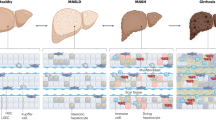
Abstract
Improvements in understanding the pathophysiology of the different benign liver nodules have refined their nosological classification. New criteria have been identified using imaging, histology and molecular analyses for a precise diagnosis of these tumours. Improvement in the classification of liver tumours provides a more accurate prediction of disease progression and has modified patient management. Haemangioma and focal nodular hyperplasia, the most common benign liver tumours that develop in the absence of chronic liver disease, are usually easy to diagnose on imaging and do not require specific treatment. However, hepatocellular adenomas and cirrhotic macronodules can be difficult to discriminate from hepatocellular carcinoma. The molecular subtyping of hepatocellular adenomas in five major subgroups defined by HNF1A inactivation, β-catenin mutation in exon 3 or exon 7/8, and activation of inflammatory or Hedgehog pathways helps to identify the tumours at risk of malignant transformation or bleeding. New clinical, biological and molecular tools have gradually been included in diagnostic and treatment algorithms to classify benign liver tumours and improve patient management. This Review aims to explain the main pathogenic mechanisms of benign liver tumours and how this knowledge could influence clinical practice.
Key points
-
Hepatic haemangioma (HH) and focal nodular hyperplasia (FNH) are frequent benign liver tumours not related to contraception and are mostly diagnosed at imaging even if some FNH require a biopsy to reach a diagnosis.
-
FNH and HH do not require any treatment owing to the absence of complications.
-
Hepatocellular adenoma occurs mostly in young women (20–50 years) who have taken oestrogen-based contraception and could be complicated by tumour bleeding or malignant transformation to hepatocellular carcinoma.
-
Hepatocellular adenomas are divided into molecular subgroups: HNF1A inactivated, inflammatory, CTNNB1 mutations in exon 3 (high risk of transformation), CTNNB1 mutations in exon 7/8 and Sonic Hedgehog activated (high risk of bleeding).
-
Low-grade and high-grade dysplastic nodules are preneoplastic lesions developed in a cirrhotic liver that could progress to hepatocellular carcinoma through the acquisition of somatic mutations in the TERT promoter.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Choi, S. H. et al. Focal hepatic solid lesions incidentally detected on initial ultrasonography in 542 asymptomatic patients. Abdom. Radiol. 41, 265–272 (2016).
Kaltenbach, T. E.-M. et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom. Radiol. 41, 25–32 (2016).
Nault, J.-C., Bioulac-Sage, P. & Zucman-Rossi, J. Hepatocellular benign tumors — from molecular classification to personalized clinical care. Gastroenterology 144, 888–902 (2013).
Rooks, J. B. et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. J. Am. Med. Assoc. 242, 644–648 (1979). A study demonstrating the role of oestrogen-based oral contraception in the occurrence of hepatocellular adenomas in the 1970s.
Di Tommaso, L. et al. Advanced precancerous lesions in the liver. Best Pract. Res. Clin. Gastroenterol. 27, 269–284 (2013).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of benign liver tumours. J. Hepatol. 65, 386–398 (2016). The only international guidelines available on the diagnosis and treatment of benign liver tumours, including hepatic haemangioma, FNH and HCA.
Hoekstra, L. T. et al. Management of giant liver hemangiomas: an update. Expert Rev. Gastroenterol. Hepatol. 7, 263–268 (2013).
Gandolfi, L. et al. Natural history of hepatic haemangiomas: clinical and ultrasound study. Gut 32, 677–680 (1991).
Baranes, L. et al. Imaging benign hepatocellular tumors: atypical forms and diagnostic traps. Diagn. Interv. Imaging 94, 677–695 (2013).
Ridge, C. A., Shia, J., Gerst, S. R. & Do, R. K. G. Sclerosed hemangioma of the liver: concordance of MRI features with histologic characteristics. J. Magn. Reson. Imaging 39, 812–818 (2014).
Gill, R. M. et al. Hepatic small vessel neoplasm, a rare infiltrative vascular neoplasm of uncertain malignant potential. Hum. Pathol. 54, 143–151 (2016). The first description of hepatic small vessel neoplasm in the literature, an entity different from hepatic haemangioma and hepatic haemangioendothelioma.
Joseph, N. M. et al. Frequent GNAQ and GNA14 mutations in hepatic small vessel neoplasm. Am. J. Surg. Pathol. 42, 1201–1207 (2018).
Paisant, A. et al. Imaging features of hepatic small vessel neoplasm: case series. Hepatology 74, 2894–2896 (2021).
