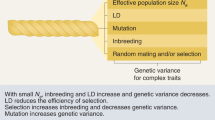
Abstract
Pleiotropy (whereby one genetic polymorphism affects multiple traits) and epistasis (whereby non-linear interactions between genetic polymorphisms affect the same trait) are fundamental aspects of the genetic architecture of quantitative traits. Recent advances in the ability to characterize the effects of polymorphic variants on molecular and organismal phenotypes in human and model organism populations have revealed the prevalence of pleiotropy and unexpected shared molecular genetic bases among quantitative traits, including diseases. By contrast, epistasis is common between polymorphic loci associated with quantitative traits in model organisms, such that alleles at one locus have different effects in different genetic backgrounds, but is rarely observed for human quantitative traits and common diseases. Here, we review the concepts and recent inferences about pleiotropy and epistasis, and discuss factors that contribute to similarities and differences between the genetic architecture of quantitative traits in model organisms and humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fisher, R. A. The correlation between relatives on the supposition of Mendelian inheritance. Trans. R. Soc. Edinb. 52, 399–433 (1918). This landmark paper lays out the theoretical foundations of the genetics of quantitative traits, reconciling previous observations of Mendelian segregation and continuous variation for quantitative traits.
Falconer, D. S. & Mackay, T. F. C. Introduction to Quantitative Genetics 4th edn (Longman, 1996).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer, 1998).
Feagan, B. G. et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol. Hepatol. 3, 671–680 (2018).
Hertz, D. L. & Rae, J. Pharmacogenetics of cancer drugs. Annu. Rev. Med. 66, 65–81 (2015).
Kullo, I. J. et al. Polygenic scores in biomedical research. Nat. Rev. Genet. 23, 524–532 (2022).
Goddard, M. E. & Hayes, B. J. Genomic selection. J. Anim. Breed. Genet. 124, 323–330 (2007).
Enbody, E. D. et al. Community-wide genome sequencing reveals 30 years of Darwin’s finch evolution. Science 381, eadf6218 (2023).
Abdellaoui, A., Yengo, L., Verweij, K. J. H. & Visscher, P. M. 15 years of GWAS discovery: realizing the promise. Am. J. Hum. Genet. 110, 179–194 (2023). This work presents an excellent review of the lessons learned from large GWAS in humans and future directions in human quantitative genetics.
Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709–717 (2016).
van Rheenen, W., Peyrot, W. J., Schork, A. J., Hong Lee, S. & Wray, N. R. Genetic correlations of polygenic disease traits: from theory to practice. Nat. Rev. Genet. 20, 567–581 (2019). This work presents a review of the theory underlying genetic correlations and pleiotropy and methods for detecting pleiotropy in human populations.
Flatt, T. Life-history evolution and the genetics of fitness components in Drosophila melanogaster. Genetics 214, 3–48 (2020).
Sieberts, S. K. & Schadt, E. E. Moving toward a system genetics view of disease. Mamm. Genome 18, 389–401 (2007).
Greene, C. S., Penrod, N. M., Williams, S. M. & Moore, J. H. Failure to replicate a genetic association may provide important clues about genetic architecture. PLoS ONE 4, e5639 (2009). This analysis shows that the effect of a focal locus may differ in populations with different allele frequencies if it interacts with other loci; a phenomenon that can be used to detect epistatically interacting loci in replicated association studies in populations with different allele frequencies.
Gibson, G. & Dworkin, I. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5, 681–690 (2004).
Ober, U. et al. Accounting for genetic architecture improves sequence based genomic prediction for a Drosophila fitness trait. PLoS ONE 10, e0126880 (2015). This study demonstrates that incorporating epistatic interactions into genomic prediction models can substantially improve predictive ability when traits exhibit epistasis.
Pate, L. in Festschrift zum sechzigsten Geburtstag Richard Hertwigs [German] 536–610 (Fischer, 1910).
Stearns, F. W. One hundred years of pleiotropy: a retrospective. Genetics 186, 767–773 (2010).
Solovieff, N., Cotsapas, C., Lee, P. H., Purcell, S. M. & Smoller, J. W. Pleiotropy in complex traits: challenges and strategies. Nat. Rev. Genet. 14, 483–495 (2013).
Reissmann, M. & Ludwig, A. Pleiotropic effects of coat colour-associated mutations in humans, mice and other mammals. Semin. Cell Dev. Biol. 24, 576–586 (2013).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Carbone, M. A. et al. Phenotypic variation and natural selection at Catsup, a pleiotropic quantitative trait gene in Drosophila. Curr. Biol. 2, 912–919 (2006).
Falconer, D. S. The problem of environment and selection. Am. Nat. 86, 293–298 (1952).
Mackay, T. F. C. & Huang, W. Charting the genotype–phenotype map: lessons from the Drosophila melanogaster Genetic Reference Panel. Wiley Interdiscip. Rev. Dev. Biol. 7, e289 (2018).
Ho, P. W. et al. Massive QTL analysis identifies pleiotropic genetic determinants for stress resistance, aroma formation, and ethanol, glycerol and isobutanol production in Saccharomyces cerevisiae. Biotechnol. Biofuels 14, 211 (2021).
Flint, J. & Mackay, T. F. C. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 19, 723–733 (2009).
