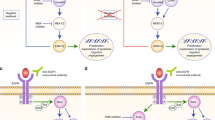
Abstract
In the era of targeted therapy based on genomic alterations, the treatment strategy for metastatic colorectal cancer (mCRC) has been changing. Before systemic treatment initiation, determination of tumour genomic status for KRAS and NRAS, BRAFV600E mutations, ERBB2, and microsatellite instability and/or mismatch repair (MMR) status is recommended. In patients with deficient MMR and BRAFV600E mCRC, randomized phase III trials have established the efficacy of pembrolizumab as first-line therapy and the combination of encorafenib and cetuximab as second-line or third-line therapy. In addition, new agents have been actively developed in other rare molecular fractions such as ERBB2 alterations and KRASG12C mutations. In March 2022, the combination of pertuzumab and trastuzumab for ERBB2-positive mCRC was approved in Japan, thereby combining real-world evidence from the SCRUM-Japan Registry. As the populations are highly fragmented owing to rare genomic alterations, various strategies in clinical development are expected. Clinical development of a tumour-agnostic approach, such as NTRK fusion and tumour mutational burden, has successfully introduced corresponding drugs to clinical practice. Considering the difficulty of randomized trials owing to cost–benefit and rarity, a promising solution could be real-world evidence utilized as an external control from the molecular-based disease registry.
Key points
-
For the best practice of metastatic colorectal cancer, the determination of tumour genomic status for KRAS and NRAS, BRAFV600E mutations, ERBB2, and microsatellite instability and/or mismatch repair (MMR) status is strongly recommended.
-
Immune-checkpoint inhibitors for patients with microsatellite instability-high and/or MMR-deficient tumours is the established standard therapy; combining immune-checkpoint inhibitors and stimulating agents is an emerging strategy investigated in patients with microsatellite stability and/or proficient MMR tumours.
-
In Japan, trastuzumab plus pertuzumab was approved considering real-world data from the SCRUM-Japan Registry; combining an anti-KRAS-G12C inhibitor and an anti-EGFR antibody is promising in KRASG12C-mutated tumours.
-
Rechallenge of anti-EGFR therapy in former responders with wild-type RAS in circulating tumour DNA assay after an interval of >4 months is also a promising treatment option.
-
Based on longitudinal genomic alteration data from the SCRUM-Japan platform, National Cancer Center Hospital East aims to establish a gene alteration database and identify artificial intelligence-based predictors for a pre-emptive treatment strategy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Leporrier, J. et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br. J. Surg. 93, 465–474 (2006).
Heinemann, V. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1065–1075 (2014).
Venook, A. P. et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317, 2392–2401 (2017).
Benson, A. B. et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 19, 329–359 (2021).
Ebi, H. & Bando, H. Precision oncology and the universal health coverage system in Japan. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00291 (2019).
Nakamura, Y. & Shitara, K. Development of circulating tumour DNA analysis for gastrointestinal cancers. ESMO Open 5, e000600 (2020).
Bando, H., Nakamura, Y., Kotani, D. & Yoshino, Y. Comparing GOZILA and COLOMATE: ongoing umbrella/basket trials examining genetic testing in gastrointestinal malignancies. Oncology 35, 382–389 (2021).
Bando, H. et al. Effects of metastatic sites on circulating tumor DNA in patients with metastatic colorectal cancer. JCO Precis. Oncol. 6, e2100535 (2022).
Koopman, M. et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 100, 266–273 (2009).
Lochhead, P. et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl Cancer Inst. 105, 1151–1156 (2013).
Venderbosch, S. et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 20, 5322–5330 (2014).
Tol, J., Nagtegaal, I. D. & Punt, C. J. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 361, 98–99 (2009).
Van Cutsem, E. et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 29, 2011–2019 (2011).
Chida, K. et al. The prognostic impact of KRAS G12C mutation in patients with metastatic colorectal cancer: a multicenter retrospective observational study. Oncologist 26, 845–853 (2021).
Salem, M. E. et al. Landscape of KRAS(G12C), associated genomic alterations, and interrelation with immuno-oncology biomarkers in KRAS-mutated cancers. JCO Precis. Oncol. 6, e2100245 (2022).
Osterlund, E. et al. KRAS-G12C Mutation in one real-life and three population-based nordic cohorts of metastatic colorectal cancer. Front. Oncol. 12, 826073 (2022).
