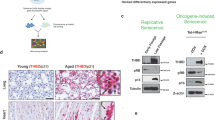
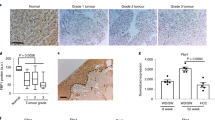
Abstract
The liver is not oblivious to the passage of time, as ageing is a major risk factor for the development of acute and chronic liver diseases. Ageing produces alterations in all hepatic cells, affecting their phenotype and function and worsening the prognosis of liver disease. The ageing process also implies the accumulation of a cellular state characterized by a persistent proliferation arrest and a specific secretory phenotype named cellular senescence. Indeed, senescent cells have key roles in many physiological processes; however, their accumulation owing to ageing or pathological conditions contributes to the damage occurring in chronic diseases. The aim of this Review is to provide an updated description of the pathophysiological events in which hepatic senescent cells are involved and their role in liver disease progression. Finally, we discuss novel geroscience therapies that could be applied to prevent or improve liver diseases and age-mediated hepatic deregulations.
Key points
-
Senescent hepatocytes undergo morphological changes and metabolic alterations, negatively affecting liver function and promoting disease progression.
-
Structural and functional changes in senescent liver sinusoidal endothelial cells contribute to liver disease.
-
In the initial stages of liver disease, hepatic stellate cells undergo senescence, facilitating liver regeneration and fibrosis regression. Conversely, during cirrhosis, the increase in their number induces inflammation, promoting hepatocellular carcinoma (HCC).
-
Senescence has a role in the development and progression of metabolic dysfunction-associated steatotic liver disease and viral hepatitis, contributing to liver injury, inflammation, fibrosis and HCC.
-
Senescence can protect against HCC, inhibiting the growth of damaged cells; it can also lead to HCC progression by promoting inflammation and creating a pro-tumoural microenvironment within the liver.
-
Senotherapeutic strategies, drug repurposing, and lifestyle interventions hold potential as therapies for liver disease by targeting and eliminating senescent cells and counteracting the harmful effects of senescence and ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Muñoz-Espín, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Aravinthan, A. D. & Alexander, G. J. M. Senescence in chronic liver disease: is the future in aging? J. Hepatol. 65, 825–834 (2016).
Maeso-Díaz, R. & Gracia-Sancho, J. Aging and chronic liver disease. Semin. Liver Dis. 40, 373–384 (2020).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature Hepatology 78, 1966–1986 (2023).
Schmucker, D. L. Age-related changes in liver structure and function: implications for disease. Exp. Gerontol. 40, 650–659 (2005).
Koehler, E. M. et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J. Hepatol. 57, 1305–1311 (2012).
Gracia-Sancho, J., Marrone, G. & Fernández-Iglesias, A. Hepatic microcirculation and mechanisms of portal hypertension. Nat. Rev. Gastroenterol. Hepatol. 16, 221–234 (2019).
Gibert-Ramos, A. et al. The hepatic sinusoid in chronic liver disease: the optimal milieu for cancer. Cancers 13, 5719 (2021).
Ginès, P. et al. Liver cirrhosis. Lancet 398, 1359–1376 (2021).
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Pimpin, L. et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 69, 718–735 (2018).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660.e4 (2017).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Sherr, C. J. Ink4-Arf locus in cancer and aging. WIREs Dev. Biol. 1, 731–741 (2012).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Zhang, R. et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
Kennedy, A. L. et al. Senescent mouse cells fail to overtly regulate the HIRA histone chaperone and do not form robust senescence associated heterochromatin foci. Cell Div. 5, 16 (2010).
Di Micco, R. et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 13, 292–302 (2011).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 5, 99–118 (2010).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Udomsinprasert, W., Sobhonslidsuk, A., Jittikoon, J., Honsawek, S. & Chaikledkaew, U. Cellular senescence in liver fibrosis: implications for age-related chronic liver diseases. Expert. Opin. Ther. Targets 25, 799–813 (2021).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Takahashi, A. et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/CCdh1 in senescent cells. Mol. Cell 45, 123–131 (2012).
De Cecco, M. et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).
Glück, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Gulen, M. F. et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374–380 (2023).
Robbins, E., Levine, E. M. & Eagle, H. Morphologic changes accompanying senescence of cultured human diploid cells. J. Exp. Med. 131, 1211–1222 (1970).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Lee, B. Y. et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 (2006).