Giaever, G. & Nislow, C. The yeast deletion collection: a decade of functional genomics. Genetics 197, 451–465 (2014).
Kamath, R. & Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30, 313–321 (2003).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Bellen, H. J. et al. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics 188, 731–743 (2011).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene activation in Drosophila. Nature 448, 151–156 (2007).
Zirin, J. et al. Large-scale transgenic Drosophila resource collections for loss- and gain-of-function studies. Genetics 214, 755–767 (2020).
Brown, S. D. M. Advances in mouse genetics for the study of human disease. Hum. Mol. Genet. 30, R274–R264 (2021).
Ericson, E. et al. Genetic pleiotropy in Saccharomyces cerevisiae quantified by high-resolution phenotypic profiling. Mol. Genet. Genomics 275, 605–614 (2006).
Norga, K. K. et al. Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr. Biol. 13, 1388–1396 (2003).
Anholt, R. R. H., Lyman, R. F. & Mackay, T. F. C. Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics 143, 293–301 (1996).
Yamamoto, A. et al. Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 105, 12393–12398 (2008).
Harbison, S. T. & Seghal, A. Quantitative genetic analysis of sleep in Drosophila melanogaster. Genetics 178, 2341–23460 (2008).
Harbison, S. T. et al. Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics 166, 1807–1823 (2004).
Morozova, T. V., Mackay, T. F. C. & Anholt, R. R. H. Transcriptional networks for alcohol sensitivity in Drosophila melanogaster. Genetics 187, 1193–1205 (2011).
Nolan, P. M. et al. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat. Genet. 25, 440–443 (2000).
De Angelis, M. H. et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat. Genet. 25, 444–447 (2000).
De Angelis, M. H. et al. Analysis of mammalian gene function through broad-based phenotypic screens across a consortium of mouse clinics. Nat. Genet. 47, 969–978 (2015).
Birling, M.-C. et al. A resource of targeted mutant mouse lines for 5,061 genes. Nat. Genet. 53, 416–419 (2021).
Groza, T. et al. The International Mouse Phenotyping Consortium: comprehensive knockout phenotyping underpinning the study of human disease. Nucleic Acids Res. 51, D1038–D1045 (2023).
Paaby, A. B. & Rockman, M. V. The many faces of pleiotropy. Trends Genet. 29, 66–73 (2013).
The Gene Ontology Consortium. The Gene Ontology resource: 20 years and still GOing strong [database issue]. Nucleic Acids Res. 47, D330–D338 (2019).
Hughes, T. R. et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126 (2000).
Featherstone, D. E. & Broadie, K. Wrestling with pleiotropy: genomic and topological analysis of the yeast gene expression network. Bioessays 24, 267–274 (2002).
Kemmeren, P. et al. Large-scale genetic perturbations reveal regulatory networks and an abundance of gene-specific repressors. Cell 157, 740–752 (2014).
Vande Zande, P., Hill, M. S. & Wittkopp, P. J. Pleiotropic effects of trans-regulatory mutations on fitness and gene expression. Science 377, 105–109 (2022).
Vande Zande, P. & Wittkopp, P. J. Network topology can explain differences in pleiotropy between cis- and trans-regulatory mutations. Mol. Biol. Evol. 39, msac266 (2022).
Anholt, R. R. H. et al. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat. Genet. 35, 180–184 (2003).
Magwire, M. M. et al. Quantitative and molecular genetic analyses of mutations increasing Drosophila life span. PLoS Genet. 6, e1001037 (2010).
Amberger, J. S. & Hamosh, A. Searching Online mendelian Inheritance in Man (OMIM): a knowledgebase of human genes and genetic phenotypes. Curr. Protoc. Bioinformatics 58, 1.2.1–1.2.12 (2017).
Replogle, J. M. et al. Mapping information-rich genotype–phenotype landscapes with genome-scale Perturb-seq. Cell 185, 2559–2575 (2022).
Li, H. & Auwerx, J. Mouse systems genetics as a prelude to precision medicine. Trends Genet. 36, 259–272 (2020).
Aitman, T. J. et al. Progress and prospects in rat genetics: a community view. Nat. Genet. 40, 516–522 (2008).
Mulligan, M. K., Mozhui, K., Prins, P. & Williams, R. W. GeneNetwork: a toolbox for systems genetics. Methods Mol. Biol. 1488, 75–120 (2017).
Bouge, M. A. et al. Mouse phenome database: curated data repository with interactive multi-population and multi-trait analyses. Mamm. Genome 34, 509–519 (2023).
Smith, J. R. et al. The year of the rat: the rat genome database at 20: a multi-species knowledgebase and analysis platform. Nucleic Acids Res. 48, D731–D742 (2020).
Mackay, T. F. C. et al. The Drosophila melanogaster Genetic Reference Panel. Nature 482, 173–178 (2012).
Huang, W. et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 24, 1193–1208 (2014).
King, E. G. et al. Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 22, 1558–1566 (2012).
Evans, K. S., van Wijk, M. H., McGrath, P. T., Andersen, E. C. & Sterken, M. G. From QTL to gene: C. elegans facilitates discoveries of the genetic mechanisms underlying natural variation. Trends Genet. 37, 933–947 (2021).
The 1001 Genomes Consortium. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166, 481–491 (2016).
