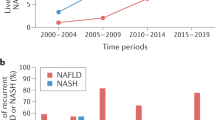
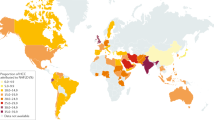
Abstract
Recompensation has gained increasing attention in the field of cirrhosis, particularly in chronic liver disease with a definite aetiology. The current global prevalence of obesity and nonalcoholic fatty liver disease (NAFLD) is increasing, but there is currently a lack of a clear definition for recompensation in NAFLD-related cirrhosis. Here, we provide an up-to-date perspective on the natural history of NAFLD, emphasizing the reversible nature of the disease, summarizing possible mechanisms underlying recompensation in NAFLD, discussing challenges that need to be addressed and outlining future research directions in the field. Recompensation is a promising goal in patients with NAFLD-related cirrhosis, and further studies are needed to explore its underlying mechanisms and uncover its clinical features.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schuppan, D. & Afdhal, N. H. Liver cirrhosis. Lancet 371, 838–851 (2008).
Mauro, E. & Gadano, A. What’s new in portal hypertension? Liver Int. 40, 122–127 (2020).
Xu, X. et al. Recompensation factors for patients with decompensated cirrhosis: a multicentre retrospective case-control study. BMJ Open 11, e043083 (2021).
Cheung, M. C. M. et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 65, 741–747 (2016).
Gentile, I. et al. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity–LINA cohort). Hepatol. Int. 13, 66–74 (2019).
Lens, S. et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J. Hepatol. 73, 1415–1424 (2020).
Gadiparthi, C. et al. NAFLD epidemiology, emerging pharmacotherapy, liver transplantation implications and the trends in the United States. J. Clin. Transl. Hepatol. 8, 215–221 (2020).
Le, M. H. et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, 2809–2817.e28 (2022).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023). This study proposes a thoughtful renaming and redefinition of NAFLD through a rigorous Delphi process involving extensive international expert consensus, aiming to establish a nomenclature that is informative, systematic and non-stigmatizing.
Rinella, M. E. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.06.003 (2023).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Singh, S. P., Anirvan, P., Khandelwal, R. & Satapathy, S. K. Nonalcoholic fatty liver disease (NAFLD) name change: requiem or reveille. J. Clin. Transl. Hepatol. 9, 931–938 (2021).
Feng, G. et al. Bioinformatics analysis reveals novel core genes associated with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Gene 742, 144549 (2020).
Younossi, Z. M. & Henry, L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 3, 100305 (2021).
Gutierrez-Cuevas, J., Lucano-Landeros, S., Lopez-Cifuentes, D., Santos, A. & Armendariz-Borunda, J. Epidemiologic, genetic, pathogenic, metabolic, epigenetic aspects involved in NASH-HCC: current therapeutic strategies. Cancers 15, 23 (2022).
Wong, R. J. & Singal, A. K. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014-2019. JAMA Netw. Open 3, e1920294 (2020).
Noureddin, M. et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am. J. Gastroenterol. 113, 1649–1659 (2018).
Stepanova, M. et al. Nonalcoholic steatohepatitis is the most common indication for liver transplantation among the elderly: data from the United States scientific registry of transplant recipients. Hepatol. Commun. 6, 1506–1515 (2022).
Sun, Y. M., Chen, S. Y. & You, H. Regression of liver fibrosis: evidence and challenges. Chin. Med. J. 133, 1696–1702 (2020).
Pose, E. et al. A notable proportion of liver transplant candidates with alcohol-related cirrhosis can be delisted because of clinical improvement. J. Hepatol. 75, 275–283 (2021).
Aravinthan, A. D. et al. Characteristics of liver transplant candidates delisted following recompensation and predictors of such delisting in alcohol-related liver disease: a case-control study. Transpl. Int. 30, 1140–1149 (2017).
de Franchis, R. et al. Baveno VII – renewing consensus in portal hypertension. J. Hepatol. 76, 959–974 (2022). This article provides a summary of the most important conclusions/recommendations from the Baveno VII workshop.
Zheng, K. I. et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin. Med. J. 133, 2271–2273 (2020).
Sun, D. Q. et al. An international Delphi consensus statement on metabolic dysfunction-associated fatty liver disease and risk of chronic kidney disease. Hepatobiliary Surg. Nutr. 12, 386–403 (2023).
Balcar, L. et al. Patterns of acute decompensation in hospitalized patients with cirrhosis and course of acute-on-chronic liver failure. United European Gastroenterol. J. 9, 427–437 (2021).