Latham, A. et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J. Clin. Oncol. https://doi.org/10.1200/jco.18.00283 (2018).
Fujii, S. et al. International harmonization of diagnostic criteria for HER2-amplified metastatic colorectal cancer and application of targeted next-generation sequencing panel as a diagnostic method. J. Clin. Oncol. 36, 3594 (2018).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Sartore-Bianchi, A. et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncologist 24, 1395–1402 (2019).
Johnson, B. et al. Atypical, non-V600 BRAF mutations as a potential mechanism of resistance to EGFR inhibition in metastatic colorectal cancer. JCO Precis. Oncol. https://doi.org/10.1200/po.19.00102 (2019).
Raghav, K. et al. MET amplification in metastatic colorectal cancer: an acquired response to EGFR inhibition, not a de novo phenomenon. Oncotarget 7, 54627–54631 (2016).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Dienstmann, R. et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17, 79–92 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02465060 (2015).
Flaherty, K. T. et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH). J. Clin. Oncol. https://doi.org/10.1200/jco.19.03010 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05564377 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05564390 (2022).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med 23, 703–713 (2017).
AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 7, 818–831 (2017).
Micheel, C. M. et al. American Association for Cancer Research Project Genomics Evidence Neoplasia Information Exchange: from inception to first data release and beyond — lessons learned and member institutions’ perspectives. JCO Clin. Cancer Inform. 2, https://doi.org/10.1200/CCI.17.00083 (2018).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. https://doi.org/10.1200/po.17.00011 (2017).
Johnson, A. et al. Clinical use of precision oncology decision support. JCO Precis. Oncol. https://doi.org/10.1200/po.17.00036 (2017).
Chakravarty, D. et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J. Clin. Oncol. https://doi.org/10.1200/jco.21.02767 (2022).
Nakamura, Y. et al. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 112, 4425–4432 (2021).
Sakamoto, Y. et al. Trajectory for the regulatory approval of a combination of pertuzumab plus trastuzumab for pre-treated HER2-positive metastatic colorectal cancer using real-world data: author contributions. Clin. Colorectal Cancer https://doi.org/10.1016/j.clcc.2022.10.003 (2022).
Cervantes, A. et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.10.003 (2022).
Yoshino, T. et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 29, 44–70 (2018).
Morris, V. K. et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. https://doi.org/10.1200/jco.22.01690 (2022).
Arnold, D. et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann. Oncol. https://doi.org/10.1093/annonc/mdx175 (2017).
Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Engstrom, P. F. et al. NCCN clinical practice guidlines in oncology (NCCN guidelines): colon cancer. J. Natl Compr. Canc. Netw. 7, 778–831 (2022).
Yoshino, T. et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): results from the phase 3 PARADIGM trial. J. Clin. Oncol. 40, abstr LBA1 (2022).
Patel, S. G., Karlitz, J. J., Yen, T., Lieu, C. H. & Boland, C. R. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 7, 262–274 (2022).
Jin, Z. et al. Response to epithelial growth factor receptor inhibitor (EGFRi) treatment in patients with early-onset, treatment-naïve metastatic colorectal cancer (mCRC): an ARCAD database analysis. J. Clin. Oncol. 40, 3572 (2022).
Zaanan, A. et al. Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol. 4, 379–383 (2018).
Taieb, J. et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann. Oncol. 30, 1466–1471 (2019).
Cohen, R. et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. https://doi.org/10.1200/jco.20.01600 (2020).
André, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Diaz, L. A. Jr. et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 23, 659–670 (2022).
Lenz, H. J. et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J. Clin. Oncol. https://doi.org/10.1200/jco.21.01015 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04008030 (2019).
Le, D. T. et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 38, 11–19 (2020).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Overman, M. J. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779 (2018).
Cohen, R. et al. RECIST and iRECIST criteria for the evaluation of nivolumab plus ipilimumab in patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the GERCOR NIPICOL phase II study. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-001499 (2020).
Cohen, R. et al. One-year duration of nivolumab plus ipilimumab in patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up of the GERCOR NIPICOL phase II study. J. Clin. Oncol. 40 (Suppl. 4), 13 (2022).
Andre, T. et al. Safety and efficacy of anti-PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: results from GARNET study. J. Clin. Oncol. 39, 9 (2021).