Evangelou, K. et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16, 192–197 (2017).
Kohli, J. et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 16, 2471–2498 (2021).
Liu, P. et al. Hepatocellular senescence: immunosurveillance and future senescence-induced therapy in hepatocellular carcinoma. Front. Oncol. 10, 589908 (2020).
Seo, E., Kang, H., Choi, H., Choi, W. & Jun, H. S. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 18, e12895 (2019).
Aravinthan, A. et al. Selective insulin resistance in hepatocyte senescence. Exp. Cell Res. 331, 38–45 (2015).
Brunt, E. M., Walsh, S. N., Hayashi, P. H., Labundy, J. & Di Bisceglie, A. M. Hepatocyte senescence in end-stage chronic liver disease: a study of cyclin-dependent kinase inhibitor p21 in liver biopsies as a marker for progression to hepatocellular carcinoma. Liver Int. 27, 662–671 (2007).
Paradis, V. et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum. Pathol. 32, 327–332 (2001).
Hoare, M., Das, T. & Alexander, G. Ageing, telomeres, senescence, and liver injury. J. Hepatol. 53, 950–961 (2010).
Wang, C. et al. The extent of liver injury determines hepatocyte fate toward senescence or cancer. Cell Death Dis. 9, 575 (2018).
Aravinthan, A. et al. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS ONE 8, e72904 (2013).
Marshall, A. et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 128, 33–42 (2005).
Tachtatzis, P. M. et al. Chronic hepatitis B virus infection: the relation between hepatitis B antigen expression, telomere length, senescence, inflammation and fibrosis. PloS ONE 10, e01257511 (2015).
Wijayasiri, P. et al. Role of hepatocyte senescence in the activation of hepatic stellate cells and liver fibrosis progression. Cells 11, 2221 (2022).
Yu, H. et al. Lipid accumulation-induced hepatocyte senescence regulates the activation of hepatic stellate cells through the Nrf2-antioxidant response element pathway. Exp. Cell Res. 405, 112689 (2021).
Irvine, K. M. et al. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J. Gastroenterol. 20, 17851 (2014).
Rudolph, K. L., Chang, S., Millard, M., Schreiber-Agus, N. & DePinho, R. A. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287, 1253–1258 (2000).
Jin, J., Iakova, P., Jiang, Y., Medrano, E. E. & Timchenko, N. A. The reduction of SIRT1 in livers of old mice leads to impaired body homeostasis and to inhibition of liver proliferation. Hepatology 54, 989–998 (2011).
Imai, Y. et al. Crosstalk between the Rb pathway and AKT signaling forms a quiescence-senescence switch. Cell Rep. 7, 194–207 (2014).
Eggert, T. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30, 533–547 (2016).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Ishigami, T. et al. Regulatory effects of senescence marker protein 30 on the proliferation of hepatocytes. Pathol. Int. 51, 491–497 (2001).
Diehl, A. M. et al. Adenovirus-mediated transfer of CCAAT/enhancer-binding protein-ɑ identifies a dominant antiproliferative role for this isoform in hepatocytes. J. Biol. Chem. 271, 7343–7350 (1996).
Wang, H. et al. C/EBPɑ arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol. Cell 8, 817–828 (2001).
da Silva, P. F. L. et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848 (2019).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345 (2012).
De Leeuw, A. M., Brouwer, A. & Knook, D. L. Sinusoidal endothelial cells of the liver: fine structure and function in relation to age. J. Electron. Microsc. Tech. 14, 218–236 (1990).
Maeso-Díaz, R. et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 17, e12829 (2018).
Ito, Y. et al. Age-related changes in the hepatic microcirculation in mice. Exp. Gerontol. 42, 789–797 (2007).
Vats, R. et al. Intravital imaging reveals inflammation as a dominant pathophysiology of age-related hepatovascular changes. Am. J. Physiol. Cell Physiol. 322, C508–C520 (2022).
Maeso-Díaz, R. et al. Aging influences hepatic microvascular biology and liver fibrosis in advanced chronic liver disease. Aging Dis. 10, 684–698 (2019).
Grosse, L. & Bulavin, D. V. LSEC model of aging. Aging 12, 11152–11160 (2020).
Le Couteur, D. G. et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 33, 537–543 (2001).