Kover, P. X. et al. A multiparent advanced generation inter-cross to fine-map quantitative traits in Arabidopsis thaliana. PLoS Genet. 5, e1000551 (2009).
Evans, K. S. et al. Shared genomic regions underlie natural variation in diverse toxin responses. Genetics 210, 1509–1525 (2018).
Ba, A. N. N. et al. Barcoded bulk QTL mapping reveals highly polygenic and epistatic architecture of complex traits in yeast. eLife 11, e73983 (2022).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
The All of Us Research Program Investigators. The “All of Us” research program. N. Eng. J. Med. 381, 6680676 (2019).
Solis, E. et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. 51, D977–D985 (2023).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014). This article reports a statistical method for inferring whether two association signals, such as an organismal trait and an eQTL, map to the same molecular polymorphism.
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015). This study is among the first to report substantial genetic correlation among human psychiatric disorders and quantitative traits.
Denny, J. C. et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene–disease associations. Bioinformatics 26, 1205–1210 (2010).
Bush, W. S., Oetjens, M. T. & Crawford, D. C. Unravelling the human genome–phenome relationship using phenome-wide association studies. Nat. Rev. Genet. 17, 129–145 (2016).
International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379 (2013).
Watanabe, K. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51, 1339–1348 (2019).
Mackay, T. F. C. The genetic architecture of quantitative traits. Annu. Rev. Genet. 35, 303–339 (2001).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547, 173–178 (2017). This paper describes an excellent study fine-mapping GWAS associations at single-variant resolution.
Pickar-Oliver, A. & Gersbach, C. A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20, 490–507 (2019).
Lamb, A. M., Walker, E. A. & Wittkopp, P. J. Tools and strategies for scarless allele replacement in Drosophila using CRISPR/Cas9. Fly 11, 53–64 (2017).
Hoedjes, K. M., Kostic, H., Flatt, T. & Keller, L. A single nucleotide variant in the PPARγ-homolog Eip75B affects fecundity in Drosophila. Mol. Biol. Evol. 40, msad018 (2023).
Bassett, A. R. Editing the genome of hiPSC with CRISPR/Cas9: disease models. Mamm. Genome 28, 348–364 (2017).
Morris, J. A. et al. Discovery of target genes and pathways at GWAS loci by pooled single-cell CRISPR screens. Science 380, eadh7699 (2023).
Rockman, M. V. Reverse engineering the genotype–phenotype map with natural genetic variation. Nature 456, 738–744 (2008).
Mackay, T. F. C., Stone, E. A. & Ayroles, J. F. The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 10, 565–577 (2009).
MacKinnon, D. in Multivariate Applications in Substance Use Research: New Methods for New Questions (eds Rose, J. S., Chassin, L., Presson, C. C. & Sherman, S. J.) 141–160 (Lawrence Erlbaum, 2000).
Zeng, P., Shao, Z. & Zhou, X. Statistical methods for mediation analysis in the era of high-throughput genomics: current successes and future challenges. Comput. Struct. Biotechnol. J. 19, 3209–3224 (2021). This work presents an excellent review on methods for mediation analysis.
Davey Smith, G. & Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22 (2003).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Primers 2, 6 (2022).
O’Connor, L. J. & Price, A. L. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat. Genet. 50, 1728–1734 (2018).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Jensen, R. C. & Nap, J.-P. Genetical genomics: the added value from segregation. Trends Genet. 17, 388–391 (2001).
Cheung, V. G. & Spielman, R. S. The genetics of variation in gene expression. Nat. Genet. 32, 522–525 (2002).
Jin, W. et al. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29, 389–395 (2001).
Sandberg, R. et al. Regional and strain-specific gene expression mapping in the adult mouse brain. Proc. Natl Acad. Sci. USA 97, 11038–11043 (2000).
Oleksiak, M. F., Churchill, G. A. & Crawford, D. L. Variation in gene expression within and among natural populations. Nat. Genet. 32, 261–266 (2002).
Brem, R. B., Yvert, G., Clinton, R. & Kruglyak, L. Genetic dissection of transcriptional regulation in budding yeast. Science 296, 752–755 (2002). This paper describes the first study to map QTL association with variation in genome-wide gene expression.
Yvert, G. et al. trans-Acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat. Genet. 35, 57–64 (2003).
Brem, R. B. & Kruglyak, L. The landscape of genetic complexity across 5,700 gene expression traits in yeast. Proc. Natl Acad. Sci. USA 102, 1572–1577 (2005).
Schadt, E. E. et al. Genetics of gene expression surveyed in maize, mouse and man. Nature 422, 297–302 (2003).
Fu, Y. et al. Genetic dissection of intermated recombinant inbred lines using a new genetic map of maize. Genetics 174, 1671–1683 (2006).
West, M. A. et al. Global eQTL mapping reveals the complex genetic architecture of transcript-level variation in Arabidopsis. Genetics 175, 1441–1450 (2007).
Ruden, D. M. et al. Genetical toxicogenomics in Drosophila identifies master-modulatory loci that are regulated by developmental exposure to lead. Neurotoxicology 30, 898–914 (2009).
Li, Y. et al. Mapping determinants of gene expression plasticity by genetical genomics in C. elegans. PLoS Genet. 2, e222 (2006).
Cheung, V. G. et al. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat. Genet. 33, 422–425 (2003).