Li, J. et al. Updated analysis from a phase 2 study of tislelizumab (TIS) monotherapy in patients (pts) with previously treated, locally advanced, unresectable/metastatic microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) solid tumors. J. Clin. Oncol. 40 (Suppl. 4), 1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02997228 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04041310 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04729322 (2021).
Chen, D. et al. BRAFV600E mutation and its association with clinicopathological features of colorectal cancer: a systematic review and meta-analysis. PLoS ONE 9, e90607 (2014).
Advani, S. M. et al. Epidemiology and molecular-pathologic characteristics of CpG island methylator phenotype (CIMP) in colorectal cancer. Clin. Colorectal Cancer https://doi.org/10.1016/j.clcc.2020.09.007 (2020).
Vedeld, H. M. et al. CpG island methylator phenotype identifies high risk patients among microsatellite stable BRAF mutated colorectal cancers. Int. J. Cancer 141, 967–976 (2017).
Modest, D. P. et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 27, 1746–1753 (2016).
Pietrantonio, F. et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur. J. Cancer 51, 587–594 (2015).
Loupakis, F. et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur. J. Cancer 50, 57–63 (2014).
Loupakis, F. et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 371, 1609–1618 (2014).
Cremolini, C. et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16, 1306–1315 (2015).
Cremolini, C. et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J. Clin. Oncol. https://doi.org/10.1200/jco.20.01225 (2020).
Stintzing, S. et al. Randomized study to investigate FOLFOXIRI plus either bevacizumab or cetuximab as first-line treatment of BRAF V600E-mutant mCRC: the phase-II FIRE-4.5 study (AIO KRK-0116). J. Clin. Oncol. 39, 3502–3502 (2021).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019).
Tabernero, J. et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J. Clin. Oncol. 39, 273–284 (2021).
Kopetz, S. et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J. Clin. Oncol. 39, 285–294 (2021).
Cutsem, E. V. et al. O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer. Ann. Oncol. 32, S222 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04607421 (2020).
Kopetz, S. et al. BREAKWATER: Randomized phase 3 study of encorafenib (enco)+cetuximab (cetux)±chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 39 (Suppl. 15), TPS3619 (2022).
Russo, M. et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 366, 1473–1480 (2019).
Morris, V. K. et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J. Clin. Oncol. 40, 12 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05308446 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05217446 (2022).
Kotani, H. et al. Distinct dependencies on receptor tyrosine kinases in the regulation of MAPK signaling between BRAF V600E and non-V600E mutant lung cancers. Oncogene 37, 1775–1787 (2018).
Kotani, D. et al. BIG BANG study (EPOC1703): multicentre, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy with binimetinib, encorafenib and cetuximab in patients with BRAF non-V600E mutated metastatic colorectal cancer. ESMO Open 5, e000624 (2020).
UMIN. https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000036377 (2018).
Misale, S., Di Nicolantonio, F., Sartore-Bianchi, A., Siena, S. & Bardelli, A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 4, 1269–1280 (2014).
Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 21, 795–801 (2015).
Montagut, C. et al. Efficacy of Sym004 in patients with metastatic colorectal cancer with acquired resistance to anti-EGFR therapy and molecularly selected by circulating tumor DNA analyses: a phase 2 randomized clinical trial. JAMA Oncol. 4, e175245 (2018).
Liu, X. et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer 15, 713 (2015).
Masuishi, T. et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br. J. Cancer https://doi.org/10.1038/s41416-020-01042-w (2020).
Morelli, M. P. et al. Characterizing the patterns of clonal selection in circulating tumor DNA from patients with colorectal cancer refractory to anti-EGFR treatment. Ann. Oncol. 26, 731–736 (2015).
Parseghian, C. M. et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann. Oncol. 30, 243–249 (2019).
Cremolini, C. et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2018.5080 (2018).
Martinelli, E. et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE Trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2021.2915 (2021).
Martinelli, E. et al. Evidence of therapeutic effectiveness of third-line cetuximab rechallenge in appropriately selected patients: Findings from long-term follow-up of CRICKET and CAVE trials. Ann. Oncol. 33, S381–S382 (2022).
Sartore-Bianchi, A. et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat. Med. 28, 1612–1618 (2022).
Nakajima, H. et al. REMARRY and PURSUIT trials: liquid biopsy-guided rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab plus irinotecan for patients with plasma RAS wild-type metastatic colorectal cancer. BMC Cancer 21, 674 (2021).