Gracia-Sancho, J., Caparrós, E., Fernández-Iglesias, A. & Francés, R. Role of liver sinusoidal endothelial cells in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 18, 411–431 (2021).
McLean, A. J. et al. Age-related pseudocapillarization of the human liver. J. Pathol. 200, 112–117 (2003).
Warren, A. et al. Hepatic pseudocapillarization in aged mice. Exp. Gerontol. 40, 807–812 (2005).
Mohamad, M. et al. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell 15, 706–715 (2016).
Duan, J.-L. et al. Age-related liver endothelial zonation triggers steatohepatitis by inactivating pericentral endothelium-derived C-kit. Nat. Aging 3, 258–274 (2022).
Hide, D. et al. Ischemia/reperfusion injury in the aged liver: the importance of the sinusoidal endothelium in developing therapeutic strategies for the elderly. J. Gerontol. A. Biol. Sci. Med. Sci. 75, 268–277 (2020).
Koudelkova, P., Weber, G. & Mikulits, W. Liver sinusoidal endothelial cells escape senescence by loss of p19ARF. PLoS ONE 10, e0142134 (2015).
Grosse, L. et al. Defined p16high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Sun, X. & Harris, E. N. New aspects of hepatic endothelial cells in physiology and nonalcoholic fatty liver disease. Am. J. Physiol. Cell Physiol. 318, C1200–C1213 (2020).
Duan, J.-L. et al. Notch-regulated c-Kit–positive liver sinusoidal endothelial cells contribute to liver zonation and regeneration. Cell. Mol. Gastroenterol. Hepatol. 13, 1741–1756 (2022).
Bloom, S. I., Islam, M. T., Lesniewski, L. A. & Donato, A. J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 20, 38–51 (2023).
Chala, N. et al. Mechanical fingerprint of senescence in endothelial cells. Nano Lett. 21, 4911–4920 (2021).
Simon-Santamaria, J. et al. Age-related changes in scavenger receptor-mediated endocytosis in rat liver sinusoidal endothelial cells. J. Gerontol. A. Biol. Sci. Med. Sci. 65, 951–960 (2010).
Baiocchi, L. et al. Impact of aging on liver cells and liver disease: focus on the biliary and vascular compartments. Hepatol. Commun. 5, 1125–1137 (2021).
Hilmer, S. N. et al. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology 42, 1349–1354 (2005).
Wan, Y. et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 36, e22125 (2022).
Duan, J.-L. et al. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 75, 584–599 (2022).
Yin, K. et al. Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance. Genes. Dev. 36, 533–549 (2022).
Saile, B. et al. Rat liver myofibroblasts and hepatic stellate cells differ in CD95-mediated apoptosis and response to TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G435–G444 (2002).
Verma, S. et al. Sustained telomere length in hepatocytes and cholangiocytes with increasing age in normal liver. Hepatology 56, 1510–1520 (2012).
Wiemann, S. U. et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 (2002).
Warren, A. et al. The effects of old age on hepatic stellate cells. Curr. Gerontol. Geriatr. Res. 2011, 439835 (2011).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017).
Kamm, D. R. & McCommis, K. S. Hepatic stellate cells in physiology and pathology. J. Physiol. 600, 1825–1837 (2022).
Schnabl, B., Purbeck, C. A., Choi, Y. H., Hagedorn, C. H. & Brenner, D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology 37, 653–664 (2003).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 135, 190 (2008).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Yamagishi, R. et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci. Immunol. 7, eabl7209 (2022).
Cogliati, B., Yashaswini, C. N., Wang, S., Sia, D. & Friedman, S. L. Friend or foe? The elusive role of hepatic stellate cells in liver cancer. Nat. Rev. Gastroenterol. Hepatol. 20, 647–661 (2023).
Jin, H. et al. Hepatic stellate cell interferes with NK cell regulation of fibrogenesis via curcumin induced senescence of hepatic stellate cell. Cell. Signal. 33, 79–85 (2017).
Huang, Y.-H. et al. Interleukin-10 induces senescence of activated hepatic stellate cells via STAT3-p53 pathway to attenuate liver fibrosis. Cell. Signal. 66, 109445 (2020).
Kong, X. et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56, 1150–1159 (2012).