Monks, S. A. et al. Genetic inheritance of gene expression in human cell lines. Am. J. Hum. Genet. 75, 1094–1105 (2004).
Drake, T. A., Schadt, E. E. & Lusis, A. J. Integrating genetic and gene expression data: application to cardiovascular and metabolic traits in mice. Mamm. Genome 17, 466–479 (2006).
Nicolae, D. L. et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 6, e1000888 (2010).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Hormozdiari, F. et al. Leveraging molecular quantitative trait loci to understand the genetic architecture of diseases and complex traits. Nat. Genet. 50, 1041–1047 (2018).
Maurano, M. T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012). This study shows that non-coding variants associated with human complex traits and diseases predominantly map to regulatory DNA sequences.
Gusev, A. et al. Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am. J. Hum. Genet. 95, 535–552 (2014).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Gamazon, E. R. et al. A gene-based association method for mapping traits using reference transcriptome data. Nat. Genet. 47, 1091–1098 (2015). This study introduces PrediXcan, a popular method for predicting associations of quantitative traits with imputed transcript abundance genome-wide.
Gusev, A. et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 48, 245–252 (2016).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 300–1310 (2021).
Boyle, E. A., Li, Y. I. & Pritchard, J. K. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 (2017). This work on an omnigenic model of quantitative genetic variation proposes that the molecular basis of trait variation arises from regulatory genetic variation downstream of core genes affecting the traits in trait-relevant tissues.
Liu, X., Li, Y. I. & Pritchard, J. K. trans effects on gene expression can drive omnigenetic inheritance. Cell 177, 1022–1034 (2019).
Huang, W. et al. Genetic basis of transcriptome diversity in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 112, E6010–E6019 (2015). This study shows that variance eQTLs interact epistatically with cis-eQTLs affecting the same gene expression trait.
Everett, L. J. et al. Gene expression networks in the Drosophila Genetic Reference Panel. Genome Res. 30, 485–496 (2020).
Chun, S. et al. Limited statistical evidence for shared genetic effects of eQTLs and autoimmune-disease-associated loci in three major immune-cell types. Nat. Genet. 49, 600–605 (2017).
Mancuso, N. et al. Probabilistic fine-mapping of transcriptome-wide association studies. Nat. Genet 51, 675–682 (2019).
Connally, N. J. et al. The missing link between genetic association and regulatory function. eLife 11, e74970 (2022). This study challenges the view that variants affecting human quantitative traits are enriched for eQTLs.
Yao, D. W., O’Connor, L. J., Price, A. L. & Gusev, A. Quantifying genetic effects on disease mediated by assayed gene expression levels. Nat. Genet 52, 626–633 (2020).
Nathan, A. et al. Single-cell eQTL models reveal dynamic T cell state dependence of disease loci. Nature 606, 120–128 (2022).
Gazal, S. et al. Linkage disequilibrium-dependent architecture of human complex traits shows action of negative selection. Nat. Genet 49, 1421–1427 (2017).
Zeng, J. et al. Signatures of negative selection in the genetic architecture of human complex traits. Nat. Genet 50, 746–753 (2018).
Turelli, M. Effects of pleiotropy on predictions concerning mutation–selection balance for polygenic traits. Genetics 111, 165–195 (1985).
Barton, N. H. Pleiotropic models of quantitative variation. Genetics 124, 773–782 (1990).
Hill, W. G. & Keightley, P. D. Quantitative genetic variability maintained by mutation-stabilizing selection balance in finite populations. Genet. Res 52, 33–43 (1988).
Simons, Y. B., Bullaughey, K., Hudson, R. R. & Sella, G. A population genetic interpretation of GWAS findings for human quantitative traits. PLoS Biol. 16, e2002985 (2018).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957). This paper provides an exposition of the role of antagonistic pleiotropy in the evolution of life history traits.
Maharjan, R., McKenzie, C., Yeung, A. & Ferenci, T. The basis of antagonistic pleiotropy in hfq mutations that have opposite effects on fitness at slow and fast growth rates. Heredity 110, 10–18 (2013).
Delaney, J. R., Murakami, C. J., Olsen, B., Kennedy, B. K. & Kaeberlein, M. Quantitative evidence for early life fitness defects from 32 longevity-associated alleles in yeast. Cell Cycle 10, 156–165 (2011).
Qian, W., Ma, D., Xiao, C., Wang, Z. & Zhang, J. The genomic landscape and evolutionary resolution of antagonistic pleiotropy in yeast. Cell Rep. 2, 1399–1410 (2012).
Maklakov, A. A. et al. Antagonistically pleiotropic allele increases lifespan and late-life reproduction at the cost of early-life reproduction and individual fitness. Proc. Biol. Sci. 284, 20170376 (2017).
Huang, W. et al. Context-dependent genetic architecture of Drosophila life span. PLoS Biol. 18, e3000645 (2020).
Rodríguez, J. A. et al. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat. Ecol. Evol. 1, 55 (2017).
Byars, S. G. & Voskarides, K. Antagonistic pleiotropy in human disease. J. Mol. Evol. 88, 12–25 (2020).
Song, W. et al. Locus-level antagonistic selection shaped the polygenic architecture of human complex diseases. Hum. Genet. 141, 1935–1947 (2022).
