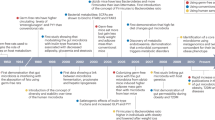
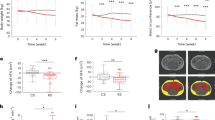
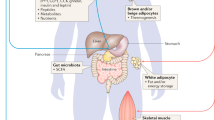
Abstract
Obesity and metabolic syndrome represent a growing epidemic worldwide. Body weight is regulated through complex interactions between hormonal, neural and metabolic pathways and is influenced by numerous environmental factors. Imbalances between energy intake and expenditure can occur due to several factors, including alterations in eating behaviours, abnormal satiation and satiety, and low energy expenditure. The gut microbiota profoundly affects all aspects of energy homeostasis through diverse mechanisms involving effects on mucosal and systemic immune, hormonal and neural systems. The benefits of dietary fibre on metabolism and obesity have been demonstrated through mechanistic studies and clinical trials, but many questions remain as to how different fibres are best utilized in managing obesity. In this Review, we discuss the physiochemical properties of different fibres, current findings on how fibre and the gut microbiota interact to regulate body weight homeostasis, and knowledge gaps related to using dietary fibres as a complementary strategy. Precision medicine approaches that utilize baseline microbiota and clinical characteristics to predict individual responses to fibre supplementation represent a new paradigm with great potential to enhance weight management efficacy, but many challenges remain before these approaches can be fully implemented.
Key points
-
Obesity is a complex chronic progressive disease characterized by excess body weight and dysregulation in enteroendocrine and neurohormonal signalling pathways favouring increased appetite and energy storage.
-
Therapeutics based on the manipulation of enteroendocrine pathways in the gastrointestinal tract are the most efficacious for weight loss and improving metabolic function.
-
Prospective and epidemiological studies have demonstrated associations between fibre consumption and metabolic health, highlighting the role of the gut microbiota in linking dietary intake of fibre with beneficial effects.
-
Microbiota-derived metabolites, including short-chain fatty acids, indole derivatives and bile acids, have been implicated in the pathogenesis of obesity and metabolic dysregulation.
-
Heterogeneity exists between fibres in terms of their chemical and physical structures, which determines the effects of fibre on the gastrointestinal tract, the gut microbiota and energy homeostasis.
-
Increased consumption of dietary fibre has the potential to induce structural, physicochemical and gastrointestinal site-specific benefits that are relevant for the treatment of obesity and metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
World Obesity. Obesity Atlas 2023. World Obesity https://data.worldobesity.org/publications/?cat=19 (2023).
Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450 (2019).
Ricardo-Silgado, M. L., McRae, A. & Acosta, A. Role of enteroendocrine hormones in appetite and glycemia. Obes. Med. 23, 100332 (2021).
Crooks, B., Stamataki, N. S. & McLaughlin, J. T. Appetite, the enteroendocrine system, gastrointestinal disease and obesity. Proc. Nutr. Soc. 80, 50–58 (2021).
de Heredia, F. P., Gomez-Martinez, S. & Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 71, 332–338 (2012).
Brown, S. S. G., Westwater, M. L., Seidlitz, J., Ziauddeen, H. & Fletcher, P. C. Hypothalamic volume is associated with body mass index. Neuroimage Clin. 39, 103478 (2023).
She, Y., Mangat, R., Tsai, S., Proctor, S. D. & Richard, C. The interplay of obesity, dyslipidemia and immune dysfunction: a brief overview on pathophysiology, animal models, and nutritional modulation. Front. Nutr. 9, 840209 (2022).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Lassenius, M. I. et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34, 1809–1815 (2011).
Boutagy, N. E., McMillan, R. P., Frisard, M. I. & Hulver, M. W. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie 124, 11–20 (2016).
Camargo, A. et al. Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: from the CORDIOPREV study. Clin. Nutr. 38, 529–538 (2019).
Pussinen, P. J., Havulinna, A. S., Lehto, M., Sundvall, J. & Salomaa, V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34, 392–397 (2011).
