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 55, 1089–1098 (1988).
Wagner, R. W., Smith, J. E., Cooperman, B. S. & Nishikura, K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc. Natl Acad. Sci. USA 86, 2647–2651 (1989).
George, C. X. & Samuel, C. E. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc. Natl Acad. Sci. USA 96, 4621–4626 (1999).
Kawakubo, K. & Samuel, C. E. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene 258, 165–172 (2000).
George, C. X., Wagner, M. V. & Samuel, C. E. Expression of interferon-inducible RNA adenosine deaminase ADAR1 during pathogen infection and mouse embryo development involves tissue-selective promoter utilization and alternative splicing. J. Biol. Chem. 280, 15020–15028 (2005).
Sun, T. et al. Decoupling expression and editing preferences of ADAR1 p150 and p110 isoforms. Proc. Natl Acad. Sci. USA 118, e2021757118 (2021).
Liang, Z., Goradia, A., Walkley, C. R. & Heraud-Farlow, J. E. Generation of a new Adar1p150–/– mouse demonstrates isoform-specific roles in embryonic development and adult homeostasis. RNA 29, 1325–1338 (2023).
Tian, B., Bevilacqua, P. C., Diegelman-Parente, A. & Mathews, M. B. The double-stranded-RNA-binding motif: interference and much more. Nat. Rev. Mol. Cell Biol. 5, 1013–1023 (2004).
Gleghorn, M. L. & Maquat, L. E. ‘Black sheep’ that don’t leave the double-stranded RNA-binding domain fold. Trends Biochem. Sci. 39, 328–340 (2014).
Poulsen, H., Nilsson, J., Damgaard, C. K., Egebjerg, J. & Kjems, J. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol. Cell. Biol. 21, 7862–7871 (2001).
Strehblow, A., Hallegger, M. & Jantsch, M. F. Nucleocytoplasmic distribution of human RNA-editing enzyme ADAR1 is modulated by double-stranded RNA-binding domains, a leucine-rich export signal, and a putative dimerization domain. Mol. Biol. Cell 13, 3822–3835 (2002).
Patterson, J. B. & Samuel, C. E. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol. Cell. Biol. 15, 5376–5388 (1995).
Slavov, D., Crnogorac-Jurcevic, T., Clark, M. & Gardiner, K. Comparative analysis of the DRADA A-to-I RNA editing gene from mammals, pufferfish and zebrafish. Gene 250, 53–60 (2000).
Kim, Y. G. et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl Acad. Sci. USA 100, 6974–6979 (2003).
Athanasiadis, A. et al. The crystal structure of the Zβ domain of the RNA-editing enzyme ADAR1 reveals distinct conserved surfaces among Z-domains. J. Mol. Biol. 351, 496–507 (2005).
Inoue, M. et al. An Aicardi–Goutières syndrome-causative point mutation in Adar1 gene invokes multiorgan inflammation and late-onset encephalopathy in mice. J. Immunol. 207, 3016–3027 (2021).
Guo, X. et al. Aicardi–Goutières syndrome-associated mutation at ADAR1 gene locus activates innate immune response in mouse brain. J. Neuroinflammation 18, 169 (2021).
Guo, X. et al. An AGS-associated mutation in ADAR1 catalytic domain results in early-onset and MDA5-dependent encephalopathy with IFN pathway activation in the brain. J. Neuroinflammation 19, 285 (2022).
Matthews, M. M. et al. Structures of human ADAR2 bound to dsRNA reveal base-flipping mechanism and basis for site selectivity. Nat. Struct. Mol. Biol. 23, 426–433 (2016).
Fisher, A. J. & Beal, P. A. Effects of Aicardi–Goutières syndrome mutations predicted from ADAR–RNA structures. RNA Biol. 14, 164–170 (2017).
Cho, D. S. et al. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J. Biol. Chem. 278, 17093–17102 (2003).