Kagawa, Y. et al. Plasma RAS dynamics and anti-EGFR rechallenge efficacy in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. J. Clin. Oncol. https://doi.org/10.1200/JCO.2022.40.16_suppl.3518 (2022).
Moretto, R. et al. Rationale and study design of the PARERE trial: randomized phase II study of panitumumab Re-treatment followed by regorafenib versus the reverse sequence in RAS and BRAF wild-type chemo-refractory metastatic colorectal cancer patients. Clin. Colorectal Cancer 20, 314–317 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04787341 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02934529 (2016).
Fujii, S. et al. International harmonization of provisional diagnostic criteria for ERBB2-amplified metastatic colorectal cancer allowing for screening by next-generation sequencing panel. JCO Precis. Oncol. 4, 6–19 (2020).
Sawada, K. et al. Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 17, 198–205 (2018).
Raghav, K. et al. Validation of HER2 amplification as a predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis. Oncol. 3, 1–13 (2019).
Sartore-Bianchi, A. et al. Central nervous system as possible site of relapse in ERBB2-positive metastatic colorectal cancer: long-term results of treatment with trastuzumab and lapatinib. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.0571 (2020).
Richman, S. D. et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J. Pathol. 238, 562–570 (2016).
Ingold Heppner, B. et al. HER2/neu testing in primary colorectal carcinoma. Br. J. Cancer 111, 1977–1984 (2014).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25, 1415–1421 (2019).
Nakamura, Y. et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat. Med. 27, 1899–1903 (2021).
Tosi, F. et al. Long-term clinical outcome of trastuzumab and lapatinib for HER2-positive metastatic colorectal cancer. Clin. Colorectal Cancer 19, 256–262.e2 (2020).
Meric-Bernstam, F. et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20, 518–530 (2019).
Gupta, R. et al. Pertuzumab plus trastuzumab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 amplification or overexpression: results from the TAPUR study. J. Clin. Oncol. 38, 132–132 (2020).
Strickler, J. et al. Primary analysis of MOUNTAINEER: a phase 2 study of tucatinib and trastuzumab for HER2-positive mCRC. Ann. Oncol. 33, S375–S376 (2022).
Siena, S. et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 22, 779–789 (2021).
Siena, S. et al. Exploratory biomarker analysis of DESTINY-CRC01, a phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd, DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC). Ann. Oncol. 32, S530–S582 (2021).
Raghav, K. P. S. et al. A randomized phase II study of trastuzumab and pertuzumab (TP) compared to cetuximab and irinotecan (CETIRI) in advanced/metastatic colorectal cancer (mCRC) with HER2 amplification: S1613. J. Clin. Oncol. 36 (Suppl. 15), TPS3620 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03365882 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05253651 (2022).
Raghav, K. P. S. et al. Trastuzumab deruxtecan in patients with HER2-overexpressing locally advanced, unresectable, or metastatic colorectal cancer (mCRC): a randomized, multicenter, phase 2 study (DESTINY-CRC02). J. Clin. Oncol. 39 (Suppl. 15), TPS3620 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04744831 (2021).
Ganesh, K. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 16, 361–375 (2019).
Riihimaki, M., Hemminki, A., Sundquist, J. & Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 6, 29765 (2016).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. https://doi.org/10.1038/s41591-020-1131-x (2021).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Antoniotti, C. et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 23, 876–887 (2022).
Cremolini, C. et al. FOLFOXIRI plus bevacizumab (bev) plus atezolizumab (atezo) versus FOLFOXIRI plus bev as first-line treatment of unresectable metastatic colorectal cancer (mCRC) patients: results of the phase II randomized AtezoTRIBE study by GONO. Ann. Oncol. 32, S1294–S1295 (2021).
Lenz, H.-J. et al. Nivolumab (NIVO)+5-fluorouracil/leucovorin/oxaliplatin (mFOLFOX6)/bevacizumab (BEV) versus mFOLFOX6/BEV for first-line (1L) treatment of metastatic colorectal cancer (mCRC): phase 2 results from CheckMate 9×8. J. Clin. Oncol. 40, 8–8 (2022).
Schmoll, H. J. et al. MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: an adaptable signal-seeking approach. J. Cancer Res. Clin. Oncol. 144, 1197–1204 (2018).
Mettu, N. B. et al. Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: a randomized clinical trial. JAMA Netw. Open 5, e2149040 (2022).
