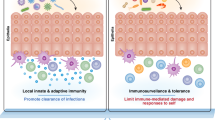
Abstract
Antigen-presenting cells (APCs) are master regulators of the immune response by directly interacting with T cells to orchestrate distinct functional outcomes. Several types of professional APC exist, including conventional dendritic cells, B cells and macrophages, and numerous other cell types have non-classical roles in antigen presentation, such as thymic epithelial cells, endothelial cells and granulocytes. Accumulating evidence indicates the presence of a new family of APCs marked by the lineage-specifying transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt) and demonstrates that these APCs have key roles in shaping immunity, inflammation and tolerance, particularly in the context of host–microorganism interactions. These RORγt+ APCs include subsets of group 3 innate lymphoid cells, extrathymic autoimmune regulator-expressing cells and, potentially, other emerging populations. Here, we summarize the major findings that led to the discovery of these RORγt+ APCs and their associated functions. We discuss discordance in recent reports and identify gaps in our knowledge in this burgeoning field, which has tremendous potential to advance our understanding of fundamental immune concepts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blum, J. S., Wearsch, P. A. & Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 31, 443–473 (2013).
Guermonprez, P., Valladeau, J., Zitvogel, L., Théry, C. & Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002).
Rossjohn, J. et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 33, 169–200 (2015).
Pishesha, N., Harmand, T. J. & Ploegh, H. L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 22, 751–764 (2022).
Huppa, J. B. & Davis, M. M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
Murphy, T. L. et al. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 34, 93–119 (2016).
Kambayashi, T. & Laufer, T. M. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat. Rev. Immunol. 14, 719–730 (2014).
Hirose, T., Smith, R. J. & Jetten, A. M. ROR-γ: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem. Biophys. Res. Commun. 205, 1976–1983 (1994).
Medvedev, A., Yan, Z. H., Hirose, T., Giguère, V. & Jetten, A. M. Cloning of a cDNA encoding the murine orphan receptor RZR/RORγ and characterization of its response element. Gene 181, 199–206 (1996).
Eberl, G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 10, 27–34 (2017).
He, Y. W., Deftos, M. L., Ojala, E. W. & Bevan, M. J. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity 9, 797–806 (1998).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Kurebayashi, S. et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA 97, 10132–10137 (2000).
Okada, S. et al. Immunodeficiencies. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349, 606–613 (2015).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Mebius, R. E., Streeter, P. R., Michie, S., Butcher, E. C. & Weissman, I. L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc. Natl Acad. Sci. USA 93, 11019–11024 (1996).
Adachi, S., Yoshida, H., Kataoka, H. & Nishikawa, S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int. Immunol. 9, 507–514 (1997).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3- LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Eberl, G. & Littman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Lochner, M. et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205, 1381–1393 (2008).
Ohnmacht, C. et al. Mucosal Immunology. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 349, 989–993 (2015).
Sefik, E. et al. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Martin, B., Hirota, K., Cua, D. J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Spits, H. et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Grigg, J. B. et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature 600, 707–712 (2021). This publication defines a circulating ILC3 subset in mice and humans, termed inflammatory ILC3s, that promotes inflammatory T cell responses through MHC-II and is essential for autoimmune neuroinflammation.
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013). This publication defines MHC-II+ LTi-like ILC3s in mice and humans as the first described RORγt+ APC subset and identifies unexpected roles for these cells in enforcing CD4+ T cell tolerance to the microbiota.
Hepworth, M. R. et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
Yamano, T. et al. Aire-expressing ILC3-like cells in the lymph node display potent APC features. J. Exp. Med. 216, 1027–1037 (2019). This publication is the first to define RORγt+ eTACs in mouse lymph nodes.
Brown, C. C. et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 179, 846–863.e24 (2019).
Wang, J. et al. Single-cell multiomics defines tolerogenic extrathymic Aire-expressing populations with unique homology to thymic epithelium. Sci. Immunol. 6, eabl5053 (2021). This publication provides the first single-cell analysis focusing on Aire-expressing cells in the mouse peripheral immune system.
Dobeš, J. et al. Extrathymic expression of Aire controls the induction of effective TH17 cell-mediated immune response to Candida albicans. Nat. Immunol. 23, 1098–1108 (2022). This publication is the first to define that RORγt+ eTACs promote TH17 cell responses to Candida albicans in mice.
Kedmi, R. et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 610, 737–743 (2022). This publication further defines RORγt+ APCs and their ability to migrate to the mesenteric lymph nodes to promote microbiota-specific RORγt+ Treg cells in mice.