Valente, L. & Nishikura, K. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J. Biol. Chem. 282, 16054–16061 (2007).
Mannion, N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014). This study shows that the MAVS signalling pathway causes the embryonic lethality of Adar-knockout mice.
Wang, Q. et al. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J. Biol. Chem. 279, 4952–4961 (2004).
Hartner, J. C. et al. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J. Biol. Chem. 279, 4894–4902 (2004). Together with Wang et al. (2004), this study describes the original (conditional) Adar-knockout mouse models, which have been extensively used in genetic studies.
Levanon, E. Y. et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 22, 1001–1005 (2004).
Athanasiadis, A., Rich, A. & Maas, S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2, e391 (2004).
Kim, D. D. et al. Widespread RNA editing of embedded Alu elements in the human transcriptome. Genome Res. 14, 1719–1725 (2004).
Blow, M., Futreal, P. A., Wooster, R. & Stratton, M. R. A survey of RNA editing in human brain. Genome Res. 14, 2379–2387 (2004).
Bazak, L., Levanon, E. Y. & Eisenberg, E. Genome-wide analysis of Alu editability. Nucleic Acids Res. 42, 6876–6884 (2014).
Zhang, X. O., Pratt, H. & Weng, Z. Investigating the potential roles of SINEs in the human genome. Annu. Rev. Genomics Hum. Genet. 22, 199–218 (2021).
Bahn, J. H. et al. Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways. Nat. Commun. 6, 6355 (2015).
Neeman, Y., Levanon, E. Y., Jantsch, M. F. & Eisenberg, E. RNA editing level in the mouse is determined by the genomic repeat repertoire. RNA 12, 1802–1809 (2006).
Licht, K. et al. A high resolution A-to-I editing map in the mouse identifies editing events controlled by pre-mRNA splicing. Genome Res. 29, 1453–1463 (2019).
Porath, H. T., Knisbacher, B. A., Eisenberg, E. & Levanon, E. Y. Massive A-to-I RNA editing is common across the Metazoa and correlates with dsRNA abundance. Genome Biol. 18, 185 (2017).
Bazak, L. et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 24, 365–376 (2014).
Song, Y. et al. irCLASH reveals RNA substrates recognized by human ADARs. Nat. Struct. Mol. Biol. 27, 351–362 (2020).
Solomon, O. et al. RNA editing by ADAR1 leads to context-dependent transcriptome-wide changes in RNA secondary structure. Nat. Commun. 8, 1440 (2017).
Uzonyi, A. et al. Deciphering the principles of the RNA editing code via large-scale systematic probing. Mol. Cell 81, 2374–2387.e3 (2021).
Scadden, A. D. & O’Connell, M. A. Cleavage of dsRNAs hyper-edited by ADARs occurs at preferred editing sites. Nucleic Acids Res. 33, 5954–5964 (2005).
Scadden, A. D. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat. Struct. Mol. Biol. 12, 489–496 (2005).
Morita, Y. et al. Human endonuclease V is a ribonuclease specific for inosine-containing RNA. Nat. Commun. 4, 2273 (2013).
Vitali, P. & Scadden, A. D. Double-stranded RNAs containing multiple IU pairs are sufficient to suppress interferon induction and apoptosis. Nat. Struct. Mol. Biol. 17, 1043–1050 (2010).
Hartner, J. C., Walkley, C. R., Lu, J. & Orkin, S. H. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat. Immunol. 10, 109–115 (2009). This study shows that ADAR1 functions as a negative regulator of type I interferon signalling.
Pestal, K. et al. Isoforms of RNA-editing enzyme adar1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity 43, 933–944 (2015). This study shows that ADAR1-p150 prevents MDA5-mediated immunopathology.
Heraud-Farlow, J. E. et al. Protein recoding by ADAR1-mediated RNA editing is not essential for normal development and homeostasis. Genome Biol. 18, 166 (2017).
Berke, I. C. & Modis, Y. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J. 31, 1714–1726 (2012).