Toro, A. et al. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann. Hepatol. 13, 327–339 (2014).
Rajakannu, M. et al. Revisiting the surgical management of giant hepatic hemangiomas: enucleation versus anatomical resection? J. Clin. Exp. Hepatol. 11, 321–326 (2021).
Torkian, P., Li, J., Kaufman, J. A. & Jahangiri, Y. Effectiveness of transarterial embolization in treatment of symptomatic hepatic hemangiomas: systematic review and meta-analysis. Cardiovasc. Interv. Radiol. 44, 80–91 (2021).
Zavras, N., Dimopoulou, A., Machairas, N., Paspala, A. & Vaos, G. Infantile hepatic hemangioma: current state of the art, controversies, and perspectives. Eur. J. Pediatr. 179, 1–8 (2020).
Krowchuk, D. P. et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics 143, e20183475 (2019).
Fukukura, Y., Nakashima, O., Kusaba, A., Kage, M. & Kojiro, M. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J. Hepatol. 29, 470–475 (1998).
Mathieu, D., Kobeiter, H., Cherqui, D., Rahmouni, A. & Dhumeaux, D. Oral contraceptive intake in women with focal nodular hyperplasia of the liver. Lancet 352, 1679–1680 (1998). A study demonstrating that oestrogen-based oral contraception has no effect on the risk of developing FNH.
Mathieu, D. et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology 118, 560–564 (2000).
Rifai, K. et al. No evidence of substantial growth progression or complications of large focal nodular hyperplasia during pregnancy. Scand. J. Gastroenterol. 48, 88–92 (2013).
Sempoux, C. et al. Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd– Chiari syndrome and other rare hepatic vascular disorders. J. Hepatol. 63, 1173–1180 (2015).
Buscarini, E. et al. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med. Biol. 30, 1089–1097 (2004).
Furlan, A. et al. Focal nodular hyperplasia after treatment with oxaliplatin: a multiinstitutional series of cases diagnosed at MRI. Am. J. Roentgenol. 210, 775–779 (2018).
Scoazec, J. Y. et al. Focal nodular hyperplasia of the liver: composition of the extracellular matrix and expression of cell-cell and cell-matrix adhesion molecules. Hum. Pathol. 26, 1114–1125 (1995).
Rebouissou, S. et al. The beta-catenin pathway is activated in focal nodular hyperplasia but not in cirrhotic FNH-like nodules. J. Hepatol. 49, 61–71 (2008).
Paradis, V., Laurent, A., Flejou, J. F., Vidaud, M. & Bedossa, P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology 26, 891–895 (1997).
Wanless, I. R., Mawdsley, C. & Adams, R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology 5, 1194–1200 (1985).
Paradis, V. et al. A quantitative gene expression study suggests a role for angiopoietins in focal nodular hyperplasia. Gastroenterology 124, 651–659 (2003).
Gouw, A. S. H. et al. Molecular characterization of the vascular features of focal nodular hyperplasia and hepatocellular adenoma: a role for angiopoietin-1. Hepatology 52, 540–549 (2010).
Vilgrain, V. et al. Benign and malignant hepatocellular lesions in patients with vascular liver diseases. Abdom. Radiol. 43, 1968–1977 (2018).
van Rosmalen, B. V. et al. Long-term outcomes of resection in patients with symptomatic benign liver tumours. HPB (Oxford) 18, 908–914 (2016).
Cherqui, D. et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology 22, 1674–1681 (1995).
Tselikas, L. et al. Impact of hepatobiliary phase liver MRI versus contrast-enhanced ultrasound after an inconclusive extracellular gadolinium-based contrast-enhanced MRI for the diagnosis of benign hepatocellular tumors. Abdom. Radiol. 42, 825–832 (2016).
Fabre, A. et al. Histologic scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology 35, 414–420 (2002).
Bertin, C. et al. Contrast-enhanced ultrasound of focal nodular hyperplasia: a matter of size. Eur. Radiol. 24, 2561–2571 (2014).
Grazioli, L., Morana, G., Kirchin, M. A. & Schneider, G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology 236, 166–177 (2005).
Bioulac-Sage, P. et al. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int. 29, 459–465 (2009).
Bioulac-Sage, P. et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am. J. Surg. Pathol. 36, 1691–1699 (2012).
Ronot, M. et al. MR findings of steatotic focal nodular hyperplasia and comparison with other fatty tumours. Eur. Radiol. 23, 914–923 (2013).
Birn, J. et al. Transarterial embolization of symptomatic focal nodular hyperplasia. J. Vasc. Interv. Radiol. 24, 1647–1655 (2013).