Chen, E. X. et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian Cancer Trials Group CO.26 study. JAMA Oncol. 6, 831–838 (2020).
Bullock, A. et al. Botensilimab, a novel innate/adaptive immune activator, plus balstilimab (anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer. Ann. Oncol. 33, S376 (2022).
Eng, C. et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 20, 849–861 (2019).
Fukuoka, S. et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38, 2053–2061 (2020).
Gomez-Roca, C. et al. LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors — Results from the colorectal cancer cohort. J. Clin. Oncol. 39, 94 (2021).
Yoshino, T. et al. Pembrolizumab plus lenvatinib versus standard of care for previously treated metastatic colorectal cancer (mCRC): phase III LEAP-017 study. Ann. Oncol. 32, S580 (2021).
Pietrantonio, F. et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Ann. Oncol. 25, 404–408 (2014).
Germano, G. et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552, 116–120 (2017).
Morano, F. et al. Temozolomide followed by combination with low-dose ipilimumab and nivolumab in patients with microsatellite-stable, O(6)-methylguanine-DNA methyltransferase-silenced metastatic colorectal cancer: The MAYA trial. J. Clin. Oncol. https://doi.org/10.1200/jco.21.02583 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03519412 (2018).
Crisafulli, G. et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients. Cancer Discov. 12, 1656–1675 (2022).
Monjazeb, A. M. et al. A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin. Cancer Res. 27, 2470–2480 (2021).
Segal, N. H. et al. Phase II single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair-proficient metastatic colorectal cancer. Clin. Cancer Res. 27, 2200–2208 (2021).
Floudas, C. S. et al. A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 18, e349–e360 (2019).
Nebot-Bral, L. et al. Hypermutated tumours in the era of immunotherapy: the paradigm of personalised medicine. Eur. J. Cancer 84, 290–303 (2017).
Yao, J. et al. Comprehensive analysis of POLE and POLD1 Gene Variations identifies cancer patients potentially benefit from immunotherapy in Chinese population. Sci. Rep. 9, 15767 (2019).
Oh, C. R. et al. Phase II study of durvalumab monotherapy in patients with previously treated microsatellite instability-high/mismatch repair-deficient or POLE-mutated metastatic or unresectable colorectal cancer. Int. J. Cancer 150, 2038–2045 (2022).
Sha, D. et al. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 10, 1808–1825 (2020).
Fancello, L., Gandini, S., Pelicci, P. G. & Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J. Immunother. Cancer 7, 183 (2019).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
McGrail, D. J. et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. https://doi.org/10.1016/j.annonc.2021.02.006 (2021).
Meiri, E. et al. Pembrolizumab (P) in patients (Pts) with colorectal cancer (CRC) with high tumor mutational burden (HTMB): results from the targeted agent and profiling utilization registry (TAPUR) study. J. Clin. Oncol. 38, 133–133 (2020).
Rousseau, B. et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N. Engl. J. Med. 384, 1168–1170 (2021).
Pages, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Awad, M. M. et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Amodio, V. et al. EGFR blockade reverts resistance to KRAS G12C inhibition in colorectal cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.Cd-20-0187 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04793958 (2021).
Tanaka, N. et al. Clinical acquired resistance to KRAS(G12C) inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov. 11, 1913–1922 (2021).
Wang, X. et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J. Med. Chem. 65, 3123–3133 (2022).
Lenz, H.-J. et al. A phase 1b/2 trial of the PLK1 inhibitor onvansertib in combination with FOLFIRI-bev in 2L treatment of KRAS-mutated (mKRAS) metastatic colorectal carcinoma (mCRC). J. Clin. Oncol. 40, 100 (2022).
Hendifar, A. E. et al. Phase 1b study of RGX-202-01, a first-in-class oral inhibitor of the SLC6A8/CKB pathway, in combination with FOLFIRI and bevacizumab (BEV) in second-line advanced colorectal cancer (CRC). J. Clin. Oncol. 40, 3579–3579 (2022).
Hendifar, A. et al. Phase 1b study of RGX-202-01, a first-in-class oral inhibitor of the SLC6A8/CKB pathway, in combination with FOLFIRI and bevacizumab (BEV) in second-line advanced colorectal cancer (CRC). Ann. Oncol. 33, S294 (2022).
Marchio, C. et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 30, 1417–1427 (2019).
Yoshino, T. et al. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.03.299 (2020).
