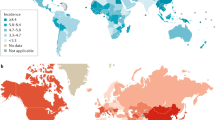
Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide. Surveillance for HCC is critical for early detection and treatment, but fewer than one-quarter of individuals at risk of HCC undergo surveillance. Multiple failures across the screening process contribute to the underutilization of surveillance, including limited disease awareness among patients and health-care providers, knowledge gaps, and difficulty recognizing patients who are at risk. Non-alcoholic fatty liver disease and alcohol-associated liver disease are the fastest-rising causes of HCC-related death worldwide and are associated with unique barriers to surveillance. In particular, more than one-third of patients with HCC related to non-alcoholic fatty liver disease do not have cirrhosis and therefore lack a routine indication for HCC surveillance on the basis of current practice guidelines. Semi-annual abdominal ultrasound with measurement of α-fetoprotein levels is recommended for HCC surveillance, but the sensitivity of this approach for early HCC is limited, especially for patients with cirrhosis or obesity. In this Review, we discuss the current status of HCC surveillance and the remaining challenges, including the changing aetiology of liver disease. We also discuss strategies to improve the utilization and quality of surveillance for HCC.
Key points
-
Fewer than one-quarter of patients with cirrhosis receive surveillance for hepatocellular carcinoma (HCC).
-
Multiple patient-related and provider-related barriers limit the utilization of HCC surveillance; these barriers include limited disease awareness, knowledge gaps, lack of resources and failure to recognize patients at risk.
-
Non-alcoholic fatty liver disease-related HCC develops in many people without cirrhosis, but routine HCC surveillance is not recommended in the absence of cirrhosis; surveillance should be individualized on the basis of additional risk factors.
-
Unique barriers to HCC surveillance (for example, non-adherence, limited social report, stigma and psychological issues) are associated with alcohol-associated cirrhosis; a multidisciplinary approach is required to address these barriers.
-
Ultrasonography has a suboptimal sensitivity for the detection of early-stage HCC and its performance can be poorer in the presence of obesity and non-alcoholic fatty liver disease-related or alcohol-related cirrhosis.
-
Novel blood-based and imaging-based biomarkers for HCC surveillance are emerging but require validation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Vogel, A., Meyer, T., Sapisochin, G., Salem, R. & Saborowski, A. Hepatocellular carcinoma. Lancet https://doi.org/10.1016/S0140-6736(22)01200-4 (2022).
Rumgay, H. et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77, 1598–1606 (2022).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6 (2021).
Moon, A. M., Singal, A. G. & Tapper, E. B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 18, 2650–2666 (2020).
Reveron-Thornton, R. F. et al. Global and regional long-term survival following resection for HCC in the recent decade: a meta-analysis of 110 studies. Hepatol. Commun. 6, 1813–1826 (2022).
Koh, J. H. et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg. Nutr. 11, 78–93 (2022).
Sangro, B., Sarobe, P., Hervás-Stubbs, S. & Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 525–543 (2021).
Vogel, A. & Martinelli, E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann. Oncol. 32, 801–805 (2021).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Cheng, A.-L. et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76, 862–873 (2022).
Huang, D. Q. et al. Characteristics and outcomes of hepatocellular carcinoma patients with macrovascular invasion following surgical resection: a meta-analysis of 40 studies and 8,218 patients. Hepatobiliary Surg. Nutr. 11, 848–860 (2022).
Tapper, E. B. & Parikh, N. D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. Br. Med. J. 362, k2817 (2018).
Huang, D. Q. et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 34, 969–977.e2 (2022).
El-Serag, H. B. & Rudolph, K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
Zhang, B.-H., Yang, B.-H. & Tang, Z.-Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 130, 417–422 (2004).
Jepsen, P. & West, J. We need stronger evidence for (or against) hepatocellular carcinoma surveillance. J. Hepatol. 74, 1234–1239 (2021).
Singal, A. G. et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J. Hepatol. 77, 128–139 (2022).
Wolf, E., Rich, N. E., Marrero, J. A., Parikh, N. D. & Singal, A. G. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology 73, 713–725 (2021).
Nguyen, M. H. et al. Gaps in hepatocellular carcinoma surveillance among insured patients with hepatitis B infection without cirrhosis in the United States. Hepatol. Commun. 6, 3443–3456 (2022).