Ben Ismail, I., Zenaidi, H., Jouini, R., Rebii, S. & Zoghlami, A. Pedunculated hepatic focal nodular hyperplasia: a case report and review of the literature. Clin. Case Rep. 9, e04202 (2021).
Bröker, M. E. E. et al. Growth of focal nodular hyperplasia is not a reason for surgical intervention, but patients should be referred to a tertiary referral centre. World J. Surg. 42, 1506–1513 (2018).
Edmondson, H. A., Henderson, B. & Benton, B. Liver-cell adenomas associated with use of oral contraceptives. N. Engl. J. Med. 294, 470–472 (1976).
Flejou, J. F. et al. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology 89, 1132–1138 (1985). A study describing liver adenomatosis, a rare entity characterized by the presence of >10 HCAs.
Lepreux, S. et al. The identification of small nodules in liver adenomatosis. J. Hepatol. 39, 77–85 (2003).
Sempoux, C. et al. Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd–Chiari syndrome and other rare hepatic vascular disorders. J. Hepatol. 63, 1173–1180 (2015).
Labrune, P., Trioche, P., Duvaltier, I., Chevalier, P. & Odievre, M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J. Pediatr. Gastroenterol. Nutr. 24, 276–279 (1997).
Bacq, Y. et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology 125, 1470–1475 (2003). In this study, the authors show that familial liver adenomatosis is due to a HNF1A germline mutation and is associated with MODY3 diabetes.
Sasaki, M., Yoneda, N., Kitamura, S., Sato, Y. & Nakanuma, Y. Characterization of hepatocellular adenoma based on the phenotypic classification: the Kanazawa experience. Hepatol. Res. 41, 982–988 (2011).
Laurent, C., Trillaud, H., Lepreux, S., Balabaud, C. & Bioulac-Sage, P. Association of adenoma and focal nodular hyperplasia: experience of a single French academic center. Comp. Hepatol. 2, 6 (2003).
Pilati, C. et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell 25, 428–441 (2014).
Zucman-Rossi, J. et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 43, 515–524 (2006). A pivotal study demonstrating the genotype–phenotype correlation in HCA and the link between the presence of CTNNB1 exon 3 mutations and the risk of malignant transformation.
Nault, J.-C. et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology 152, 880–894.e6 (2017). The largest cohort demonstrating the correlation between molecular subclasses, risk factors and clinical behaviour; a new subclass of HCA characterized by activation of the Hedgehog pathway is described.
Bioulac-Sage, P. et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 46, 740–748 (2007).
van Aalten, S. M. et al. Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J. Hepatol. 55, 120–125 (2011).
Bellamy, C. O. C. et al. The value of immunophenotyping hepatocellular adenomas: consecutive resections at one UK centre. Histopathology 62, 431–445 (2013).
Odom, D. T. et al. Control of pancreas and liver gene expression by HNF transcription factors. Science 303, 1378–1381 (2004).
Bluteau, O. et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat. Genet. 32, 312–315 (2002).
Haddouche, A. et al. Liver adenomatosis in patients with hepatocyte nuclear factor-1 alpha maturity onset diabetes of the young (HNF1A-MODY): clinical, radiological and pathological characteristics in a French series. J. Diabetes 12, 48–57 (2020).
Pelletier, L. et al. Loss of hepatocyte nuclear factor 1alpha function in human hepatocellular adenomas leads to aberrant activation of signaling pathways involved in tumorigenesis. Hepatology 51, 557–566 (2010).
Rebouissou, S. et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J. Biol. Chem. 282, 14437–14446 (2007).
Laumonier, H. et al. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 48, 808–818 (2008). This study shows that imaging features by MRI could be useful for the non-invasive diagnosis of HNF1A-mutated and IHCAs.
Ronot, M. et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53, 1182–1191 (2011).
Rebouissou, S. et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457, 200–204 (2009).
Pilati, C. et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 208, 1359–1366 (2011).
Nault, J.-C. et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J. Hepatol. 56, 184–191 (2012).
Bayard, Q. et al. Recurrent chromosomal rearrangements of ROS1, FRK and IL6 activating JAK/STAT pathway in inflammatory hepatocellular adenomas. Gut 69, 1667–1676 (2020).
Paradis, V. et al. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology 46, 140–146 (2007).
Sa Cunha, A. et al. Inflammatory syndrome with liver adenomatosis: the beneficial effects of surgical management. Gut 56, 307–309 (2007).
Calderaro, J. et al. Systemic AA amyloidosis caused by inflammatory hepatocellular adenoma. N. Engl. J. Med. 379, 1178–1180 (2018).