Wright S. Evolution and the Genetics of Populations. Volume 1: Genetic and Biometric Foundations (Univ. Chicago Press, 1968).
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, 1930).
Orr, H. A. Adaptation and the cost of complexity. Evolution 54, 13–20 (2000).
Mendel, G. Versuche über Pflanzen-Hybriden [German]. Verh. Naturforsch. Ver. Brünn 4, 3–47 (1865).
Bateson, W. Facts limiting the theory of heredity. Science 26, 649–660 (1907).
Mackay, T. F. C. Epistasis and quantitative traits: using model organisms to study gene–gene interactions. Nat. Rev. Genet. 15, 22–33 (2014).
Hu, Z. et al. Genomic value prediction for quantitative traits under the epistatic model. BMC Genet. 12, 15 (2011).
Wang, D. et al. Prediction of genetic values of quantitative traits with epistatic effects in plant breeding populations. Heredity 109, 313–319 (2012).
Martini, J. W. R., Wimmer, V., Erbe, M. & Simianer, H. Epistasis and covariance: how gene interaction translates into genomic relationship. Theor. Appl. Genet. 129, 963–976 (2016).
Vojgani, E. et al. Accounting for epistasis improves genomic prediction of phenotypes with univariate and bivariate models across environments. Theor. Appl. Genet. 134, 2913–2930 (2021).
Costanzo, M. et al. The genetic landscape of a cell. Science 327, 425–431 (2010). This paper describes a pioneering study mapping gene–gene interactions genome-wide using the yeast deletion collection.
Kuzmin, E. et al. Synthetic genetic array analysis for global mapping of genetic networks in yeast. Methods Mol. Biol. 1205, 143–168 (2014).
Dixon, S. J., Costanzo, M., Baryshnikova, A., Andrews, B. & Boone, C. Systematic mapping of genetic interaction networks. Annu. Rev. Genet. 43, 601–625 (2009).
Kuzmin, E. et al. Systematic analysis of complex genetic interactions. Science 360, eaao1729 (2018).
Lehner, B., Crombie, C., Tischler, J., Fortunato, A. & Fraser, A. G. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 38, 896–903 (2006).
Byrne, A. B. et al. A global analysis of genetic interactions in Caenorhabditis elegans. J. Biol. 6, 8 (2007).
Bakal, C. et al. Phosphorylation networks regulating JNK activity in diverse genetic backgrounds. Science 322, 453–456 (2008).
Zender, L. et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell 135, 852–864 (2008).
Luo, B. et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl Acad. Sci. USA 105, 20380–20385 (2008).
Lee, H. M. T. et al. Epistatic, synthetic, and balancing interactions among tubulin missense mutations affecting neurite growth in Caenorhabditis elegans. Mol. Biol. Cell 32, 331–347 (2021).
Kwong, L. N. et al. Identification of Mom7, a novel modifier of ApcMin/+ on mouse chromosome 18. Genetics 176, 1237–1244 (2007).
Weatherly, S. M. et al. Identification of Arhgef12 and Prkci as genetic modifiers of retinal dysplasia in the Crb1rd8 mouse model. PLoS Genet. 18, e1009798 (2022).
Clark, A. G. & Wang, L. Epistasis in measured genotypes: Drosophila P-element insertions. Genetics 147, 157–163 (1997).
Zwarts, L. et al. Complex genetic architecture of Drosophila aggressive behavior. Proc. Natl Acad. Sci. USA 108, 17070–17075 (2011).
Li, B. et al. Epistatic transcription factor networks differentially modulate Arabidopsis growth and defense. Genetics 214, 529–541 (2020).
Li, C., Qian, W., Maclean, C. J. & Zhang, J. The fitness landscape of a tRNA gene. Science 352, 837–840 (2016).
Puchta, O. et al. Network of epistatic interactions within a yeast snoRNA. Science 352, 840–844 (2016).
Gonzalez, C. E. & Ostermeier, M. Pervasive pairwise intragenic epistasis among sequential mutations in TEM-1 β-lactamase. J. Mol. Biol. 431, 1981–1992 (2019).
Huang, W. & Mackay, T. F. C. The genetic architecture of quantitative traits cannot be inferred from variance component analysis. PLoS Genet. 12, e1006421 (2016).
Hill, W. G., Goddard, M. E. & Visscher, P. M. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 4, e1000008 (2008). This paper introduces a theoretical treatment showing that epistatic effects, even if large, contribute more to additive genetic variance than epistatic variance, and hence can be ignored in analyses of human quantitative traits.
Mäki-Tanila, A. & Hill, W. G. Influence of gene interaction on complex trait variation with multilocus models. Genetics 198, 355–367 (2014).
Cheverud, J. M. & Routman, E. J. Epistasis and its contribution to genetic variance components. Genetics 139, 1455–1461 (1995). This analysis distinguishes between epistatic effects (physiological epistasis) and epistatic variance (statistical epistasis) and argues that the former can be large and needs to be accounted for to fully understand the genotype–phenotype map, although the latter is typically negligible.
Phillips, P. C. Epistasis — the essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 9, 855–867 (2008).
Eshed, Y. & Zamir, D. Less-than-additive epistatic interactions of quantitative trait loci in tomato. Genetics 143, 1807–1817 (1996).