Lyu, M. et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751 (2022). This publication defines all RORγt+ APCs in the mesenteric lymph nodes of mice, identifies that MHC-II+ LTi-like ILC3s instruct RORγt+ Treg cells and immune tolerance to the microbiota in mice, and finds that this pathway is altered in human IBD.
Akagbosu, B. et al. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 610, 752–760 (2022). This publication characterizes Rorc+ DC-like cells in mice and shows that RORγt+ APCs promote the induction of RORγt+ Treg cells in early life.
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Klose, C. S. et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Withers, D. R. et al. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med. 22, 319–323 (2016).
Fiancette, R. et al. Reciprocal transcription factor networks govern tissue-resident ILC3 subset function and identity. Nat. Immunol. 22, 1245–1255 (2021).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Fawkner-Corbett, D. et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell 184, 810–826.e23 (2021).
Goc, J. et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184, 5015–5030.e16 (2021).
Teng, F. et al. ILC3s control airway inflammation by limiting T cell responses to allergens and microbes. Cell Rep. 37, 110051 (2021).
Huang, S. et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459.e25 (2021).
Gardner, J. M. et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843–847 (2008). This publication is the first to identify Aire-reporter-positive cells and AIRE protein in mouse lymph nodes.
Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 17, 399–403 (1997).
Nagamine, K. et al. Positional cloning of the APECED gene. Nat. Genet. 17, 393–398 (1997).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
Gardner, J. M. et al. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4+ T cells. Immunity 39, 560–572 (2013).
Lyu, M. et al. ILC3s select for RORγt+ Tregs and establish tolerance to intestinal microbiota. Preprint at bioRxiv https://doi.org/10.1101/2022.04.25.489463 (2022).
Wang, X. et al. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Front. Immunol. 3, 19 (2012).
Salvermoser, J. et al. Mediated ablation of conventional dendritic cells suggests a lymphoid path to generating dendritic cells. Front. Immunol. 9, 699 (2018).
Zhou, W. et al. ZBTB46 defines and regulates ILC3s that protect the intestine. Nature 609, 159–165 (2022).
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012).
Meredith, M. M. et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 209, 1153–1165 (2012).
Schlenner, S. M. et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436 (2010).
Leung, G. A. et al. The lymphoid-associated interleukin 7 receptor (IL7R) regulates tissue-resident macrophage development. Development 146, dev176180 (2019).
Ghaedi, M. et al. Single-cell analysis of RORα tracer mouse lung reveals ILC progenitors and effector ILC2 subsets. J. Exp. Med. 217, jem.20182293 (2020).
Fergusson, J. R. et al. Maturing human CD127+CCR7+PDL1+ dendritic cells express AIRE in the absence of tissue restricted antigens. Front. Immunol. 9, 2902 (2018). This publication is the first single-cell analysis identifying human cells expressing AIRE in the peripheral immune system.
Poliani, P. L. et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am. J. Pathol. 176, 1104–1112 (2010).
Melo-Gonzalez, F. et al. Antigen-presenting ILC3 regulate T cell-dependent IgA responses to colonic mucosal bacteria. J. Exp. Med. 216, 728–742 (2019).
Wang, W. et al. The interaction between lymphoid tissue inducer-like cells and T cells in the mesenteric lymph node restrains intestinal humoral immunity. Cell Rep. 32, 107936 (2020).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Liu, K. et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196, 1091–1097 (2002).
Kurts, C., Kosaka, H., Carbone, F. R., Miller, J. F. & Heath, W. R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186, 239–245 (1997).
Probst, H. C., Lagnel, J., Kollias, G. & van den Broek, M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity 18, 713–720 (2003).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Curotto de Lafaille, M. A. & Lafaille, J. J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
von Burg, N. et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc. Natl Acad. Sci. USA 111, 12835–12840 (2014).
Lehmann, F. M. et al. Microbiota-induced tissue signals regulate ILC3-mediated antigen presentation. Nat. Commun. 11, 1794 (2020).
Castellanos, J. G. et al. Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity 49, 1077–1089.e5 (2018).
Rao, A. et al. Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs. Nat. Commun. 11, 2049 (2020).
Lim, A. I. et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168, 1086–1100.e10 (2017).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43, 246–252 (2011).
Lill, C. M. et al. MANBA, CXCR5, SOX8, RPS6KB1 and ZBTB46 are genetic risk loci for multiple sclerosis. Brain 136, 1778–1782 (2013).
Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira da Costa, M. & Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C. C. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4, 350–354 (2003).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Malchow, S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016).
Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015).
Gillis-Buck, E. et al. Extrathymic Aire-expressing cells support maternal-fetal tolerance. Sci. Immunol. 6, eabf1968 (2021).
Anderson, M. S. et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005).
Kisand, K. et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010).
Xu, M. et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018).
Russler-Germain, E. V. et al. Gut Helicobacter presentation by multiple dendritic cell subsets enables context-specific regulatory T cell generation. eLife 10, e54792 (2021).
Chai, J. N. et al. Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci. Immunol. 2, eaal5068 (2017).
Mackley, E. C. et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 6, 5862 (2015).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Husebye, E. S., Anderson, M. S. & Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 2543–2544 (2018).
Husebye, E. S., Perheentupa, J., Rautemaa, R. & Kämpe, O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J. Intern. Med. 265, 514–529 (2009).
Abramson, J. & Husebye, E. S. Autoimmune regulator and self-tolerance — molecular and clinical aspects. Immunol. Rev. 271, 127–140 (2016).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Bruserud, Ø. et al. Altered immune activation and IL-23 signaling in response to. Front. Immunol. 8, 1074 (2017).
Mathä, L. et al. Migration of lung resident group 2 innate lymphoid cells link allergic lung inflammation and liver immunity. Front. Immunol. 12, 679509 (2021).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Acknowledgements
The authors thank members of the Abramson, Dobeš and Sonnenberg laboratories for discussions and critical reading of the manuscript. G.F.S. is kindly supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, U01AI095608, R01AI162936, R37AI174468 and R01CA274534), the NIAID Mucosal Immunology Studies Team (MIST), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, the Cancer Research Institute, Linda and Glenn Greenberg, the Dalton Family Foundation, and the Roberts Institute for Research in IBD. G.F.S. is a CRI Lloyd J. Old STAR. M.L. is supported by a Crohn’s and Colitis Foundation Research Fellowship Award (#935259). J.A. is kindly supported by the Eugene and Marcia Applebaum Professorial Chair, European Research Council (ERC-2016-CoG-724821), IOCB fellowship for sabbatical visit program (RVO 61388963) and Bill and Marika Glied and Family Fund. J.D. is kindly supported by the Czech Science Foundation JUNIOR STAR grant (21-22435M), Czech Science Foundation grant (22-30879S) and by the Charles University PRIMUS grant (Primus/21/MED/003).
Author information
Authors and Affiliations
Contributions
All authors are equal contributors and collectively conceived the concepts, wrote the manuscript and prepared the figure drafts.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks J. Gardner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Autoimmune regulator
-
(AIRE). A transcriptional regulator initially reported to be expressed by medullary thymic epithelial cells, where it has a role in central immune tolerance by inducing the expression of tissue-restricted antigens.
- Non-professional APCs
-
A subset of antigen-presenting cells (APCs), such as thymic epithelial cells, endothelial cells and granulocytes, that modulate the quality of the CD4+ T cell response in peripheral tissues through antigen presentation on MHC class II.
- Professional APCs
-
A subset of antigen-presenting cells (APCs), including conventional dendritic cells, macrophages and B cells, that are specialized in activating naive CD4+ T cell responses through phagocytosis of exogenous antigens and through processing and presentation of antigenic peptides on their MHC class II molecules, together with co-stimulatory signals.
- RORγt+ APCs
-
A newly defined family of antigen-presenting cells (APCs) that express retinoic acid receptor-related orphan receptor-γt (RORγt) and can present antigens to CD4+ T cells through MHC class II.
- Tissue-restricted antigens
-
(TRAs). Self-antigens whose coding genes are expressed in less than five different parenchymal tissues (out of approximately 60) based on currently available expression atlases. Expression of these genes may also be restricted to a particular developmental period, be sex specific or be regulated by complex biochemical pathways.
- Type 3 immune responses
-
Type 3 immune responses involve RORγt+ lymphocytes, including T helper 17 cells, that produce the cytokines IL-17 and IL-22 to mediate antimicrobial responses and neutrophil recruitment. Type 3 immune responses are protective in the case of extracellular bacterial or fungal infections but, if dysregulated, can drive chronic inflammation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abramson, J., Dobeš, J., Lyu, M. et al. The emerging family of RORγt+ antigen-presenting cells. Nat Rev Immunol 24, 64–77 (2024). https://doi.org/10.1038/s41577-023-00906-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00906-5
This article is cited by
-
Group 3 innate lymphoid cells in intestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
Same yet different — how lymph node heterogeneity affects immune responses
Nature Reviews Immunology (2023)