Calderaro, J. et al. Inflammatory hepatocellular adenomas developed in the setting of chronic liver disease and cirrhosis. Mod. Pathol. 29, 43–50 (2016).
Dokmak, S. & Belghiti, J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. 35, 2228–2232 (2015).
Vernuccio, F. et al. Long-term evolution of hepatocellular adenomas at MRI follow-up. Radiology 292, 361–372 (2020).
Klompenhouwer, A. J. et al. Development and validation of a model to predict regression of large size hepatocellular adenoma. Am. J. Gastroenterol. 114, 1292–1298 (2019).
Poussin, K. et al. Biochemical and functional analyses of gp130 mutants unveil JAK1 as a novel therapeutic target in human inflammatory hepatocellular adenoma. Oncoimmunology 2, e27090 (2013).
Chen, Y. W., Jeng, Y. M., Yeh, S. H. & Chen, P. J. P53 gene and Wnt signaling in benign neoplasms: beta-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology 36, 927–935 (2002).
Evason, K. J., Grenert, J. P., Ferrell, L. D. & Kakar, S. Atypical hepatocellular adenoma-like neoplasms with beta-catenin activation show cytogenetic alterations similar to well-differentiated hepatocellular carcinomas. Hum. Pathol. 44, 750–758 (2013).
Svrcek, M. et al. Regressive liver adenomatosis following androgenic progestin therapy withdrawal: a case report with a 10-year follow-up and a molecular analysis. Eur. J. Endocrinol. 156, 617–621 (2007).
Van der Borght, S. et al. Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology 51, 855–866 (2007).
Rebouissou, S. et al. Genotype-phenotype correlation of CTNNB1 mutations reveals different β-catenin activity associated with liver tumor progression. Hepatology 64, 2047–2061 (2016).
Reizine, E. et al. Hepatospecific MR contrast agent uptake on hepatobiliary phase can be used as a biomarker of marked β-catenin activation in hepatocellular adenoma. Eur. Radiol. 31, 3417–3426 (2021).
Henriet, E. et al. Argininosuccinate synthase 1 (ASS1): a marker of unclassified hepatocellular adenoma and high bleeding risk. Hepatology 66, 2016–2028 (2017).
Bieze, M., Phoa, S. S., Verheij, J., van Lienden, K. P. & van Gulik, T. M. Risk factors for bleeding in hepatocellular adenoma. Br. J. Surg. 101, 847–855 (2014).
van Aalten, S. M., de Man, R. A. I., Jzermans, J. N. & Terkivatan, T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br. J. Surg. 99, 911–916 (2012). A large analysis of the literature assessing the frequency of bleeding and the risk factors predicting this complication in patients with HCA.
Dokmak, S. et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137, 1698–1705 (2009).
Farges, O. et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut 60, 85–89 (2011).
Silva, T. S., Sung, M., Nelson, D. W., DiFronzo, A. L. & O’Connor, V. V. A multicenter, 10-year experience with hepatocellular adenoma: risk factors and disease course. Am. Surg. https://doi.org/10.1177/00031348211011084 (2021).
Laurent, A. et al. European experience of 573 liver resections for hepatocellular adenoma: a cross-sectional study by the AFC-HCA-2013 study group. HPB (Oxford) 18, 748–755 (2016).
Hirsch, T. Z. et al. Integrated genomic analysis identifies driver genes and cisplatin-resistant progenitor phenotype in pediatric liver cancer. Cancer Discov. 11, 2524–2543 (2021).
Nault, J.-C. et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 4, 2218 (2013). A study identifying TERT promoter mutations as the most frequent somatic genetic alterations in HCC; it also demonstrates that TERT promoter mutations are involved in the malignant transformation of HCA together with CTNNB1 exon 3 mutations.
Sempoux, C. et al. Malignant transformation of a β-catenin inflammatory adenoma due to an S45 β-catenin-activating mutation present 12 years before. Hum. Pathol. 62, 122–125 (2017).
Bedossa, P. et al. Well-differentiated hepatocellular neoplasm of uncertain malignant potential: proposal for a new diagnostic category. Hum. Pathol. 45, 658–660 (2014).
Barbier, L. et al. Natural history of liver adenomatosis: a long-term observational study. J. Hepatol. 71, 1184–1192 (2019).
Klompenhouwer, A. J. et al. Retrospective study on timing of resection of hepatocellular adenoma. Br. J. Surg. 104, 1695–1703 (2017).
van Rosmalen, B. V. et al. Hepatocellular adenoma in men: a nationwide assessment of pathology and correlation with clinical course. Liver Int. 41, 2474–2484 (2021).