Pietrantonio, F. et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djx089 (2017).
Cocco, E. et al. Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res. 79, 1047–1053 (2019).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. https://doi.org/10.1016/s1470-2045(19)30856-3 (2020).
Drilon, A. et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 7, 400–409 (2017).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 21, 271–282 (2020).
Seligmann, J. F. et al. Inhibition of WEE1 is effective in TP53- and RAS-mutant metastatic colorectal cancer: a randomized trial (FOCUS4-C) comparing adavosertib (AZD1775) with active monitoring. J. Clin. Oncol. 39, 3705–3715 (2021).
Strickler, J. H. et al. Cabozantinib and panitumumab for RAS wild-type metastatic colorectal cancer. Oncologist 26, 465–e917 (2021).
Pietrantonio, F. et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann. Oncol. 29, 1394–1401 (2018).
Thein, K. Z., Velcheti, V., Mooers, B. H. M., Wu, J. & Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 7, 1074–1088 (2021).
Tie, J. et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N. Engl. J. Med. 386, 2261–2272 (2022).
Taniguchi, H. et al. CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 112, 2915–2920 (2021).
Kann, B. H., Hosny, A. & Aerts, H. Artificial intelligence for clinical oncology. Cancer Cell 39, 916–927 (2021).
Luchini, C., Pea, A. & Scarpa, A. Artificial intelligence in oncology: current applications and future perspectives. Br. J. Cancer https://doi.org/10.1038/s41416-021-01633-1 (2021).
Kawazoe, A. et al. Multicenter phase I/II trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP Trial). Clin. Cancer Res. 26, 5887–5894 (2020).
Corcoran, R. et al. Clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann. Oncol. 31, S226–S227 (2020).
Yaeger, R. et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 21, 1313–1320 (2015).
Ramanathan, R. K. et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 22, 858–865 (2004).
Sartore-Bianchi, A. et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 5, e000911 (2020).
Strickler, J. H. et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann. Oncol. 30, v200 (2019).
Yuan, Y. et al. Dual-targeted therapy with pyrotinib and trastuzumab for HER2-positive advanced colorectal cancer: preliminary results from a multicenter phase 2 trial. J. Clin. Oncol. 39, e15554 (2021).
Clark, J. W. et al. Phase II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzamab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy [Abstract]. Proc. Am. Soc. Clin. Oncol. 22, A-3584 (2003).
Bekaii-Saab, T. S. et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol. Cancer Ther. 8, 2983–2991 (2009).
Meric-Bernstam, F. et al. Zanidatamab a novel bispecific antibody for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1 dose-escalation and expansion study. Lancet Oncol. 23, 1558–1570 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.B. reports research funding from Ono Pharmaceutical and honoraria from Taiho Pharmaceutical and Eli Lilly, Japan. A.O. reports research funding from BMS and honoraria from Chugai and Ono Pharmaceutical. T.Y. reports research funding from Taiho Pharmaceutical, Sumitomo Dainippon Pharma, Ono Pharmaceutical, Chugai Pharmaceutical, Amgen, Parexel International, MSD, Daiichi Sankyo and Sanofi.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Andrea Sartore-Bianchi, Tanios Bekaii-Saab, Daniel Walden and Elena Élez for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
JapicCTI-194709: https://rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-194709
UMIN000031857: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000036377
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bando, H., Ohtsu, A. & Yoshino, T. Therapeutic landscape and future direction of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol 20, 306–322 (2023). https://doi.org/10.1038/s41575-022-00736-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00736-1
This article is cited by
-
Evaluating AI in medicine: a comparative analysis of expert and ChatGPT responses to colorectal cancer questions
Scientific Reports (2024)
-
Future direction of total neoadjuvant therapy for locally advanced rectal cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
PLA-HA/Fe3O4 magnetic nanoparticles loaded with curcumin: physicochemical characterization and toxicity evaluation in HCT116 colorectal cancer cells
Discover Applied Sciences (2024)
-
Inhibition of Aurora B kinase (AURKB) enhances the effectiveness of 5-fluorouracil chemotherapy against colorectal cancer cells
British Journal of Cancer (2024)
-
NEXUS trial: a multicenter phase II clinical study evaluating the efficacy and safety of the perioperative use of encorafenib, binimetinib, and cetuximab in patients with previously untreated surgically resectable BRAF V600E mutant colorectal oligometastases
BMC Cancer (2023)