Peisley, A. et al. Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments. Proc. Natl Acad. Sci. USA 109, E3340–E3349 (2012).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Liu, S. et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife 2, e00785 (2013).
Fang, R. et al. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 13, e1006720 (2017).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Lei, Y. et al. MAVS-mediated apoptosis and its inhibition by viral proteins. PLoS ONE 4, e5466 (2009).
Huang, Y. et al. MAVS–MKK7–JNK2 defines a novel apoptotic signaling pathway during viral infection. PLoS Pathog. 10, e1004020 (2014).
El Maadidi, S. et al. A novel mitochondrial MAVS/caspase-8 platform links RNA virus-induced innate antiviral signaling to Bax/Bak-independent apoptosis. J. Immunol. 192, 1171–1183 (2014).
Liddicoat, B. J. et al. Adenosine-to-inosine RNA editing by ADAR1 is essential for normal murine erythropoiesis. Exp. Hematol. 44, 947–963 (2016).
Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III interferons. Immunity 50, 907–923 (2019).
Philips, R. L. et al. The JAK–STAT pathway at 30: much learned, much more to do. Cell 185, 3857–3876 (2022).
Ourthiague, D. R. et al. Limited specificity of IRF3 and ISGF3 in the transcriptional innate-immune response to double-stranded RNA. J. Leukoc. Biol. 98, 119–128 (2015).
Bajad, P. et al. An internal deletion of ADAR rescued by MAVS deficiency leads to a minute phenotype. Nucleic Acids Res. 48, 3286–3303 (2020).
Garcia-Gonzalez, C. et al. ADAR1 prevents autoinflammatory processes in the heart mediated by IRF7. Circ. Res. 131, 580–597 (2022).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Maurano, M. et al. Protein kinase R and the integrated stress response drive immunopathology caused by mutations in the RNA deaminase ADAR1. Immunity 54, 1948–1960.e5 (2021). This study provides in vivo evidence that PKR activation contributes to the immunopathology of mice with hemizygous Zα domain P195A-mutant ADAR1 expression.
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015). This study reports the generation of an ADAR1 editing-deficient knock-in mouse, showing that A-to-I editing is crucial to prevent spontaneous MDA5 activation.
Goodman, R. A., Macbeth, M. R. & Beal, P. A. ADAR proteins: structure and catalytic mechanism. Curr. Top. Microbiol. Immunol. 353, 1–33 (2012).
Lai, F., Drakas, R. & Nishikura, K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J. Biol. Chem. 270, 17098–17105 (1995).
Ahmad, S. et al. Breaching self-tolerance to alu duplex RNA underlies MDA5-mediated inflammation. Cell 172, 797–810.e13 (2018).
Barak, M. et al. Purifying selection of long dsRNA is the first line of defense against false activation of innate immunity. Genome Biol. 21, 26 (2020).
Rice, G. I. et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 (2014).
Oda, H. et al. Aicardi–Goutières syndrome is caused by IFIH1 mutations. Am. J. Hum. Genet. 95, 121–125 (2014).
Li, Q. et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature 608, 569–577 (2022).
Chen, J., Sun, M., Hurst, L. D., Carmichael, G. G. & Rowley, J. D. Genome-wide analysis of coordinate expression and evolution of human cis-encoded sense–antisense transcripts. Trends Genet. 21, 326–329 (2005).
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 (2008).
Bruns, A. M., Leser, G. P., Lamb, R. A. & Horvath, C. M. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5–RNA interaction and filament assembly. Mol. Cell 55, 771–781 (2014).
Stok, J. E. et al. RNA sensing via the RIG-I-like receptor LGP2 is essential for the induction of a type I IFN response in ADAR1 deficiency. EMBO J. 41, e109760 (2022).
Schmelzer, L. et al. Variable clinical phenotype in two siblings with Aicardi–Goutières syndrome type 6 and a novel mutation in the ADAR gene. Eur. J. Paediatr. Neurol. 22, 186–189 (2018).