Dokmak, S. et al. Hemorrhage of hepatocellular adenoma: a complication that can be treated by conservative management without surgery. HPB 20, 1198–1205 (2018).
Bioulac-Sage, P. et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50, 481–489 (2009).
Klompenhouwer, A. J. et al. Evidence of good prognosis of hepatocellular adenoma in post-menopausal women. J. Hepatol. 65, 1163–1170 (2016).
de’Angelis, N. et al. Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB 16, 783–788 (2014).
Gaspersz, M. P. et al. Growth of hepatocellular adenoma during pregnancy: a prospective study. J. Hepatol. 72, 119–124 (2020). A prospective study demonstrating that pregnancy is safe in women with HCAs of <5 cm.
van Rosmalen, B. V. et al. Safety and efficacy of transarterial embolization of hepatocellular adenomas. Br. J. Surg. 106, 1362–1371 (2019).
van Vledder, M. G. et al. Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J. Vasc. Int. Radiol. 22, 787–793 (2011).
Chiche, L. et al. Liver transplantation for adenomatosis: European experience. Liver Transpl. 22, 516–526 (2016).
Brunner, S. F. et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature 574, 538–542 (2019).
Zhu, M. et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic liver disease. Cell 177, 608–621.e12 (2019).
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49, 658–664 (2009).
Nault, J.-C. et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 60, 1983–1992 (2014).
Torrecilla, S. et al. Trunk mutational events present minimal intra- and inter-tumoral heterogeneity in hepatocellular carcinoma. J. Hepatol. 67, 1222–1231 (2017).
Marquardt, J. U. et al. Sequential transcriptome analysis of human liver cancer indicates late stage acquisition of malignant traits. J. Hepatol. 60, 346–353 (2014).
Llovet, J. M. et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 131, 1758–1767 (2006).
Nault, J.-C., Ningarhari, M., Rebouissou, S. & Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 16, 544–558 (2019).
Xiong, J., Luo, J., Bian, J. & Wu, J. Overall diagnostic accuracy of different MR imaging sequences for detection of dysplastic nodules: a systematic review and meta-analysis. Eur. Radiol. 32, 1285–1296 (2022).
Forner, A. et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 47, 97–104 (2008).
Di Tommaso, L. et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 45, 725–734 (2007).
Borzio, M. et al. Impact of large regenerative, low grade and high grade dysplastic nodules in hepatocellular carcinoma development. J. Hepatol. 39, 208–214 (2003). A study analysing the risk of transformation in HCC of premalignant nodules (regenerative, LGDN and HGDN) in patients with cirrhosis.
Terasaki, S., Kaneko, S., Kobayashi, K., Nonomura, A. & Nakanuma, Y. Histological features predicting malignant transformation of nonmalignant hepatocellular nodules: a prospective study. Gastroenterology 115, 1216–1222 (1998).
Sato, T. et al. Natural history of large regenerative nodules and dysplastic nodules in liver cirrhosis: 28-year follow-up study. Hepatol. Int. 9, 330–336 (2015).
Roux, M. et al. Differentiating focal nodular hyperplasia from hepatocellular adenoma: is hepatobiliary phase MRI (HBP-MRI) using linear gadolinium chelates always useful? Abdom. Radiol. 43, 1670–1681 (2018).
Acknowledgements
J.-C.N. and J.Z.-R. are funded by the INCA PRENEO grant (PREMALHEP).
Review criteria
We searched MEDLINE with the terms “hepatic haemangioma”, “liver haemangioma”, “hepatic angioma”, “liver angioma”, “focal nodular hyperplasia”, “cirrhotic nodule”, “cirrhotic premalignant lesion”, “liver premalignant lesion”, “hepatic adenoma”, “hepatocellular adenoma”, “liver adenoma” and “benign liver tumour”, for articles published from 1970 to 2022. In addition, we selected the most relevant clinical trials, systematic reviews and high-quality review articles. We also manually searched reference lists of identified articles to retrieve additional studies.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests in the field of benign hepatocellular tumours.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Jan Ijzermans, who co-reviewed with Julia Klompenhouwer; Massimo Colombo; Alejandro Forner; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nault, JC., Paradis, V., Ronot, M. et al. Benign liver tumours: understanding molecular physiology to adapt clinical management. Nat Rev Gastroenterol Hepatol 19, 703–716 (2022). https://doi.org/10.1038/s41575-022-00643-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00643-5
This article is cited by
-
Benigne solide Lebertumoren
Die Chirurgie (2023)