Ye, Q. et al. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J. Hepatol. 76, 63–74 (2022).
Zhao, C. & Nguyen, M. H. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J. Clin. Gastroenterol. 50, 120–133 (2016).
Huang, D. Q., Mathurin, P., Cortez-Pinto, H. & Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 20, 37–49 (2023).
Tan, D. J. H. et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 23, 521–530 (2022).
Simmons, O. et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 45, 169–177 (2017).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750 (2018).
Singal, A. G. et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology https://doi.org/10.1097/hep.0000000000000466 (2023).
Kim, J. H. et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 69, 1066–1073 (2018).
Kanwal, F. & Singal, A. G. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 157, 54–64 (2019).
Davila, J. A. et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann. Intern. Med. 154, 85–93 (2011).
Goldberg, D. S. et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 65, 864–874 (2017).
Tran, S. A. et al. Rate of hepatocellular carcinoma surveillance remains low for a large, real-life cohort of patients with hepatitis C cirrhosis. BMJ Open Gastroenterol. 5, e000192 (2018).
Nguyen, M. H. et al. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr. Med. Res. Opin. 38, 2163–2173 (2022).
Yeo, Y. H. et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J. Hepatol. 75, 856–864 (2021).
Zhao, C. et al. Poor adherence to hepatocellular carcinoma surveillance: a systematic review and meta-analysis of a complex issue. Liver Int. 38, 503–514 (2018).
Wang, C. et al. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine 95, e4744 (2016).
Singal, A. G. et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev. Res. 5, 1124–1130 (2012).
Zhang, W. et al. Awareness and knowledge of nonalcoholic fatty liver disease among office employees in Beijing, Cchina. Dig. Dis. Sci. 64, 708–717 (2019).
Jun, D. W. et al. A study of the awareness of chronic liver diseases among Korean adults. Korean J. Hepatol. 17, 99–105 (2011).
Farvardin, S. et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 65, 875–884 (2017).
Singal, A. G. et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin. Gastroenterol. Hepatol. 19, 987–995.e1 (2021).
Rodriguez Villalvazo, Y. et al. Effect of travel distance and rurality of residence on initial surveillance for hepatocellular carcinoma in VA primary care patient with cirrhosis. Health Serv. Res. 55, 103–112 (2020).
Teerasarntipan, T. et al. Physician- and patient-reported barriers to hepatocellular carcinoma surveillance: a nationwide survey. Medicine 101, e30538 (2022).
Karlsen, T. H. et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399, 61–116 (2022).
Walker, M. et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment. Pharmacol. Ther. 43, 621–630 (2016).
Alexander, M. et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 16, 130 (2018).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861 (2022).
Le, M. H. et al. 2019 Global NAFLD prevalence — a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, 2809–2817.e28 (2021).
Simmons, O. L., Feng, Y., Parikh, N. D. & Singal, A. G. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin. Gastroenterol. Hepatol. 17, 766–773 (2019).
Tzartzeva, K. et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 154, 1706–1718.e1 (2018).
Tan, D. J. H. et al. UNOS down-staging criteria for liver transplantation of hepatocellular carcinoma: systematic review and meta-analysis of 25 studies. Clin. Gastroenterol. Hepatol. 21, 1475–1484 (2022).
Reig, M. et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 76, 681–693 (2022).
Del Poggio, P. et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 12, 1927–1933.e2 (2014).
Fetzer, D. T. et al. Screening and surveillance of hepatocellular carcinoma: an introduction to ultrasound liver imaging reporting and data system. Radiol. Clin. North Am. 55, 1197–1209 (2017).
Tang, A. et al. Introduction to the liver imaging reporting and data system for hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 17, 1228–1238 (2019).
Schoenberger, H. et al. Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 20, 1561–1569 (2021).
Chong, N. et al. Association between ultrasound quality and test performance for HCC surveillance in patients with cirrhosis: a retrospective cohort study. Aliment. Pharmacol. Ther. 55, 683–690 (2022).
Atiq, O. et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 65, 1196–1205 (2017).
Singal, A. G. et al. Benefits and harms of hepatocellular carcinoma surveillance in a prospective cohort of patients with cirrhosis. Clin. Gastroenterol. Hepatol. 19, 1925–1932.e1 (2021).