Wang, W. et al. Analysis of clinical characteristics of children with Aicardi–Goutières syndrome in China. World J. Pediatr. 18, 490–497 (2022).
Ward, S. V. et al. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc. Natl Acad. Sci. USA 108, 331–336 (2011). This study reports the generation of Adar-p150-specific knockout mice, which phenocopy the embryonic lethality of full Adar-knockout mice.
Hu, S.-B. et al. ADAR1p150 prevents MDA5 and PKR activation via distinct mechanisms to avert fatal autoinflammation. Preprint at bioRxiv https://doi.org/10.1101/2023.01.25.525475 (2023).
Kim, J. I. et al. RNA editing at a limited number of sites is sufficient to prevent MDA5 activation in the mouse brain. PLoS Genet. 17, e1009516 (2021). This study reports the generation of Adar-p110-specific knockout mice, which in contrast to p150-knockout mice do not develop MDA5-mediated embryonic lethality.
Barraud, P., Banerjee, S., Mohamed, W. I., Jantsch, M. F. & Allain, F. H. A bimodular nuclear localization signal assembled via an extended double-stranded RNA-binding domain acts as an RNA-sensing signal for transportin 1. Proc. Natl Acad. Sci. USA 111, E1852–E1861 (2014).
Eckmann, C. R., Neunteufl, A., Pfaffstetter, L. & Jantsch, M. F. The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein. Mol. Biol. Cell 12, 1911–1924 (2001).
Kleinova, R. et al. The ADAR1 editome reveals drivers of editing-specificity for ADAR1-isoforms. Nucleic Acids Res. 51, 4191–4207 (2023).
Sun, T. et al. A small subset of cytosolic dsRNAs must be edited by ADAR1 to evade MDA5-mediated autoimmunity. Preprint at bioRxiv https://doi.org/10.1101/2022.08.29.505707 (2022).
Yang, J. H. et al. Widespread inosine-containing mRNA in lymphocytes regulated by ADAR1 in response to inflammation. Immunology 109, 15–23 (2003).
George, C. X., Ramaswami, G., Li, J. B. & Samuel, C. E. Editing of cellular self-RNAs by adenosine deaminase ADAR1 suppresses innate immune stress responses. J. Biol. Chem. 291, 6158–6168 (2016).
Chung, H. et al. Human ADAR1 prevents endogenous RNA from triggering translational shutdown. Cell 172, 811–824.e14 (2018).
Zhang, T. et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 606, 594–602 (2022).
Krall, J. B., Nichols, P. J., Henen, M. A., Vicens, Q. & Vogeli, B. Structure and formation of Z-DNA and Z-RNA. Molecules 28, 843 (2023).
Nichols, P. J., Krall, J. B., Henen, M. A., Vogeli, B. & Vicens, Q. Z-RNA biology: a central role in the innate immune response? RNA 29, 273–281 (2023).
Sathishkumar, D. et al. Co-occurrence of Aicardi–Goutières syndrome type 6 and dyschromatosis symmetrica hereditaria due to compound heterozygous pathogenic variants in ADAR1: a case series from India. Clin. Exp. Dermatol. 46, 704–709 (2021).
Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Placido, D., Brown, B. A. 2nd, Lowenhaupt, K., Rich, A. & Athanasiadis, A. A left-handed RNA double helix bound by the Zα domain of the RNA-editing enzyme ADAR1. Structure 15, 395–404 (2007).
Schade, M., Turner, C. J., Lowenhaupt, K., Rich, A. & Herbert, A. Structure-function analysis of the Z-DNA-binding domain Zα of dsRNA adenosine deaminase type I reveals similarity to the (α + β) family of helix–turn–helix proteins. EMBO J. 18, 470–479 (1999).
de Reuver, R. et al. ADAR1 interaction with Z-RNA promotes editing of endogenous double-stranded RNA and prevents MDA5-dependent immune activation. Cell Rep. 36, 109500 (2021).