Gill, S. K., Rossi, M., Bajka, B. & Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 18, 101–116 (2021).
Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393, 434–445 (2019).
Mah, E., Liska, D. J., Goltz, S. & Chu, Y. The effect of extracted and isolated fibers on appetite and energy intake: a comprehensive review of human intervention studies. Appetite 180, 106340 (2023).
Deehan, E. C. & Walter, J. The fiber gap and the disappearing gut microbiome: implications for human nutrition. Trends Endocrinol. Metab. 27, 239–242 (2016).
Clemmensen, C. et al. Gut–brain cross-talk in metabolic control. Cell 168, 758–774 (2017).
Michalowska, J., Miller-Kasprzak, E. & Bogdanski, P. Incretin hormones in obesity and related cardiometabolic disorders: the clinical perspective. Nutrients 13, 351 (2021).
Grosse, J. et al. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc. Natl Acad. Sci. USA 111, 11133–11138 (2014).
Muller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Ellis, A. C. et al. Circulating ghrelin and GLP-1 are not affected by habitual diet. Regul. Pept. 176, 1–5 (2012).
Hengist, A., Sciarrillo, C. M., Guo, J., Walter, M. & Hall, K. D. Discordance between gut-derived appetite hormones and energy intake in humans. Preprint at medRxiv https://doi.org/10.1101/2023.05.10.23289718 (2023).
Becetti, I. et al. The neurobiology of eating behavior in obesity: mechanisms and therapeutic targets: a report from the 23rd Annual Harvard Nutrition Obesity symposium. Am. J. Clin. Nutr. 118, 314–328 (2023).
Lanfray, D. & Richard, D. Emerging signaling pathway in arcuate feeding-related neurons: role of the Acbd7. Front. Neurosci. 11, 328 (2017).
Steuernagel, L. et al. HypoMap—a unified single-cell gene expression atlas of the murine hypothalamus. Nat. Metab. 4, 1402–1419 (2022).
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Jais, A. et al. PNOC(ARC) neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025.e10 (2020).
Lavoie, O., Michael, N. J. & Caron, A. A critical update on the leptin–melanocortin system. J. Neurochem. 165, 467–486 (2023).
van Galen, K. A. et al. Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat. Metab. 5, 1059–1072 (2023).
Latorre, R., Sternini, C., De Giorgio, R. & Greenwood-Van Meerveld, B. Enteroendocrine cells: a review of their role in brain–gut communication. Neurogastroenterol. Motil. 28, 620–630 (2016).
Meek, C. L., Lewis, H. B., Reimann, F., Gribble, F. M. & Park, A. J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 77, 28–37 (2016).
Di Vincenzo, A. et al. Body weight reduction by bariatric surgery reduces the plasma levels of the novel orexigenic gut hormone insulin-like peptide 5 in patients with severe obesity. J. Clin. Med. 12, 3752 (2023).
Huang, J. et al. Change in adipokines and gastrointestinal hormones after bariatric surgery: a meta-analysis. Obes. Surg. 33, 789–806 (2023).
Liu, Y. et al. The weight-loss effect of GLP-1RAs glucagon-like peptide-1 receptor agonists in non-diabetic individuals with overweight or obesity: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Am. J. Clin. Nutr. 118, 614–626 (2023).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Jastreboff, A. M. et al. Triple-hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023).
Jones, P. M., Hobai, I. A. & Murphy, P. M. Anesthesia and glucagon-like peptide-1 receptor agonists: proceed with caution! Can. J. Anaesth. 70, 1281–1286 (2023).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
Talmor-Barkan, Y. et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 28, 295–302 (2022).
Rothschild, D. et al. An atlas of robust microbiome associations with phenotypic traits based on large-scale cohorts from two continents. PLoS ONE 17, e0265756 (2022).
Rinott, E. et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 14, 29 (2022).
Menni, C. et al. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 41, 1099–1105 (2017).