Torgeman, S. & Zamir, D. Epistatic QTLs for yield heterosis in tomato. Proc. Natl Acad. Sci. USA 120, e2205787119 (2023).
Long, A. D. et al. High resolution mapping of genetic factors affecting abdominal bristle number in Drosophila melanogaster. Genetics 139, 1273–1291 (1995).
Gurganus, M. C., Nuzhdin, S. V., Leips, J. W. & Mackay, T. F. C. High-resolution mapping of quantitative trait loci for sternopleural bristle number in Drosophila melanogaster. Genetics 152, 1585–1604 (1999).
Deutschbauer, A. M. & Davis, R. W. Quantitative trait loci mapped to single-nucleotide resolution in yeast. Nat. Genet. 37, 1333–1340 (2005). This study maps three QTLs affecting natural variation in yeast sporulation to three nucleotides that interact epistatically.
Gerke, J., Lorenz, K. & Cohen, B. Genetic interactions between transcription factors cause natural variation in yeast. Science 323, 498–501 (2009).
Kim, H. S. & Fay, J. C. A combined-cross analysis reveals genes with drug-specific and background-dependent effects on drug sensitivity in Saccharomyces cerevisiae. Genetics 183, 1141–1151 (2009).
Forsberg, S. K., Bloom, J. S., Sadhu, M. J., Kruglyak, L. & Carlborg, Ö. Accounting for genetic interactions improves modeling of individual quantitative trait phenotypes in yeast. Nat. Genet. 49, 497–503 (2017).
Matsui, T. et al. The interplay of additivity, dominance, and epistasis on fitness in a diploid yeast cross. Nat. Commun. 13, 1463 (2022).
Gaertner, B. E., Parmenter, M. D., Rockman, M. V., Kruglyak, L. & Phillips, P. C. More than the sum of its parts: a complex epistatic network underlies natural variation in thermal preference behavior in Caenorhabditis elegans. Genetics 192, 1533–1542 (2012).
Leips, J. & Mackay, T. F. C. Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics 155, 1773–1788 (2000).
Leips, J. & Mackay, T. F. C. The complex genetic architecture of Drosophila life span. Exp. Aging Res. 28, 361–390 (2002).
Shao, H. et al. Genetic architecture of complex traits: large phenotypic effects and pervasive epistasis. Proc. Natl Acad. Sci. USA 105, 19910–19914 (2008). This study of chromosome substitution panels in rodents reveals substantial epistasis for all traits studied such that the sum of the individual chromosome effects greatly exceeds the total phenotypic difference between the parental strains.
Carlborg, Ö. et al. A global search reveals epistatic interaction between QTL for early growth in the chicken. Genome Res. 13, 413–421 (2003).
Pettersson, M., Besnier, F., Siegel, P. B. & Carlborg, Ö. Replication and explorations of high-order epistasis using a large advanced intercross line pedigree. PLoS Genet. 7, e1002180 (2011).
Brem, R. B., Storey, J. D., Whittle, J. & Kruglyak, L. Genetic interactions between polymorphisms that affect gene expression in yeast. Nature 436, 701–703 (2005).
Wentzell, A. M., Boeye, I., Zhang, Z. & Kliebenstein, D. J. Genetic networks controlling structural outcome of glucosinolate activation across development. PLoS Genet. 4, e1000234 (2008).
Rowe, H. C., Hansen, B. G., Halkier, B. A. & Kliebenstein, D. J. Biochemical networks and epistasis shape the Arabidopsis thaliana metabolome. Plant. Cell 20, 1199–1216 (2008).
Cordell, H. J., Todd, J. A., Bennett, S. T., Kawaguchi, Y. & Farrall, M. Two-locus maximum LOD score analysis of a multifactorial trait: joint consideration of IDDM2 and IDDM4 with IDDM1 in type 1 diabetes. Am. J. Hum. Genet. 57, 920–934 (1995).
Cox, N. J. et al. Loci on chromosomes 2 (NIDDM1) and 15 interact to increase susceptibility to diabetes in Mexican Americans. Nat. Genet. 21, 213–215 (1999).
Cho, J. H. et al. Identification of novel susceptibility loci for inflammatory bowel disease on chromosomes 1p, 3q, and 4q: evidence for epistasis between 1p and IBD1. Proc. Natl Acad. Sci. USA 95, 7502–7507 (1998).
Lai, C., Lyman, R. F., Long, A. D., Langley, C. H. & Mackay, T. F. C. Naturally occurring variation in bristle number and DNA polymorphisms at the scabrous locus of Drosophila melanogaster. Science 266, 1697–1702 (1994).
Hivert, V. et al. Estimation of non-additive genetic variance in human complex traits from a large sample of unrelated individuals. Am. J. Hum. Genet. 108, 786–798 (2021).
Hivert, V., Wray, N. R. & Visscher, P. M. Gene action, genetic variation, and GWAS: a user-friendly web tool. PLoS Genet. 17, e1009548 (2021).
Huang, W. et al. Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc. Natl Acad. Sci. USA 109, 15553–15559 (2012).
Shorter, J. et al. Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc. Natl Acad. Sci. USA 112, E3555–E3563 (2015).
Rönnegård, L. & Valdar, W. Detecting major genetic loci controlling phenotypic variability in experimental crosses. Genetics 188, 435–447 (2011).