Tang, Q. et al. Adenosine-to-inosine editing of endogenous Z-form RNA by the deaminase ADAR1 prevents spontaneous MAVS-dependent type I interferon responses. Immunity 54, 1961–1975 e1965 (2021).
Nakahama, T. et al. Mutations in the adenosine deaminase ADAR1 that prevent endogenous Z-RNA binding induce Aicardi–Goutières-syndrome-like encephalopathy. Immunity 54, 1976–1988.e7 (2021).
Jiao, H. et al. ADAR1 averts fatal type I interferon induction by ZBP1. Nature 607, 776–783 (2022).
Liang, Z. et al. The phenotype of the most common human ADAR1p150 Zα mutation P193A in mice is partially penetrant. EMBO Rep. 24, e55835 (2023).
Guo, X. et al. ADAR1 Zα domain P195A mutation activates the MDA5-dependent RNA-sensing signaling pathway in brain without decreasing overall RNA editing. Cell Rep. 42, 112733 (2023). Together with Maurano et al. (2021), de Reuver et al. (2021), Tang et al. (2021), Nakahama et al. (2021), Jiao et al. (2022) and Liang et al. (EMBO Rep., 2023), this work shows that Zα domain mutation of ADAR1 causes spontaneous MDA5 activation.
Feng, S. et al. Alternate rRNA secondary structures as regulators of translation. Nat. Struct. Mol. Biol. 18, 169–176 (2011).
de Reuver, R. et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 607, 784–789 (2022).
Koeris, M., Funke, L., Shrestha, J., Rich, A. & Maas, S. Modulation of ADAR1 editing activity by Z-RNA in vitro. Nucleic Acids Res. 33, 5362–5370 (2005).
Nichols, P. J. et al. Recognition of non-CpG repeats in Alu and ribosomal RNAs by the Z-RNA binding domain of ADAR1 induces A-Z junctions. Nat. Commun. 12, 793 (2021).
Nie, Y., Hammond, G. L. & Yang, J. H. Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J. Virol. 81, 917–923 (2007).
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, eaat5314 (2020).
Hubbard, N. W. et al. ADAR1 mutation causes ZBP1-dependent immunopathology. Nature 607, 769–775 (2022).
Kaiser, W. J., Upton, J. W. & Mocarski, E. S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 (2008).
Rebsamen, M. et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 10, 916–922 (2009).
Peng, R. et al. Human ZBP1 induces cell death-independent inflammatory signaling via RIPK3 and RIPK1. EMBO Rep. 23, e55839 (2022).
Thapa, R. J. et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20, 674–681 (2016).
Kuriakose, T. et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1, aag2045 (2016).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012).
Lei, X., Chen, Y., Lien, E. & Fitzgerald, K. A. MLKL-driven inflammasome activation and caspase-8 mediate inflammatory cell death in influenza a virus infection. mBio 14, e0011023 (2023).
Zheng, M., Karki, R., Vogel, P. & Kanneganti, T. D. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181, 674–687.e13 (2020).
Karki, R. et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 37, 109858 (2021). Together with Zhang et al. (2022), Jiao et al. (2022), de Reuver et al. (2022) and Hubbard et al. (2022), this work identifies ADAR1 as a negative regulator of ZBP1 activation.
Kreuz, S., Siegmund, D., Scheurich, P. & Wajant, H. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol. Cell. Biol. 21, 3964–3973 (2001).
Nassour, J. et al. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature 614, 767–773 (2023).
Lei, Y. et al. Cooperative sensing of mitochondrial DNA by ZBP1 and cGAS promotes cardiotoxicity. Cell 186, 3013–3032.e22 (2023).
Li, Y. et al. Ribonuclease L mediates the cell-lethal phenotype of double-stranded RNA editing enzyme ADAR1 deficiency in a human cell line. eLife 6, e25687 (2017).