Chen, J. P., Chen, G. C., Wang, X. P., Qin, L. & Bai, Y. Dietary fiber and metabolic syndrome: a meta-analysis and review of related mechanisms. Nutrients 10, 24 (2017).
Zhao, L. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156 (2018).
Tremblay, A. et al. Dietary fibres and the management of obesity and metabolic syndrome: the RESOLVE study. Nutrients 12, 2911 (2020).
Maimaitiyiming, M. et al. The association of obesity-related dietary patterns and main food groups derived by reduced-rank regression with cardiovascular diseases incidence and all-cause mortality: findings from 116,711 adults. Eur. J. Nutr. 62, 2605–2619 (2023).
Tilg, H., Zmora, N., Adolph, T. E. & Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 20, 40–54 (2020).
Aron-Wisnewsky, J., Warmbrunn, M. V., Nieuwdorp, M. & Clement, K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 160, 573–599 (2021).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Anhe, F. F. et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2, 233–242 (2020).
Massier, L. et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 69, 1796–1806 (2020).
Xu, Z. et al. Gut microbiota in patients with obesity and metabolic disorders — a systematic review. Genes Nutr. 17, 2 (2022).
Montenegro, J. et al. Exploring the influence of gut microbiome on energy metabolism in humans. Adv. Nutr. 14, 840–857 (2023).
Le Chatelier E, N. T. & Qin, J. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Aron-Wisnewsky, J. et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 68, 70–82 (2019).
Fromentin, S. et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 28, 303–314 (2022).
Russell, W. R. et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 93, 1062–1072 (2011).
Dodd, D. et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652 (2017).
Org, E. et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 18, 70 (2017).
Surowiec, I. et al. Metabolomic and lipidomic assessment of the metabolic syndrome in Dutch middle-aged individuals reveals novel biological signatures separating health and disease. Metabolomics 15, 23 (2019).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Kaisar, M. M. M., Pelgrom, L. R., van der Ham, A. J., Yazdanbakhsh, M. & Everts, B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein-coupled receptor 109A signaling. Front. Immunol. 8, 1429 (2017).
He, J. et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21176356 (2020).
Boets, E. et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J. Physiol. 595, 541–555 (2017).
Canfora, E. E. et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci. Rep. 7, 2360 (2017).
van der Beek, C. M. et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 130, 2073–2082 (2016).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 (2015).
Larraufie, P. et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 8, 74 (2018).
Brooks, L. et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 6, 48–60 (2017).
Freeland, K. R. & Wolever, T. M. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br. J. Nutr. 103, 460–466 (2010).
Canfora, E. E. et al. Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes 14, 2009297 (2022).
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
Nohr, M. K. et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154, 3552–3564 (2013).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 156, 84–96 (2014).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
Li, Z. et al. Butyrate reduces appetite and activates brown adipose tissue via the gut–brain neural circuit. Gut 67, 1269–1279 (2018).
Goswami, C., Iwasaki, Y. & Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 57, 130–135 (2018).
Fock, E. & Parnova, R. Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells https://doi.org/10.3390/cells12040657 (2023).
Hoyles, L. et al. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 6, 55 (2018).
Knox, E. G. et al. Microbial-derived metabolites induce actin cytoskeletal rearrangement and protect blood–brain barrier function. iScience 25, 105648 (2022).
van de Wouw, M., Schellekens, H., Dinan, T. G. & Cryan, J. F. Microbiota–gut–brain axis: modulator of host metabolism and appetite. J. Nutr. 147, 727–745 (2017).
Byrne, C. S. et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 104, 5–14 (2016).
Bachmann, C., Colombo, J. P. & Beruter, J. Short chain fatty acids in plasma and brain: quantitative determination by gas chromatography. Clin. Chim. Acta 92, 153–159 (1979).
Wishart, D. S. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018).
Seltzer, M. A. et al. Radiation dose estimates in humans for 11C-acetate whole-body PET. J. Nucl. Med. 45, 1233–1236 (2004).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Goossens, G. H. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 94, 206–218 (2008).