Dale, W., Bilir, P., Han, M. & Meltzer, D. The role of anxiety in prostate carcinoma: a structured review of the literature. Cancer 104, 467–478 (2005).
Tan, D. J. H. et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology 77, 1150–1163 (2022).
Ng, C. H., Huang, D. Q. & Nguyen, M. H. NAFLD versus MAFLD: prevalence, outcomes and implications of a change in name. Clin. Mol. Hepatol. 28, 790–801 (2022).
Ng, C. H. et al. Examining the interim proposal for name change to steatotic liver disease in the US population. Hepatology 77, 1712–1721 (2023).
Eslam, M. et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014.e1 (2020).
Huang, D. Q. et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 21, 1169–1177 (2022).
Orci, L. A. et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin. Gastroenterol. Hepatol. 20, 283–292.e10 (2022).
Kanwal, F. et al. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology 77, 997–1005 (2022).
Ioannou, G. N., Green, P., Kerr, K. F. & Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 71, 523–533 (2019).
Younossi, Z. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2019).
World Health Organization. Global Status Report on Alcohol and Health 2018 https://www.who.int/publications/i/item/9789241565639 (2018).
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18, 223–238 (2021).
Vitale, A. et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut 72, 141–152 (2023).
Karim, M. A. et al. Clinical characteristics and outcomes of nonalcoholic fatty liver disease-associated hepatocellular carcinoma in the United States. Clin. Gastroenterol. Hepatol. 21, 670–680.e18 (2022).
Natarajan, Y. et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology 72, 1242–1252 (2020).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155, 1828–1837.e2 (2018).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569 (2021).
Simon, T. G. et al. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology 74, 2410–2423 (2021).
Cucchetti, A., Cescon, M., Erroi, V. & Pinna, A. D. Cost-effectiveness of liver cancer screening. Best Pract. Res. Clin. Gastroenterol. 27, 961–972 (2013).
Loomba, R., Lim, J. K., Patton, H. & El-Serag, H. B. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 158, 1822–1830 (2020).
Mózes, F. E. et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 71, 1006–1019 (2022).
Loomba, R. & Adams, L. A. Advances in non-invasive assessment of hepatic fibrosis. Gut 69, 1343–1352 (2020).
Vali, Y. et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J. Hepatol. 73, 252–262 (2020).
Loomba, R. et al. Liver stiffness thresholds to predict disease progression and clinical outcomes in bridging fibrosis and cirrhosis. Gut 72, 581–589 (2023).
Teng, M. L., Tan, D. J. H., Ng, C. H. & Huang, D. Q. Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease — who and how? Clin. Mol. Hepatol. 29, 404–407 (2023).
Huang, D. Q. et al. Type 2 diabetes, hepatic decompensation and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: an individual participant-level data meta-analysis. Lancet Gastroenterol. Hepatol. https://doi.org/10.1016/S2468-1253(23)00157-7 (2023).
Huang, D. Q., Wilson, L. A. & Behling, C. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology https://doi.org/10.1053/j.gastro.2023.04.025 (2023).
Kramer, J. R. et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology 75, 1420–1428 (2022).
Kanwal, F. et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 71, 808–819 (2020).
Cholankeril, G. et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J. Hepatol. 78, 493–500 (2023).
Ioannou, G. N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 75, 1476–1484 (2021).
Nemutlu, G., Hajjar, A. & Mueller, P. Which individuals with nonalcoholic fatty liver disease should undergo HCC surveillance? cost-effectiveness analysis. Hepatology 76, S1–S1564 (2022).
Quek, J. et al. Quality assessment of ultrasound and magnetic resonance imaging for hepatocellular carcinoma surveillance: systematic review and meta-analysis. Dig. Dis. https://doi.org/10.1159/000531016 (2023).
Huang, D. Q. et al. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: a prospective study. Aliment. Pharmacol. Ther. 55, 820–827 (2022).
McNeely, J. et al. Barriers and facilitators affecting the implementation of substance use screening in primary care clinics: a qualitative study of patients, providers, and staff. Addict. Sci. Clin. Pract. 13, 8 (2018).