Peisley, A., Wu, B., Yao, H., Walz, T. & Hur, S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell 51, 573–583 (2013).
Patel, J. R. et al. ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 14, 780–787 (2013).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Goubau, D. et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514, 372–375 (2014).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Peisley, A. et al. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc. Natl Acad. Sci. USA 108, 21010–21015 (2011).
Uchikawa, E. et al. Structural analysis of dsRNA binding to anti-viral pattern recognition receptors LGP2 and MDA5. Mol. Cell 62, 586–602 (2016).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Lind, N. A., Rael, V. E., Pestal, K., Liu, B. & Barton, G. M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 22, 224–235 (2022).
Sadler, A. J. & Williams, B. R. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316, 253–292 (2007).
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013).
Hornung, V., Hartmann, R., Ablasser, A. & Hopfner, K. P. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 14, 521–528 (2014).
Karki, R. & Kanneganti, T. D. ADAR1 and ZBP1 in innate immunity, cell death, and disease. Trends Immunol. 44, 201–216 (2023).
Maelfait, J. & Rehwinkel, J. The Z-nucleic acid sensor ZBP1 in health and disease. J. Exp. Med. 220, e20221156 (2023).
DeAntoneo, C., Herbert, A. & Balachandran, S. Z-Form nucleic acid-binding protein 1 (ZBP1) as a sensor of viral and cellular Z-RNAs: walking the razor’s edge. Curr. Opin. Immunol. 83, 102347 (2023).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371, eabd0811 (2021).
Wang, P. et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 350, 826–830 (2015).
Shen, C. et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 184, 5759–5774.e20 (2021).
Hong, X. X. & Carmichael, G. G. Innate immunity in pluripotent human cells: attenuated response to interferon-β. J. Biol. Chem. 288, 16196–16205 (2013).
Poirier, E. Z. et al. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 373, 231–236 (2021).
Kim, U., Wang, Y., Sanford, T., Zeng, Y. & Nishikura, K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl Acad. Sci. USA 91, 11457–11461 (1994).
Melcher, T. et al. A mammalian RNA editing enzyme. Nature 379, 460–464 (1996).
Chen, C. X. et al. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 6, 755–767 (2000).
Tan, M. H. et al. Dynamic landscape and regulation of RNA editing in mammals. Nature 550, 249–254 (2017).
Oakes, E., Anderson, A., Cohen-Gadol, A. & Hundley, H. A. Adenosine deaminase that acts on RNA 3 (ADAR3) binding to glutamate receptor subunit B pre-mRNA inhibits RNA editing in glioblastoma. J. Biol. Chem. 292, 4326–4335 (2017).
Raghava Kurup, R. et al. RNA binding by ADAR3 inhibits adenosine-to-inosine editing and promotes expression of immune response protein MAVS. J. Biol. Chem. 298, 102267 (2022).
Sommer, B., Kohler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Higuchi, M. et al. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron–exon structure determines position and efficiency. Cell 75, 1361–1370 (1993).
Greger, I. H., Watson, J. F. & Cull-Candy, S. G. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94, 713–730 (2017).
Barbon, A., Vallini, I., La Via, L., Marchina, E. & Barlati, S. Glutamate receptor RNA editing: a molecular analysis of GluR2, GluR5 and GluR6 in human brain tissues and in NT2 cells following in vitro neural differentiation. Brain Res. Mol. Brain Res. 117, 168–178 (2003).
Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8, 189–198 (1992).
Higuchi, M. et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406, 78–81 (2000).
Gajiwala, K. S. & Burley, S. K. Winged helix proteins. Curr. Opin. Struct. Biol. 10, 110–116 (2000).
Herbert, A. et al. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc. Natl Acad. Sci. USA 94, 8421–8426 (1997).
Schwartz, T., Behlke, J., Lowenhaupt, K., Heinemann, U. & Rich, A. Structure of the DLM-1–Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat. Struct. Biol. 8, 761–765 (2001).