Tan, J. et al. Analysis of the dose-response relationship between the international normalized ratio and hepatic encephalopathy in patients with liver cirrhosis using restricted cubic spline functions. Front. Public Health 10, 919549 (2022).
Jang, J. W. et al. Effects of virologic response to treatment on short- and long-term outcomes of patients with chronic hepatitis B virus infection and decompensated cirrhosis. Clin. Gastroenterol. Hepatol. 16, 1954–1963.e3 (2018).
Kim, T. H. et al. Determinants of re-compensation in patients with hepatitis B virus-related decompensated cirrhosis starting antiviral therapy. Aliment. Pharmacol. Ther. 55, 83–96 (2022). This article describes a combined scoring system including six clinical parameters, α-fetoprotein and the timing of antiviral therapy to accurately predict early recompensation in patients with HBV infection-related decompensated cirrhosis.
He, Z. et al. Two-year free of complications during antiviral therapy predicts stable re-compensation in immediate-treatment HBV-related decompensated cirrhosis. Scand. J. Gastroenterol. 58, 403–411 (2023).
Wang, Q. et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis. J. Hepatol. 77, 1564–1572 (2022). In this study, over 50% of patients with decompensated cirrhosis secondary to HBV infection showed recompensation after antiviral treatment, and the study proposes laboratory criteria that could be utilized to define recompensation.
El-Sherif, O. et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection. Gastroenterology 154, 2111–2121.e8 (2018).
Belli, L. S. et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J. Hepatol. 69, 810–817 (2018).
Reiberger, T. & Hofer, B. S. The Baveno VII concept of cirrhosis recompensation. Dig. Liver Dis. 55, 431–441 (2023).
Hofer, B. S. et al. Hepatic recompensation according to Baveno VII criteria is linked to a significant survival benefit in decompensated alcohol-related cirrhosis. Liver Int. 43, 2220–2231 (2023).
Rinella, M. E., Tacke, F., Sanyal, A. J. & Anstee, Q. M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J. Hepatol. 71, 823–833 (2019). This report summarizes important findings from ongoing and completed trials, defines the scientific evidence supporting distinct end points and provides guidance for future trial design.
Boyle, M., Masson, S. & Anstee, Q. M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J. Hepatol. 68, 251–267 (2018).
Berzigotti, A. et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 65, 1293–1305 (2017).
Noureddin, M. & Wong, V. W. A revisit of the natural history of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 21, 1152–1153 (2023).
Tanaka, N. et al. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 25, 163–177 (2019).
Calcaterra, V. et al. Benefits of physical exercise as approach to prevention and reversion of non-alcoholic fatty liver disease in children and adolescents with obesity. Children 9, 1174 (2022).
Vuppalanchi, R., Noureddin, M., Alkhouri, N. & Sanyal, A. J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 18, 373–392 (2021).
Trepo, E. & Valenti, L. Update on NAFLD genetics: from new variants to the clinic. J. Hepatol. 72, 1196–1209 (2020).
Bence, K. K. & Birnbaum, M. J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 50, 101143 (2021).
Ferguson, D. & Finck, B. N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 484–495 (2021).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021).
Loomba, R. et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 511–522 (2023).
Brown, G. T. & Kleiner, D. E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 65, 1080–1086 (2016).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015).
Jayakumar, S. et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J. Hepatol. 70, 133–141 (2019).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Francque, S. M. et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 385, 1547–1558 (2021).
Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394, 2012–2024 (2019).
Karim, G. & Bansal, M. B. Resmetirom: an orally administered, smallmolecule, liver-directed, beta-selective THR agonist for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. touchREV Endocrinol. 19, 60–70 (2023).
Harrison, S. et al. Primary results from MAESTRO-NASH a pivotal phase 3 52-week serial liver biopsy study in 966 patients with NASH and fibrosis. J. Hepatol. 78, S1 (2023).
Hafeez, S. & Ahmed, M. H. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J. Obes. 2013, 839275 (2013).
Bassegoda, O. et al. Decompensation in advanced nonalcoholic fatty liver disease may occur at lower hepatic venous pressure gradient levels than in patients with viral disease. Clin. Gastroenterol. Hepatol. 20, 2276–2286.e6 (2022).
Sanyal, A. J. et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology 75, 1235–1246 (2022). In this study in patients with compensated cirrhosis due to NASH, regression of fibrosis was associated with a reduction in liver-related complications, and the findings support the utility of histological fibrosis regression and noninvasive tests as clinical trial end points in NASH cirrhosis.
Tan, D. J. H. et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 23, 521–530 (2022).