DiMartini, A. F., Leggio, L. & Singal, A. K. Barriers to the management of alcohol use disorder and alcohol-associated liver disease: strategies to implement integrated care models. Lancet Gastroenterol. Hepatol. 7, 186–195 (2022).
Bucci, L. et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment. Pharmacol. Ther. 43, 385–399 (2016).
Schutte, K. et al. Delayed diagnosis of HCC with chronic alcoholic liver disease. Liver Cancer 1, 257–266 (2012).
Costentin, C. E. et al. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: results of a prospective, nationwide study. Cancer 124, 1964–1972 (2018).
Costentin, C. E. et al. Geographical disparities of outcomes of hepatocellular carcinoma in France: the heavier burden of alcohol compared to hepatitis C. Dig. Dis. Sci. 65, 301–311 (2020).
Seitz, H. K. et al. Alcoholic liver disease. Nat. Rev. Dis. Prim. 4, 16 (2018).
Keyes, K. M. et al. Stigma and treatment for alcohol disorders in the United States. Am. J. Epidemiol. 172, 1364–1372 (2010).
Im, G. Y. et al. Provider attitudes and practices for alcohol screening, treatment, and education in patients with liver disease: a survey from the American Association for the Study of Liver Diseases Alcohol-associated Liver Disease Special Interest Group. Clin. Gastroenterol. Hepatol. 19, 2407–2416.e8 (2021).
Bataller, R., Arab, J. P. & Shah, V. H. Alcohol-associated hepatitis. N. Engl. J. Med. 387, 2436–2448 (2022).
Jepsen, P. et al. Risk of hepatocellular carcinoma in Danish outpatients with alcohol-related cirrhosis. J. Hepatol. 73, 1030–1036 (2020).
Parikh, N. D., Singal, A. G., Hutton, D. W. & Tapper, E. B. Cost-effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am. J. Gastroenterol. 115, 1642–1649 (2020).
Singal, A. K. & Mathurin, P. Diagnosis and treatment of alcohol-associated liver disease: a review. J. Am. Med. Assoc. 326, 165–176 (2021).
Kim, N. J., Vutien, P., Cleveland, E., Cravero, A. & Ioannou, G. N. Fibrosis stage-specific incidence of hepatocellular cancer after hepatitis c cure with direct-acting antivirals: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 21, 1723–1738.e5 (2022).
Kanwal, F. et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996–1005.e1 (2017).
Ioannou, G. N., Green, P. K. & Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2017.08.030 (2017).
Calvaruso, V. et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 155, 411–421.e4 (2018).
Romano, A. et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J. Hepatol. 69, 345–352 (2018).
Ogawa, E. et al. Association of direct-acting antiviral therapy with liver and nonliver complications and long-term mortality in patients with chronic hepatitis C. JAMA Intern. Med. 183, 97–105 (2022).
Dang, H. et al. Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both east and west. Hepatology 71, 1910–1922 (2020).
Shiha, G. et al. Incidence of HCC in chronic hepatitis C patients with advanced hepatic fibrosis who achieved SVR following DAAs: a prospective study. J. Viral Hepat. 27, 671–679 (2020).
Tanaka, Y. et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol. Int. 14, 1023–1033 (2020).
Semmler, G. et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J. Hepatol. 76, 812–821 (2022).
DʼAmbrosio, R. et al. Incidence of liver- and non-liver-related outcomes in patients with HCV-cirrhosis after SVR. J. Hepatol. 76, 302–310 (2022).
Degasperi, E. et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin. Gastroenterol. Hepatol. 17, 1183–1191.e7 (2019).
Farhang Zangneh, H. et al. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis c virus infection and advanced fibrosis. Clin. Gastroenterol. Hepatol. 17, 1840–1849.e16 (2019).
Kanda, T. et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 13, 649–661 (2019).
Ioannou, G. N. et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J. Hepatol. 69, 1088–1098 (2018).
Ioannou, G. N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 74, 458–465 (2021).
Le, M. H. et al. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999-2016. Hepatology 71, 431–443 (2020).
Nguyen, M. H., Wong, G., Gane, E., Kao, J. H. & Dusheiko, G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin. Microbiol. Rev. 33, e00046–19 (2020).