Hulse, A. M. & Cai, J. J. Genetic variants contribute to gene expression variability in humans. Genetics 193, 95–108 (2013).
Brown, A. A. et al. Genetic interactions affecting human gene expression identified by variance association mapping. eLife 3, e01381 (2014).
Singhal, P. et al. Evidence of epistasis in regions of long-range linkage disequilibrium across five complex diseases in the UK Biobank and eMERGE datasets. Am. J. Hum. Genet. 110, 575–591 (2023).
Ang, R. M. L., Chen, S. A., Kern, A. F., Xie, Y. & Fraser, H. B. Widespread epistasis among beneficial genetic variants revealed by high-throughput genome editing. Cell Genom. 3, 100260 (2023).
Zhang, S. et al. Multiple genes in a single GWAS risk locus synergistically mediate aberrant synaptic development and function in human neurons. Cell Genom. 3, 100399 (2023).
Bis-Brewer, D. M., Fazal, S. & Züchner, S. Genetic modifiers and non-Mendelian aspects of CMT. Brain Res. 1726, 146459 (2020).
Rahit, K. M. T. H. & Tarailo-Graovac, M. Genetic modifiers and rare Mendelian disease. Genes 11, 239 (2020).
Chen, R. et al. Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nat. Biotechnol. 34, 531–538 (2016).
Tarailo-Graovac, M., Zhu, J. Y. A., Matthews, A., van Karnebeek, C. D. M. & Wasserman, W. W. Assessment of the ExAC data set for the presence of individuals with pathogenic genotypes implicated in severe Mendelian pediatric disorders. Genet. Med. 19, 1300–1308 (2017).
Fahed, A. C. et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat. Commun. 11, 3635 (2020).
Goodrich, J. K. et al. Determinants of penetrance and variable expressivity in monogenic metabolic conditions across 77,184 exomes. Nat. Commun. 12, 3505 (2021).
Mullis, M. N., Matsui, T., Schell, R., Foree, R. & Ehrenreich, I. M. The complex underpinnings of genetic background effects. Nat. Commun. 9, 3548 (2018).
Galardini, M. et al. The impact of the genetic background on gene deletion phenotypes in Saccharomyces cerevisiae. Mol. Syst. Biol. 15, e8831 (2019).
Dworkin, I. et al. Genomic consequences of background effects on scalloped mutant expressivity in the wing of Drosophila melanogaster. Genetics 181, 1065–1076 (2009).
Yamamoto, A., Anholt, R. R. H. & Mackay, T. F. C. Epistatic interactions attenuate mutations affecting startle behaviour in Drosophila melanogaster. Genet. Res. 91, 373–382 (2009).
Chandler, C. H. et al. How well do you know your mutation? Complex effects of genetic background on expressivity, complementation, and ordering of allelic effects. PLoS Genet. 13, e1007075 (2017).
He, X., Zhou, S., St. Armour, G. E., Mackay, T. F. C. & Anholt, R. R. H. Epistatic partners of neurogenic genes modulate Drosophila olfactory behavior. Genes. Brain Behav. 15, 280–290 (2016).
Özsoy, E. D. et al. Epistasis for head morphology in Drosophila melanogaster. G3 11, jkab285 (2021).
Dworkin, I., Palsson, A., Birdsall, K. & Gibson, G. Evidence that Egfr contributes to cryptic genetic variation for photoreceptor determination in natural populations of Drosophila melanogaster. Curr. Biol. 13, 1888–1893 (2003).
Chow, C. Y. Bringing genetic background into focus. Nat. Rev. Genet. 17, 63–64 (2016).
Palu, R. A. S. et al. Natural genetic variation screen in Drosophila identifies Wnt signaling, mitochondrial metabolism, and redox homeostasis genes as modifiers of apoptosis. G3 9, 3995–4005 (2019).
Talsness, D. M. et al. A Drosophila screen identifies NKCC1 as a modifier of NGLY1 deficiency. eLife 9, e57831 (2020).
Hansen, T. F. Why epistasis is important for selection and adaptation. Evolution 67, 3501–3511 (2013).
Sohail, M. et al. Negative selection in humans and fruit flies involves synergistic epistasis. Science 356, 539–542 (2017).
Rutherford, S. L. & Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 396, 336–342 (1998).
McGuigan, K. & Sgro, C. M. Evolutionary consequences of cryptic genetic variation. Trends Ecol. Evol. 24, 305–311 (2009).
Paaby, A. B. & Rockman, M. V. Cryptic genetic variation: evolution’s hidden substrate. Nat. Rev. Genet. 15, 247–258 (2014).
Zheng, J., Payne, J. L. & Wagner, A. Cryptic genetic variation accelerates evolution by opening access to diverse adaptive peaks. Science 365, 347–353 (2019).
Barton, N. H. & Turelli, M. Effects of genetic drift on variance components under a general model of epistasis. Evolution 58, 2111–2132 (2004).
Carlborg, O., Jacobsson, L., Ahgren, P., Siegel, P. & Andersson, L. Epistasis and the release of genetic variation during long-term selection. Nat. Genet. 38, 418–420 (2006).
Barton, N. H. How does epistasis influence the response to selection? Heredity 118, 96–109 (2017).
Hill, W. G. “Conversion” of epistatic into additive genetic variance in finite populations and possible impact on long-term selection response. J. Anim. Breed. Genet. 134, 196–201 (2017).