Aberdein, N., Schweizer, M. & Ball, D. Sodium acetate decreases phosphorylation of hormone sensitive lipase in isoproterenol-stimulated 3T3-L1 mature adipocytes. Adipocyte 3, 121–125 (2014).
Jocken, J. W. E. et al. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front. Endocrinol. 8, 372 (2017).
Hong, Y. H. et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005).
Al-Lahham, S. H. et al. Regulation of adipokine production in human adipose tissue by propionic acid. Eur. J. Clin. Invest. 40, 401–407 (2010).
Zaibi, M. S. et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 (2010).
Ohira, H. et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 20, 425–442 (2013).
Al-Lahham, S. et al. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur. J. Clin. Invest. 42, 357–364 (2012).
Kim, K. N., Yao, Y. & Ju, S. Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients https://doi.org/10.3390/nu11102512 (2019).
Liu, H. et al. Butyrate: a double-edged sword for health? Adv. Nutr. 9, 21–29 (2018).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Schwiertz, A. et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195 (2010).
Rahat-Rozenbloom, S., Fernandes, J., Gloor, G. B. & Wolever, T. M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 38, 1525–1531 (2014).
Fernandes, J., Su, W., Rahat-Rozenbloom, S., Wolever, T. M. & Comelli, E. M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 4, e121 (2014).
Teixeira, T. F. et al. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br. J. Nutr. 109, 914–919 (2013).
Barczynska, R. et al. Bacterial microbiota and fatty acids in the faeces of overweight and obese children. Pol. J. Microbiol. 67, 339–345 (2018).
Murugesan, S. et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1337–1346 (2015).
Muller, M. et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 9, 12515 (2019).
Everard, A. et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 8, 2116–2130 (2014).
Jakobsdottir, G., Xu, J., Molin, G., Ahrne, S. & Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 8, e80476 (2013).
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Env. Microbiol. 19, 29–41 (2017).
Li, X. et al. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 7, 305 (2022).
Jones, T. E. et al. Plasma lactate as a marker of metabolic health: implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 166, 861–866 (2019).
Serena, C. et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 12, 1642–1657 (2018).
Hu, J. et al. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J. Cell. Physiol. 235, 8938–8950 (2020).
Iraporda, C. et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 220, 1161–1169 (2015).
Errea, A. et al. Lactate inhibits the pro-inflammatory response and metabolic reprogramming in murine macrophages in a GPR81-independent manner. PLoS ONE 11, e0163694 (2016).
Murphy, M. P. & O’Neill, L. A. J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018).
Gilissen, J., Jouret, F., Pirotte, B. & Hanson, J. Insight into SUCNR1 (GPR91) structure and function. Pharmacol. Ther. 159, 56–65 (2016).
de Castro Fonseca, M., Aguiar, C. J., da Rocha Franco, J. A., Gingold, R. N. & Leite, M. F. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun. Signal. 14, 3 (2016).
Vasan, S. K. et al. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia 62, 2079–2087 (2019).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Monfort-Ferre, D. et al. The gut microbiota metabolite succinate promotes adipose tissue browning in Crohn’s disease. J. Crohns Colitis 16, 1571–1583 (2022).
McCreath, K. J. et al. Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity. Diabetes 64, 1154–1167 (2015).
Villanueva-Carmona, T. et al. SUCNR1 signaling in adipocytes controls energy metabolism by modulating circadian clock and leptin expression. Cell Metab. 35, 601–619.e10 (2023).
Keiran, N. et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 20, 581–592 (2019).
Trauelsen, M. et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 35, 109246 (2021).
De Vadder, F. et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24, 151–157 (2016).
Kalantar-Zadeh, K., Berean, K. J., Burgell, R. E., Muir, J. G. & Gibson, P. R. Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 16, 733–747 (2019).
Wachsmuth, H. R., Weninger, S. N. & Duca, F. A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 54, 377–392 (2022).