Rothenburg, S. et al. A PKR-like eukaryotic initiation factor 2α kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc. Natl Acad. Sci. USA 102, 1602–1607 (2005).
Nikpour, N. & Salavati, R. The RNA binding activity of the first identified trypanosome protein with Z-DNA-binding domains. Sci. Rep. 9, 5904 (2019).
Tome, A. R. et al. Crystal structure of a poxvirus-like Zα domain from cyprinid herpesvirus 3. J. Virol. 87, 3998–4004 (2013).
Rich, A., Nordheim, A. & Wang, A. H. The chemistry and biology of left-handed Z-DNA. Annu. Rev. Biochem. 53, 791–846 (1984).
Klysik, J., Stirdivant, S. M., Singleton, C. K., Zacharias, W. & Wells, R. D. Effects of 5 cytosine methylation on the B–Z transition in DNA restriction fragments and recombinant plasmids. J. Mol. Biol. 168, 51–71 (1983).
Acknowledgements
The group of J.M. is supported by an Odysseus II Grant (G0H8618N), EOS INFLADIS (G0I5722N) and a junior research grant (G031022N) from the Research Foundation Flanders (FWO). R.d.R. is supported by a Ghent University BOF PhD fellowship. The authors thank the reviewers for their constructive criticism and important insight. Finally, the authors apologize to the scientists who moved the field of double-stranded RNA (dsRNA)-mediated immunity forward and whose important work we were not able to cite.
Author information
Authors and Affiliations
Contributions
R.d.R. and J.M. researched and discussed the cited literature. R.d.R. and J.M. wrote the main text. R.d.R. and J.M. generated the figures and supplementary tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks C. Walkley, who co-reviewed with J. Heraud-Farlow; A. van der Veen; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Protein Data Bank: https://www.rcsb.org/
Supplementary information
Glossary
- Aicardi–Goutières syndrome
-
(AGS). Severe autoinflammatory childhood-onset encephalopathy, resulting from the activation of the nucleic acid receptors melanoma differentiation-associated protein 5 (MDA5) or cGAS by endogenous nucleic acids owing to mutations in one of the following genes involved in nucleic acid sensing or metabolism: ADAR, IFIH1, TREX1, SAMHD1, RNASEH2A, RNASEH2B, RNASEH2C, LSM11 or RNU7-1.
- cis-Natural antisense transcripts
-
(cis-NATs). RNA molecules transcribed from (partially) overlapping sequences on opposing DNA strands within the same genomic locus containing regions of perfect complementarity, enabling the formation of double-stranded RNA (dsRNA) helices.
- Integrated stress response
-
(ISR). An evolutionarily conserved cellular stress response induced by the eukaryotic translation initiation factor 2α (eIF2α) kinases, HRI, protein kinase R (PKR), PERK or GCN2, resulting in a global translational shutdown while increasing activating transcription factor 4 (ATF4)-dependent gene expression.
- Short interspersed nuclear elements
-
(SINEs). A subclass of short (<1,000 bp), interspersed (non-tandem), non-autonomous retrotransposon-type repeat elements, containing sequences derived from RNA polymerase III-dependent transcripts including 7SL RNA, tRNA and 5S ribosomal RNA.
- Z-Nucleic acid
-
Left-handed double-stranded DNA (dsDNA), double-stranded RNA (dsRNA) or hybrid DNA–RNA structures, characterized by a zig-zag-shaped (hence the name ‘Z’-nucleic acid) phosphodiester backbone containing purine–pyrimidine dinucleotide repeat sequences, which adopt alternating syn-conformations and anti-nucleobase conformations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Reuver, R., Maelfait, J. Novel insights into double-stranded RNA-mediated immunopathology. Nat Rev Immunol 24, 235–249 (2024). https://doi.org/10.1038/s41577-023-00940-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00940-3