Rios, R. S., Zheng, K. I. & Zheng, M. H. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin. Med. J. 134, 2911–2921 (2021).
Villanueva, C. et al. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology 63, 197–206 (2016).
Costa, D. et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 74, 819–828 (2021).
Villanueva, C. et al. Bacterial infections adversely influence the risk of decompensation and survival in compensated cirrhosis. J. Hepatol. 75, 589–599 (2021).
Noor, M. T. & Manoria, P. Immune dysfunction in cirrhosis. J. Clin. Transl. Hepatol. 5, 50–58 (2017).
Pouwels, S. et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 22, 63 (2022).
Kenneally, S., Sier, J. H. & Moore, J. B. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 4, e000139 (2017).
Tanwar, S., Rhodes, F., Srivastava, A., Trembling, P. M. & Rosenberg, W. M. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J. Gastroenterol. 26, 109–133 (2020).
Hsu, S. J. et al. Extrahepatic angiogenesis hinders recovery of portal hypertension and collaterals in rats with cirrhosis resolution. Clin. Sci. 132, 669–683 (2018).
Zafra, C. et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology 126, 749–755 (2004).
Fernandez, M. Molecular pathophysiology of portal hypertension. Hepatology 61, 1406–1415 (2015).
Chalasani, N. et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 158, 1334–1345.e5 (2020).
Wan, S., Huang, C. & Zhu, X. Systematic review with a meta-analysis: clinical effects of statins on the reduction of portal hypertension and variceal haemorrhage in cirrhotic patients. BMJ Open 9, e030038 (2019).
Gorabi, A. M. et al. Statin-induced nitric oxide signaling: mechanisms and therapeutic implications. J. Clin. Med. 8, 2051 (2019).
Huang, H. C. et al. Microbiota transplants from feces or gut content attenuated portal hypertension and portosystemic collaterals in cirrhotic rats. Clin. Sci. 135, 2709–2728 (2021).
Freekh, D. A., Helmy, M. W., Said, M. & El-Khodary, N. M. The effect of direct acting antiviral agents on vascular endothelial function in Egyptian patients with chronic hepatitis C virus infection. Saudi Pharm. J. 29, 1120–1128 (2021).
Van der Merwe, S., Chokshi, S., Bernsmeier, C. & Albillos, A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J. Hepatol. 75, S82–S100 (2021).
Dirchwolf, M. et al. Immune dysfunction in cirrhosis: distinct cytokines phenotypes according to cirrhosis severity. Cytokine 77, 14–25 (2016).
Trebicka, J. et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front. Immunol. 10, 476 (2019).
Monteiro, S. et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 70, 379–387 (2021).
Zheng, K. I., Sun, D. Q., Jin, Y., Zhu, P. W. & Zheng, M. H. Clinical utility of the MAFLD definition. J. Hepatol. 74, 989–991 (2021).
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Younossi, Z. M. et al. The association of histologic and noninvasive tests with adverse clinical and patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis. Gastroenterology 160, 1608–1619.e13 (2021).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Bzowej, N. H. Nonalcoholic steatohepatitis: the new frontier for liver transplantation. Curr. Opin. Organ. Transpl. 23, 169–174 (2018).
Villeret, F. et al. Inevitability of disease recurrence after liver transplantation for NAFLD cirrhosis. JHEP Rep. 5, 100668 (2023).
Dooghaie Moghadam, A. et al. Recurrence of fatty liver disease following liver transplantation for NAFLD-related cirrhosis: current status and challenges. Casp. J. Intern. Med. 11, 346–354 (2020).
Sharma, S. & Roy, A. Recompensation in cirrhosis: current evidence and future directions. J. Clin. Exp. Hepatol. 13, 329–334 (2023).
Lo, R. C. & Kim, H. Histopathological evaluation of liver fibrosis and cirrhosis regression. Clin. Mol. Hepatol. 23, 302–307 (2017).
Wanless, I. R., Nakashima, E. & Sherman, M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch. Pathol. Lab. Med. 124, 1599–1607 (2000).
Petta, S. et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J. Hepatol. 69, 878–885 (2018).
Hsu, C. et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol. 17, 630–637.e8 (2019).
Feng, G. et al. A simpler diagnostic formula for screening nonalcoholic fatty liver disease. Clin. Biochem. 64, 18–23 (2019).
Kline, A. et al. Multimodal machine learning in precision health: a scoping review. npj Digit. Med. 5, 171 (2022).
Zhang, S., Yang, Y., Fan, L., Zhang, F. & Li, L. The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Ann. Transl. Med. 8, 565 (2020).