Cuddihey, H., MacNaughton, W. K. & Sharkey, K. A. Role of the endocannabinoid system in the regulation of intestinal homeostasis. Cell. Mol. Gastroenterol. Hepatol. 14, 947–963 (2022).
O’Riordan, K. J. et al. Short chain fatty acids: microbial metabolites for gut–brain axis signalling. Mol. Cell. Endocrinol. 546, 111572 (2022).
Chen, Y., Xu, J. & Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients https://doi.org/10.3390/nu13062099 (2021).
Gabanyi, I. et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 376, eabj3986 (2022).
de Wouters d’Oplinter, A. et al. Obese-associated gut microbes and derived phenolic metabolite as mediators of excessive motivation for food reward. Microbiome 11, 94 (2023).
de Wouters d’Oplinter, A. et al. Gut microbes participate in food preference alterations during obesity. Gut Microbes 13, 1959242 (2021).
Jones, J. M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 13, 34 (2014).
Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 57, 3543–3564 (2017).
Cantu-Jungles, T. M. & Hamaker, B. R. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio https://doi.org/10.1128/mBio.02179-19 (2020).
McRorie, J. W. Jr & McKeown, N. M. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 117, 251–264 (2017).
Stewart, M. L. & Slavin, J. L. Molecular weight of guar gum affects short-chain fatty acid profile in model intestinal fermentation. Mol. Nutr. food Res. 50, 971–976 (2006).
Hamaker, B. R. & Tuncil, Y. E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850 (2014).
Deehan, E. C. et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 10, 77 (2022).
Puhlmann, M. L. & de Vos, W. M. Intrinsic dietary fibers and the gut microbiome: rediscovering the benefits of the plant cell matrix for human health. Front. Immunol. 13, 954845 (2022).
Holland, C., Ryden, P., Edwards, C. H. & Grundy, M. M. Plant cell walls: impact on nutrient bioaccessibility and digestibility. Foods https://doi.org/10.3390/foods9020201 (2020).
Guo, Q. Understanding the oral processing of solid foods: insights from food structure. Compr. Rev. Food Sci. Food Saf. 20, 2941–2967 (2021).
Lasschuijt, M. P., Mars, M., de Graaf, C. & Smeets, P. A. M. Endocrine cephalic phase responses to food cues: a systematic review. Adv. Nutr. 11, 1364–1383 (2020).
Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 87, S287–S291 (2002).
Hallfrisch, J., Scholfield, D. J. & Behall, K. M. Glucose and insulin responses to a new zero-energy fiber source. J. Am. Coll. Nutr. 21, 410–415 (2002).
Grundy, M. M. et al. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 116, 816–833 (2016).
Miehle, E., Melanie, H., Stephanie, B.-M. & Eisner, P. The role of hydration properties of souble dietary fibers on glucose diffusion. Food Hydrocoll. 131, 107822 (2022).
Chen, M. et al. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses I: in vitro digestion processes. Food Hydrocoll. 107, 105971 (2020).
Massa, M., Compari, C. & Fisicaro, E. On the mechanism of the cholesterol lowering ability of soluble dietary fibers: interaction of some bile salts with pectin, alginate, and chitosan studied by isothermal titration calorimetry. Front. Nutr. 9, 968847 (2022).
Guzior, D. V. & Quinn, R. A. Review: microbial transformations of human bile acids. Microbiome 9, 140 (2021).
Fogelson, K. A., Dorrestein, P. C., Zarrinpar, A. & Knight, R. The gut microbial bile acid modulation and its relevance to digestive health and diseases. Gastroenterology 164, 1069–1085 (2023).
Calderon, G. et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine 55, 102759 (2020).
Walter, J. & Ley, R. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65, 411–429 (2011).
Weninger, S. N. et al. Oligofructose improves small intestinal lipid-sensing mechanisms via alterations to the small intestinal microbiota. Microbiome 11, 169 (2023).