Wright, S. Evolution in Mendelian populations. Genetics 16, 97–159 (1931).
Orr, H. A. & Turelli, M. The evolution of postzygotic isolation: accumulating Dobzhansky–Muller incompatibilities. Evolution 55, 1085–1094 (2001).
Fierst, J. L. & Hansen, T. F. Genetic architecture and postzygotic reproductive isolation: evolution of Bateson–Dobzhansky–Muller incompatibilities in a polygenic model. Evolution 64, 675–693 (2010).
Rollmann, S. M. et al. Pleiotropic fitness effects of the Tre1–Gr5a region in Drosophila melanogaster. Nat. Genet. 38, 824–829 (2006).
Carter, G. W., Hays, M., Sherman, A. & Galitski, T. Use of pleiotropy to model genetic interactions in a population. PLoS Genet. 8, e1003010 (2012). This paper introduces a novel method to utilize co-expression pleiotropy to define epistatic networks.
Carter, G. W. Inferring gene function and network organization in Drosophila signaling by combined analysis of pleiotropy and epistasis. G3 3, 807–814 (2013).
Philip, V. M., Tyler, A. L. & Carter, G. W. Dissection of complex gene expression using the combined analysis of pleiotropy and epistasis. Pac. Symp. Biocomput. 2014, 200–211 (2014).
Tyler, A. L. et al. Epistatic networks jointly influence phenotypes related to metabolic disease and gene expression in diversity Outbred mice. Genetics 206, 621–639 (2017).
Pavlicev, M. et al. Genetic variation in pleiotropy: differential epistasis as a source of variation in the allometric relationship between long bone lengths and body weight. Evolution 62, 199–213 (2008).
Pavlicev, M., Norgard, E. A., Fawcett, G. L. & Cheverud, J. M. Evolution of pleiotropy: epistatic interaction pattern supports a mechanistic model underlying variation in genotype–phenotype map. J. Exp. Zool. B Mol. Dev. Evol. 316, 371–385 (2011).
Maxwell, T. J. et al. APOE modulates the correlation between triglycerides, cholesterol, and CHD through pleiotropy, and gene-by-gene interactions. Genetics 195, 1397–1405 (2013).
Houle, D., Govindaraju, D. R. & Omholt, S. Phenomics: the next challenge. Nat. Rev. Genet. 11, 855–866 (2010).
Watt, M. et al. Phenotyping: new windows into the plant for breeding. Annu. Rev. Plant. Biol. 71, 689–712 (2020).
Pérez-Enciso, M. & Steibel, J. P. Phenomes: the current frontier in animal breeding. Genet. Sel. Evol. 53, 22 (2021).
Yang, C., Li, C., Wang, Q., Chung, D. & Zhao, H. Implications of pleiotropy: challenges and opportunities for mining Big Data in biomedicine. Front. Genet. 6, 229 (2015).
Yengo, L. et al. A saturated map of common genetic variants associated with human height. Nature 610, 704–712 (2022).
Morgante, F., Huang, W., Maltecca, C. & Mackay, T. F. C. Effect of genetic architecture on the prediction accuracy of quantitative traits in samples of unrelated individuals. Heredity 120, 500–514 (2018).
Mackay, T. F. C. & Moore, J. H. Why epistasis is important for tackling complex human disease genetics. Genome Med. 6, 124 (2014).
Author information
Authors and Affiliations
Contributions
Both authors wrote the article. T.F.C.M. researched data for the article and wrote the first draft of the manuscript based on discussion of the content and organization with R.R.H.A. R.R.H.A. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Luke O’Connor, Kristel van Steen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Additive effects
-
One half of the difference in mean phenotypes associated with homozygous genotypes at a biallelic locus affecting a quantitative trait.
- Additive genetic variance
-
The variance of breeding values.
- Deoxyribonuclease I (DNase I) hypersensitivity sites
-
Accessible nucleosome-free chromatin regions sensitive to cleavage by the DNase I enzyme; these regions are inferred to be involved in gene regulation.
- Dominance effects
-
The difference between the mean phenotype of the heterozygous genotype and the mean phenotype of the two homozygous genotypes at a biallelic locus affecting a quantitative trait
- Fitness
-
The contribution of offspring to the next generation, or reproductive success (viability and fertility) of an individual.
- Genetic architecture
-
The loci, genomic locations and additive, dominance, epistatic and pleiotropic effects and allele frequencies of causal variants affecting a quantitative trait.
- Haplotypes
-
Alleles at different loci on a chromosome that tend to be inherited together.
- Heritability
-
The ratio of the variance of additive genetic variance (narrow sense heritability) or the total genetic variance (broad sense heritability) to the total phenotypic variance.
- P-element
-
A Drosophila transposable element that can be mobilized to new genomic locations by simple genetic crosses.
- Shifting balance theory of evolution
-
A model of evolution proposed by Sewell Wright that assumes widespread epistasis for fitness, natural selection, genetic drift and differentiation among subpopulations, and migration.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mackay, T.F.C., Anholt, R.R.H. Pleiotropy, epistasis and the genetic architecture of quantitative traits. Nat Rev Genet (2024). https://doi.org/10.1038/s41576-024-00711-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41576-024-00711-3