Bilzer, M., Roggel, F. & Gerbes, A. L. Role of Kupffer cells in host defense and liver disease. Liver Int. 26, 1175–1186 (2006).
Dixon, L. J., Barnes, M., Tang, H., Pritchard, M. T. & Nagy, L. E. Kupffer cells in the liver. Compr. Physiol. 3, 785 (2013).
Hilmer, S. N., Cogger, V. C. & Le Couteur, D. G. Basal activity of Kupffer cells increases with old age. J. Gerontol. A. Biol. Sci. Med. Sci. 62, 973–978 (2007).
Yang, X. et al. Kupffer cells-dependent inflammation in the injured liver increases recruitment of mesenchymal stem cells in aging mice. Oncotarget 7, 1084 (2016).
Wan, J., Benkdane, M., Alons, E., Lotersztajn, S. & Pavoine, C. M2 Kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am. J. Pathol. 184, 1763–1772 (2014).
Stahl, E. C., Haschak, M. J., Popovic, B. & Brown, B. N. Macrophages in the aging liver and age-related liver disease. Front. Immunol. 9, 2795 (2018).
Radonjić, T. et al. Aging of liver in its different diseases. Int. J. Mol. Sci. 23, 13085 (2022).
Salama, R., Sadaie, M., Hoare, M. & Narita, M. Cellular senescence and its effector programs. Genes. Dev. 28, 99 (2014).
de Oliveira da Silva, B., Ramos, L. F. & Moraes, K. C. M. Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol. Int. 41, 946–959 (2017).
Bird, T. G. et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 10, eaan1230 (2018).
Lagnado, A. et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS‐dependent manner. EMBO J. 40, e106048 (2021).
Loo, T. M. et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 7, 522–538 (2017).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Takasugi, M., Yoshida, Y., Hara, E. & Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 290, 1348–1361 (2023).
He, Y. et al. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res. Rev. 84, 101833 (2023).
Gong, Z., Tas, E., Yakar, S. & Muzumdar, R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol. Cell. Endocrinol. 455, 115–130 (2017).
Sheedfar, F., Biase, S. D., Koonen, D. & Vinciguerra, M. Liver diseases and aging: friends or foes? Aging Cell 12, 950–954 (2013).
Noureddin, M. et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology 58, 1644–1654 (2013).
Sepe, A., Tchkonia, T., Thomou, T., Zamboni, M. & Kirkland, J. L. Aging and regional differences in fat cell progenitors – a mini-review. Gerontology 57, 66–75 (2011).
Lohr, K. et al. Reduced mitochondrial mass and function add to age‐related susceptibility toward diet‐induced fatty liver in C57BL/6J mice. Physiol. Rep. 4, e12988 (2016).
Huang, Y. L., Shen, Z. Q., Huang, C. H., Lin, C. H. & Tsai, T. F. Cisd2 slows down liver aging and attenuates age-related metabolic dysfunction in male mice. Aging Cell 20, e135523 (2021).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Fontana, L. et al. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology 57, 995–1004 (2013).
Ogrodnik, M. & Jurk, D. Senescence explains age- and obesity-related liver steatosis. Cell Stress. 1, 70–72 (2017).
Bonnet, L. et al. Cellular senescence in hepatocytes contributes to metabolic disturbances in NASH. Front. Endocrinol. 13, 957616 (2022).
Aravinthan, A. et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J. Hepatol. 58, 549–556 (2013).
Baboota, R. K. et al. Chronic hyperinsulinemia promotes human hepatocyte senescence. Mol. Metab. 64, 101558 (2022).
Dabravolski, S. A., Bezsonov, E. E. & Orekhov, A. N. The role of mitochondria dysfunction and hepatic senescence in NAFLD development and progression. Biomed. Pharmacother. 142, 112041 (2021).
Karakousis, N. D., Papatheodoridi, A., Chatzigeorgiou, A. & Papatheodoridis, G. Cellular senescence and hepatitis B-related hepatocellular carcinoma: an intriguing link. Liver Int. 40, 2917–2927 (2020).
Wandrer, F. et al. Senescence mirrors the extent of liver fibrosis in chronic hepatitis C virus infection. Aliment. Pharmacol. Ther. 48, 270–280 (2018).