Leung, R. & Covasa, M. Do gut microbes taste? Nutrients https://doi.org/10.3390/nu13082581 (2021).
van Baar, A. C. G. et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut 69, 295–303 (2020).
de Oliveira, G. H. P. et al. Metabolic effects of endoscopic duodenal mucosal resurfacing: a systematic review and meta-analysis. Obes. Surg. 31, 1304–1312 (2021).
Meiring, S. et al. A changed gut microbiota diversity is associated with metabolic improvements after duodenal mucosal resurfacing with glucagon-like-peptide-1 receptor agonist in type 2 diabetes in a pilot study. Front. Clin. Diabetes Healthc. 3, 856661 (2022).
Makki, K., Deehan, E. C., Walter, J. & Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Nguyen, N. K. et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 8, 118 (2020).
Ze, X., Duncan, S. H., Louis, P. & Flint, H. J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 6, 1535–1543 (2012).
McDonald, D. et al. American Gut: an open platform for citizen science microbiome research. mSystems https://doi.org/10.1128/mSystems.00031-18 (2018).
Shanahan, F., Ghosh, T. S. & O’Toole, P. W. The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160, 483–494 (2021).
Corbin, K. D. et al. Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial. Nat. Commun. 14, 3161 (2023).
Kaur, A., Rose, D. J., Rumpagaporn, P., Patterson, J. A. & Hamaker, B. R. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J. Food Sci. 76, H137–H142 (2011).
Korpela, K. Diet, microbiota, and metabolic health: trade-off between saccharolytic and proteolytic fermentation. Annu. Rev. Food Sci. Technol. 9, 65–84 (2018).
Smith, E. A. & Macfarlane, G. T. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol. 25, 355–368 (1998).
Deehan, E. C. et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27, 389–404.e6 (2020).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Prochazkova, N. et al. Advancing human gut microbiota research by considering gut transit time. Gut 72, 180–191 (2023).
Chung, W. S. et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 14, 3 (2016).
Youngblut, N. D. et al. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 10, 2200 (2019).
Vandeputte, D. et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 66, 1968–1974 (2017).
Benitez-Paez, A. et al. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. mSystems https://doi.org/10.1128/mSystems.00209-19 (2019).
Holmes, Z. C. et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome 10, 114 (2022).
Goh, Y. J. & Klaenhammer, T. R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu. Rev. Food Sci. Technol. 6, 137–156 (2015).
Depommier, C. et al. Serum metabolite profiling yields insights into health promoting effect of A. muciniphila in human volunteers with a metabolic syndrome. Gut Microbes 13, 1994270 (2021).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Gilijamse, P. W. et al. Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose–response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes 6, 16 (2020).
Koopen, A. et al. Duodenal Anaerobutyricum soehngenii infusion stimulates GLP-1 production, ameliorates glycaemic control and beneficially shapes the duodenal transcriptome in metabolic syndrome subjects: a randomised double-blind placebo-controlled cross-over study. Gut 71, 1577–1587 (2022).
Li, X. et al. Distinct factors associated with short-term and long-term weight loss induced by low-fat or low-carbohydrate diet intervention. Cell Rep. Med. 3, 100870 (2022).
Diener, C. et al. Baseline gut metagenomic functional gene signature associated with variable weight loss responses following a healthy lifestyle intervention in humans. mSystems 6, e0096421 (2021).
Jie, Z. et al. The baseline gut microbiota directs dieting-induced weight loss trajectories. Gastroenterology 160, 2029–2042.e16 (2021).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Hjorth, M. F. et al. Pretreatment Prevotella-to-Bacteroides ratio and markers of glucose metabolism as prognostic markers for dietary weight loss maintenance. Eur. J. Clin. Nutr. 74, 338–347 (2020).
Christensen, L. et al. Prevotella abundance predicts weight loss success in healthy, overweight adults consuming a whole-grain diet ad libitum: a post hoc analysis of a 6-wk randomized controlled trial. J. Nutr. 149, 2174–2181 (2019).