Yang, X. et al. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 10, 123 (2020).
Owen, A., Patten, D., Vigneswara, V., Frampton, J. & Newsome, P. N. PDGFRα/Sca-1 sorted mesenchymal stromal cells reduce liver injury in murine models of hepatic ischemia-reperfusion injury. Stem Cell 40, 1056–1070 (2022).
Santoro, R. & Mangia, A. Progress in promising anti-fibrotic therapies. Expert Rev. Gastroenterol. Hepatol. 13, 1145–1152 (2019).
Younossi, Z. M., Zelber-Sagi, S., Henry, L. & Gerber, L. H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-023-00800-4 (2023). This paper summarizes the evidence on lifestyle interventions for management of NAFLD.
Vuille-Lessard, É., Lange, N., Riebensahm, C., Dufour, J.-F. & Berzigotti, A. Dietary interventions in liver diseases: focus on MAFLD and cirrhosis. Curr. Hepatol. Rep. 20, 61–76 (2021).
Kamada, Y. et al. Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: an expert review. J. Gastroenterol. 56, 1045–1061 (2021).
Theel, W. B. et al. Effect of bariatric surgery on NAFLD/NASH: a single-centre observational prospective cohort study. BMJ Open 13, e070431 (2023).
Bai, J. et al. Bariatric surgery is effective and safe for obese patients with compensated cirrhosis: a systematic review and meta-analysis. World J. Surg. 46, 1122–1133 (2022).
Taitano, A. A. et al. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J. Gastrointest. Surg. 19, 429–437 (2015).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835 (2023). These practice guideline provide clinical recommendations for assessment and management of NAFLD.
Gawrieh, S. et al. Automated quantification and architectural pattern detection of hepatic fibrosis in NAFLD. Ann. Diagn. Pathol. 47, 151518 (2020).
Decharatanachart, P., Chaiteerakij, R., Tiyarattanachai, T. & Treeprasertsuk, S. Application of artificial intelligence in non-alcoholic fatty liver disease and liver fibrosis: a systematic review and meta-analysis. Ther. Adv. Gastroenterol. 14, 17562848211062807 (2021).
Naoumov, N. V. et al. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J. Hepatol. 77, 1399–1409 (2022).
Acknowledgements
The authors thank the members of the CHESS-MAFLD consortium for their coordination.
Author information
Authors and Affiliations
Contributions
G.F. researched data for the article. All authors contributed substantially to discussion of the content. M.-H.Z. and G.F. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.V. reports consulting fees from Gilead Sciences, Pfizer, AstraZeneca, Novo Nordisk, Intercept pharmaceuticals, Diatech Pharmacogenetics, IONIS and Viatris; honoraria from MSD, Gilead Sciences, AlfaSigma, AbbVie and Resalis; and grants from Gilead Sciences. V.W.-S.W. reports grants from Gilead Sciences; consulting fees from AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer and TARGET PharmaSolutions; and honoraria for lectures from Abbott, AbbVie, Gilead Sciences and Novo Nordisk; and is Chairman of Subspecialty Board of Gastroenterology and Hepatology, Hong Kong College of Physicians, and Co-founder of Illuminatio Medical Technology. Y.Y. is a consultant to Zydus, Cymabay and Novo Nordisk. W.K. reports grants from GSK, Gilead Sciences, Novartis, Pfizer, Roche, Springbank, Ildong, Galmed, Dicerna, Enyo, Hanmi, Novo Nordisk and KOBIOLABS; reports consulting fees from Boehringer Ingelheim, Novo Nordisk, Standigm, Daewoong, TSD Life Sciences, Ildong, Olix Pharma, HK Inoen and KOBIOLABS; honoraria for lectures from Ildong, Samil and Novo Nordisk; owns stocks in KOBIOLABS and Lepidyne; and is the founder of Remedygen. G.S. reports honoraria from Merck, Gilead Sciences, Abbvie, Intercept, Novo Nordisk and Pfizer; and unrestricted research funding from Theratechnologies. Z.M.Y. is a consultant to BMS, Gilead Sciences, AbbVie, Intercept and GSK. V.H.-G. reports honoraria for lectures from GORE and Cook Medical. M.-H.Z. and Y.M.F. declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Takumi Kawaguchi, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, G., Valenti, L., Wong, V.WS. et al. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 21, 46–56 (2024). https://doi.org/10.1038/s41575-023-00846-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00846-4
This article is cited by
-
Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics
Lipids in Health and Disease (2024)
-
MAFLD as part of systemic metabolic dysregulation
Hepatology International (2024)