Tan, D. J. H. et al. Risk of hepatocellular carcinoma with tenofovir vs entecavir treatment for chronic hepatitis B virus: a reconstructed individual patient data meta-analysis. JAMA Netw. Open 5, e2219407 (2022).
Chan, A. C. Y. et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch. Surg. 146, 675–681 (2011).
Hsu, Y. C., Huang, D. Q. & Nguyen, M. H. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-023-00760-9 (2023).
GBD 2019 Hepatitis B Collaborators.Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 7, 796–829 (2022).
Yang, H. I. et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 12, 568–574 (2011).
Lee, H. W. et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am. J. Gastroenterol. 109, 1241–1249 (2014).
Wong, V. W. et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J. Clin. Oncol. 28, 1660–1665 (2010).
Wong, G. L. et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J. Hepatol. 60, 339–345 (2014).
Yuen, M. F. et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 50, 80–88 (2009).
Yang, H. I. et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J. Clin. Oncol. 28, 2437–2444 (2010).
Papatheodoridis, G. et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 64, 800–806 (2016).
Papatheodoridis, G. V. et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J. Hepatol. 72, 1088–1096 (2020).
Voulgaris, T., Papatheodoridi, M., Lampertico, P. & Papatheodoridis, G. V. Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 40, 484–495 (2020).
Yang, H. I. et al. REAL-B (Real-world effectiveness from the asia pacific rim liver consortium for HBV) risk score for the prediction of hepatocellular carcinoma in chronic hepatitis B patients treated with oral antiviral therapy. J. Infect. Dis. 221, 389–399 (2019).
Poh, Z. et al. Real-world risk score for hepatocellular carcinoma (RWS-HCC): a clinically practical risk predictor for HCC in chronic hepatitis B. Gut 65, 887–888 (2016).
Papatheodoridis, G. V. et al. Predictive performance of newer Asian hepatocellular carcinoma risk scores in treated Caucasians with chronic hepatitis B. JHEP Rep. 3, 100290 (2021).
Chen, C. J. et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. J. Am. Med. Assoc. 295, 65–73 (2006).
Yu, J. H., Cho, S. G., Jin, Y.-J. & Lee, J.-W. The best predictive model for hepatocellular carcinoma in patients with chronic hepatitis B infection. Clin. Mol. Hepatol. 28, 351–361 (2022).
Huang, D. Q. et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin. Gastroenterol. Hepatol. 20, 1803–1812.e5 (2022).
Chang, M.-L. & Liaw, Y.-F. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J. Hepatol. 61, 1407–1417 (2014).
Kim, H. S. et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J. Hepatol. 76, 294–301 (2022).
Yu, J. H. et al. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur. J. Gastroenterol. Hepatol. 31, 865–872 (2019).
Riveiro-Barciela, M. et al. Effectiveness and safety of entecavir or tenofovir in a spanish cohort of chronic hepatitis B patients: validation of the page-B score to predict hepatocellular carcinoma. Dig. Dis. Sci. 62, 784–793 (2017).
Kennedy, N. A. et al. Optimisation of hepatocellular carcinoma surveillance in patients with viral hepatitis: a quality improvement study. Intern. Med. J. 43, 772–777 (2013).
Singal, A. G. et al. Multicenter randomized clinical trial of a mailed outreach strategy for hepatocellular carcinoma surveillance. Clin. Gastroenterol. Hepatol. 20, 2818–2825.e1 (2021).
Del Poggio, P. et al. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 27, 1103–1108 (2015).
Beste, L. A. et al. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin. Gastroenterol. Hepatol. 13, 172–179 (2015).
Aberra, F. B., Essenmacher, M., Fisher, N. & Volk, M. L. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig. Dis. Sci. 58, 1157–1160 (2013).
Labenz, C. et al. Structured early detection of asymptomatic liver cirrhosis: results of the population-based liver screening program SEAL. J. Hepatol. 77, 695–701 (2022).
Kudo, M. Japan’s successful model of nationwide hepatocellular carcinoma surveillance highlighting the urgent need for global surveillance. Liver Cancer 1, 141–143 (2012).
Liao, S.-H. et al. Long-term effectiveness of population-wide multifaceted interventions for hepatocellular carcinoma in Taiwan. J. Hepatol. 75, 132–141 (2021).
Kudo, M. et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 10, 181–223 (2021).