Hjorth, M. F. et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes. 43, 149–157 (2019).
Christensen, L. et al. Microbial enterotypes beyond genus level: Bacteroides species as a predictive biomarker for weight change upon controlled intervention with arabinoxylan oligosaccharides in overweight subjects. Gut Microbes 12, 1847627 (2020).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Cifuentes, L. et al. Phenotype tailored lifestyle intervention on weight loss and cardiometabolic risk factors in adults with obesity: a single-centre, non-randomised, proof-of-concept study. EClinicalMedicine 58, 101923 (2023).
Acosta, A. et al. Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic. Obesity 29, 662–671 (2021).
Blanco-Rojo, R. et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: the CORDIOPREV-DIAB randomised clinical trial. Diabetologia 59, 67–76 (2016).
Trouwborst, I. et al. Cardiometabolic health improvements upon dietary intervention are driven by tissue-specific insulin resistance phenotype: a precision nutrition trial. Cell Metab. 35, 71–83.e5 (2023).
Robertson, M. D. et al. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocrinol. Metab. 97, 3326–3332 (2012).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012).
Huwiler, V. V. et al. Prolonged isolated soluble dietary fibre supplementation in overweight and obese patients: a systematic review with meta-analysis of randomised controlled trials. Nutrients https://doi.org/10.3390/nu14132627 (2022).
Lancaster, S. M. et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 30, 848–862 e847 (2022).
Armstrong, H. K. et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 164, 228–240 (2023).
Jane, M., McKay, J. & Pal, S. Effects of daily consumption of psyllium, oat bran and polyGlycopleX on obesity-related disease risk factors: a critical review. Nutrition 57, 84–91 (2019).
Haghikia, A. et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 43, 518–533 (2022).
Ranaivo, H. et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 14, 2044722 (2022).
Cantu-Jungles, T. M. et al. Dietary fiber hierarchical specificity: the missing link for predictable and strong shifts in gut bacterial communities. mBio 12, e0102821 (2021).
Delannoy-Bruno, O. et al. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature 595, 91–95 (2021).
Yao, T. et al. Differences in fine arabinoxylan structures govern microbial selection and competition among human gut microbiota. Carbohydr. Polym. 316, 121039 (2023).
Swanson, K. S. et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701 (2020).
Schroeder, B. O. et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23, 27–40.e7 (2018).
Krumbeck, J. A. et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6, 121 (2018).
Hadi, A., Alizadeh, K., Hajianfar, H., Mohammadi, H. & Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 60, 584–596 (2020).
Beserra, B. T. et al. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 34, 845–858 (2015).
Perraudeau, F. et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res. Care https://doi.org/10.1136/bmjdrc-2020-001319 (2020).
Mocanu, V. et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 27, 1272–1279 (2021).
Rinott, E. et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 160, 158–173.e10 (2021).
Poutanen, K. S. et al. Recommendations for characterization and reporting of dietary fibers in nutrition research. Am. J. Clin. Nutr. 108, 437–444 (2018).
Obradovic, M. et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. 12, 585887 (2021).
Khoramipour, K. et al. Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients https://doi.org/10.3390/nu13041180 (2021).
Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351 (2022).
Zhihong, Y. et al. Emerging roles of oxyntomodulin-based glucagon-like peptide-1/glucagon co-agonist analogs in diabetes and obesity. Peptides 162, 170955 (2023).
Hammoud, R. & Drucker, D. J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 19, 201–216 (2023).
Lee, Y. S. et al. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol. Metab. 5, 263–270 (2016).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Hannah Holscher, Seiichiro Aoe, Emanuel Canfora and João Felipe Mota for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deehan, E.C., Mocanu, V. & Madsen, K.L. Effects of dietary fibre on metabolic health and obesity. Nat Rev Gastroenterol Hepatol 21, 301–318 (2024). https://doi.org/10.1038/s41575-023-00891-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00891-z