Schirdewahn, T. et al. The third signal cytokine interleukin 12 rather than immune checkpoint inhibitors contributes to the functional restoration of hepatitis D virus-specific T cells. J. Infect. Dis. 215, 139–149 (2017).
Rossiello, F., Jurk, D., Passos, J. F. & d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 24, 135–147 (2022).
Calado, R. T. et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS ONE 4, e7926 (2009).
Aravinthan, A. et al. The senescent hepatocyte gene signature in chronic liver disease. Exp. Gerontol. 60, 37–45 (2014).
Aravinthan, A. D. & Alexander, G. J. M. Hepatocyte senescence explains conjugated bilirubinaemia in chronic liver failure. J. Hepatol. 63, 532–533 (2015).
Odagiri, N. et al. Involvement of ERK1/2 activation in the gene expression of senescence-associated secretory factors in human hepatic stellate cells. Mol. Cell. Biochem. 455, 7–19 (2019).
Liu, B. et al. Hepatic stellate cell activation and senescence induced by intrahepatic microbiota disturbances drive progression of liver cirrhosis toward hepatocellular carcinoma. J. Immunother. Cancer 10, e003069 (2022).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249 (2021).
Cai, X., Guillot, A. & Liu, H. Cellular senescence in hepatocellular carcinoma: the passenger or the driver? Cells 12, 132 (2022).
Huang, Y. et al. The hepatic senescence-associated secretory phenotype promotes hepatocarcinogenesis through Bcl3-dependent activation of macrophages. Cell Biosci. 11, 173 (2021).
Rey, S. et al. Liver damage and senescence increases in patients developing hepatocellular carcinoma. J. Gastroenterol. Hepatol. 32, 1480–1486 (2017).
Wuestefeld, A. et al. A pro-regenerative environment triggers premalignant to malignant transformation of senescent hepatocytes. Cancer Res. 83, 428–440 (2023).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 22, 728–739 (2020).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Watanabe, Y. et al. Navitoclax improves acute-on-chronic liver failure by eliminating senescent cells in mice. Hepatol. Res. 53, 460–472 (2023).
Yang, Z. et al. TUBB4B is a novel therapeutic target in non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Pathol. 260, 71–83 (2023).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e16 (2017).
Fuhrmann-Stroissnigg, H. et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 (2017).
Fuhrmann-Stroissnigg, H., Niedernhofer, L. J. & Robbins, P. D. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle Georget. Tex. 17, 1048–1055 (2018).
Ma, B. et al. Albumosomes formed by cytoplasmic pre-folding albumin maintain mitochondrial homeostasis and inhibit nonalcoholic fatty liver disease. Signal. Transduct. Target. Ther. 8, 229 (2023).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Moiseeva, O. et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12, 489–498 (2013).
Kulkarni, A. S., Gubbi, S. & Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 32, 15–30 (2020).
Hunt, N. J. et al. The effects of metformin on age-related changes in the liver sinusoidal endothelial cell. J. Gerontol. A. Biol. Sci. Med. Sci. 75, 278–285 (2020).
Ota, H. et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the akt pathway. Arterioscler. Thromb. Vasc. Biol. 30, 2205–2211 (2010).
Trebicka, J. et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 46, 242–253 (2007).
Marrone, G. et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial–stellate cell deactivation induced by statins. J. Hepatol. 58, 98–103 (2013).
Marongiu, F., Serra, M. P., Sini, M., Angius, F. & Laconi, E. Clearance of senescent hepatocytes in a neoplastic-prone microenvironment delays the emergence of hepatocellular carcinoma. Aging 6, 26–34 (2014).
Gao, Y. et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 36, 101635 (2020).
Schafer, M. J. et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615 (2016).
Zhang, X. et al. Exercise counters the age-related accumulation of senescent cells. Exerc. Sport. Sci. Rev. 50, 213–221 (2022).
Zhang, L., Pitcher, L. E., Prahalad, V., Niedernhofer, L. J. & Robbins, P. D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 290, 1362–1383 (2023).
Luo, X. et al. Sirtuin 1 ameliorates defenestration in hepatic sinusoidal endothelial cells during liver fibrosis via inhibiting stress-induced premature senescence. Cell Prolif. 54, e12991 (2021).
Smoliga, J. M., Baur, J. A. & Hausenblas, H. A. Resveratrol and health – a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 55, 1129–1141 (2011).