Toyoda, H. et al. Outcome of hepatocellular carcinoma detected during surveillance: comparing USA and Japan. Clin. Gastroenterol. Hepatol. 19, 2379–2388.e6 (2021).
Johnson, P. et al. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br. J. Cancer 116, 441–447 (2017).
Kuo, S.-C. et al. Optimal intervals of ultrasonography screening for early diagnosis of hepatocellular carcinoma in Taiwan. JAMA Netw. Open 4, e2114680 (2021).
Alqahtani, S. A. et al. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol. Commun. 5, 1833–1847 (2021).
Finberg, H. J. Whither (wither?) the ultrasound specialist? J. Ultrasound Med. 23, 1543–1547 (2004).
Fetzer, D. T., Browning, T., Xi, Y., Yokoo, T. & Singal, A. G. Associations of ultrasound LI-RADS visualization score with examination, sonographer, and radiologist factors: retrospective assessment in over 10,000 examinations. AJR Am. J. Roentgenol. 218, 1010–1020 (2022).
Parikh, N. D., Tayob, N. & Singal, A. G. Blood-based biomarkers for hepatocellular carcinoma screening: approaching the end of the ultrasound era? J. Hepatol. 78, 207–216 (2023).
Oka, H., Tamori, A., Kuroki, T., Kobayashi, K. & Yamamoto, S. Prospective study of α-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology 19, 61–66 (1994).
Lok, A. S. et al. Des-γ-carboxy prothrombin and α-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 138, 493–502 (2010).
Marrero, J. A. et al. α-Fetoprotein, des-γ carboxyprothrombin, and lectin-bound α-fetoprotein in early hepatocellular carcinoma. Gastroenterology 137, 110–118 (2009).
Singal, A. G. et al. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 75, 541–549 (2022).
Kumada, T. et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J. Gastroenterol. 49, 555–563 (2014).
Tayob, N., Kanwal, F., Alsarraj, A., Hernaez, R. & El-Serag, H. B. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): a phase 3 biomarker study in the United States. Clin. Gastroenterol. Hepatol. 21, 415–423 (2023).
Toyoda, H. et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin. Gastroenterol. Hepatol. 4, 1528–1536 (2006).
Fox, R. et al. Biomarker-based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br. J. Cancer 110, 2090–2098 (2014).
Singal, A. G. et al. Doylestown plus and GALAD demonstrate high sensitivity for HCC detection in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 20, 953–955.e2 (2022).
Tayob, N. et al. Validation of the updated hepatocellular carcinoma early detection screening algorithm in a community-based cohort of patients with cirrhosis of multiple etiologies. Clin. Gastroenterol. Hepatol. 19, 1443–1450.e6 (2021).
Chalasani, N. P. et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 20, 173–182.e7 (2022).
Lin, N. et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol. Commun. 6, 1753–1763 (2022).
Sun, N. et al. HCC EV ECG score: an extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology 77, 774–788 (2023).
Singal, A. G. et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology 160, 2572–2584 (2021).
Johnson, P., Zhou, Q., Dao, D. Y. & Lo, Y. M. D. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 670–681 (2022).
Lee, Y. T., Fujiwara, N., Yang, J. D. & Hoshida, Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology 78, 319–362 (2022).
Kim, S. Y. et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 3, 456–463 (2017).
Roberts, L. R. et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 67, 401–421 (2018).
An, J. Y. et al. Abbreviated MRI for hepatocellular carcinoma screening and surveillance. Radiographics 40, 1916–1931 (2020).
Gupta, P. et al. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J. Hepatol. 75, 108–119 (2021).
Park, H. J. et al. Abbreviated magnetic resonance imaging vs. ultrasound for surveillance of hepatocellular carcinoma in high-risk patients. Liver Int. 42, 2080–2092 (2021).
Yokoo, T. et al. Multicenter validation of abbreviated MRI for detecting early-stage hepatocellular carcinoma. Radiology https://doi.org/10.1148/radiol.220917 (2023).
GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 5, 245–266 (2020).
World Health Organization. Global Hepatitis Report https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=6DB65DA61DB685B218A314037DBE0C09?sequence=1 (2017).
Mohammed, H. A. et al. Factors influencing surveillance for hepatocellular carcinoma in patients with liver cirrhosis. Liver Cancer 6, 126–136 (2017).
Signorelli, I. V. et al. Socioeconomic disparities in access to a hepatocellular carcinoma screening program in Brazil. Clinics 71, 361–364 (2016).
Palmer, L. B., Kappelman, M. D., Sandler, R. S. & Hayashi, P. H. Surveillance for hepatocellular carcinoma in a Medicaid cirrhotic population. J. Clin. Gastroenterol. 47, 713–718 (2013).
Singal, A. G., Tiro, J., Li, X., Adams-Huet, B. & Chubak, J. Hepatocellular carcinoma surveillance among patients with cirrhosis in a population-based integrated health care delivery system. J. Clin. Gastroenterol. 51, 650–655 (2017).
Chang, S. S. et al. Factors associated with nonadherence to surveillance for hepatocellular carcinoma among patients with hepatic C virus cirrhosis, 2000-2015. Medicine 101, e31907 (2022).
Goldberg, D. S. et al. Hepatocellular carcinoma surveillance rates in commercially insured patients with noncirrhotic chronic hepatitis B. J. Viral Hepat. 22, 727–736 (2015).
Tran, S., Jeong, D., Henry, L., Cheung, R. C. & Nguyen, M. H. Initial evaluation, long-term monitoring, and hepatocellular carcinoma surveillance of chronic hepatitis B in routine practice: a nationwide US study. Am. J. Gastroenterol. 116, 1885–1895 (2021).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
D.Q.H. has served as an advisory board member for Eisai and Gilead and has received funding from the Singapore Ministry of Health’s National Medical Research Council (MOH-000595-01). A.G.S. has served as a consultant or on advisory boards for AstraZeneca, Bayer, Eisai, Exact Sciences, Exelixis, Freenome, FujiFilm Medical Sciences, Genentech, Glycotest and GRAIL. P.L. has served on advisory boards and/or speaker bureaus for Abbvie, Arrowhead, Aligos, Alnylam, Antios, Bristol Myers Squibb, Eiger, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Myr, Roche, Spring Bank and Vir Biotechnology. M.B. has received research support from Gilead and has served as an advisory board member for Abbvie, Gilead, GlaxoSmithKline, Janssen and Spring Bank. C.B.S. has received research grants from the American College of Radiology, Bayer, Foundation of NIH, GE Healthcare, Gilead, Pfizer, Philips and Siemens; has lab service agreements with Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva and Takeda; conducts institutional consulting for Bristol Myers Squibb, Exact Sciences, IBM–Watson and Pfizer; provides personal consulting for Blade, Boehringer, Epigenomics and Guerbet; receives royalties and/or honoraria from Medscape and Wolters Kluwer; owns stock options in Livivos; has an unpaid advisory board position at Quantix Bio; and serves as Chief Medical Officer for Livivos (unsalaried position with stock options) with appointment approved from his university. M.H.N. has received research support from AstraZeneca, B.K. Kee Foundation, CurveBio, Delfi, Enanta, Exact Science, Gilead, Glycotest, Helio Health, Innogen, the National Cancer Institute, Pfizer and Vir. She has served as an advisory board member or consultant to Eli Lilly, Exact Sciences, Exelixis, Gilead, GlaxoSmithKline and Intercept. R.L. received funding from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK120515) and serves as a consultant to 89bio, Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutes have received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. F.K. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks E. Giannini, R. Tateishi and A. Vogel for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria PubMed was searched from inception to February 2023 using the terms “hepatocellular carcinoma”, “surveillance” and “screening” without language restrictions. Original articles were evaluated. Studies were selected to provide data from diverse geographical locations on the utilization of hepatocellular carcinoma (HCC) surveillance in the presence of cirrhosis and chronic hepatitis B. We included studies that reported the utilization of HCC surveillance in a real-world setting. We excluded trials of HCC surveillance and studies of dedicated HCC surveillance programmes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D.Q., Singal, A.G., Kanwal, F. et al. Hepatocellular carcinoma surveillance — utilization, barriers and the impact of changing aetiology. Nat Rev Gastroenterol Hepatol 20, 797–809 (2023). https://doi.org/10.1038/s41575-023-00818-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00818-8