Wong, Y. T. et al. Elevation of oxidative-damage biomarkers during aging in F2 hybrid mice: protection by chronic oral intake of resveratrol. Free. Radic. Biol. Med. 46, 799–809 (2009).
Yang, S. J. & Lim, Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism 63, 693–701 (2014).
Qi, X. et al. Curcumol inhibits ferritinophagy to restrain hepatocyte senescence through YAP/NCOA4 in non-alcoholic fatty liver disease. Cell Prolif. 54, e13107 (2021).
Shi, T. et al. Activated carbon N-acetylcysteine microcapsule protects against nonalcoholic fatty liver disease in young rats via activating telomerase and inhibiting apoptosis. PLoS ONE 13, e0189856 (2018).
Liu, Z. Y. et al. Protection against ischemia-reperfusion injury in aged liver donor by the induction of exogenous human telomerase reverse transcriptase gene. Transplant. Proc. 46, 1567–1572 (2014).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022).
Wang, C. et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 574, 268–272 (2019).
Ruscetti, M. et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362, 1416–1422 (2018).
Senturk, S. et al. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52, 966–974 (2010).
El-Shorbagy, A. A., Shafaa, M. W., Salah Elbeltagy, R., El-Hennamy, R. E. & Nady, S. Liposomal IL-22 ameliorates liver fibrosis through miR-let7a/STAT3 signaling in mice. Int. Immunopharmacol. 124, 111015 (2023).
Hwang, S. et al. Novel treatment of acute and acute-on-chronic liver failure: Interleukin-22. Liver Int. https://doi.org/10.1111/liv.15619 (2023).
Xiang, X. et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 72, 736–745 (2020).
Greten, T. F. & Eggert, T. Cellular senescence associated immune responses in liver cancer. Hepatic Oncol. 4, 123–127 (2017).
Hunt, N. J. et al. Quantum dot nanomedicine formulations dramatically improve pharmacological properties and alter uptake pathways of metformin and nicotinamide mononucleotide in aging mice. ACS Nano 15, 4710–4727 (2021).
Pinto, C. et al. Aging and the biological response to liver injury. Semin. Liver Dis. 40, 225–232 (2020).
Kim, I. H., Kisseleva, T. & Brenner, D. A. Aging and liver disease. Curr. Opin. Gastroenterol. 31, 184–191 (2015).
Lazo, M. & Clark, J. M. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin. Liver Dis. 28, 339–350 (2008).
Forrest, E. H. et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 54, 1174–1179 (2005).
Poynard, T. et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J. Hepatol. 34, 730–739 (2001).
Wong, G. L. Prediction of fibrosis progression in chronic viral hepatitis. Clin. Mol. Hepatol. 20, 228–236 (2014).
Lockart, I. et al. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: a meta-analysis. Hepatology 76, 139–154 (2022).
Cotreau, M. M., Von Moltke, L. L. & Greenblatt, D. J. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin. Pharmacokinet. 44, 33–60 (2005).
Acknowledgements
J.G.-S. is supported by the Instituto de Salud Carlos III (FIS PI23/00945 and DTS22/00010, co-funded by the European Union), the CIBEREHD, the Swiss National Science Foundation (320030_189252), the Novartis Foundation for Medical-Biological Research, the Foundation Suisse Contre le Cancer du Foie and the AGAUR-Generalitat de Catalunya (2021 SGR 01322 and 2021 PROD 00036). CIBEREHD is funded by the Instituto de Salud Carlos III. D.S.-R. acknowledges the support of the Spanish Ministry of Universities through the FPU fellowship FPU19/03098. A.G.-R. acknowledges the support of the Instituto de Salud Carlos III through the Sara Borrell fellowship CD22/00097.
Author information
Authors and Affiliations
Contributions
D.S.-R. and A.G.-R. researched data for the article. J.G.-S. conceptualized and coordinated the work. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Vassilis Gorgoulis, Naoko Ohtani and Chunfeng Lu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanfeliu-Redondo, D., Gibert-Ramos, A. & Gracia-Sancho, J. Cell senescence in liver diseases: pathological mechanism and theranostic opportunity. Nat Rev Gastroenterol Hepatol (2024). https://doi.org/10.1038/s41575-024-00913-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41575-024-00913-4