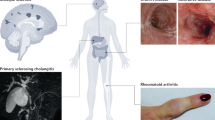
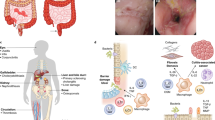
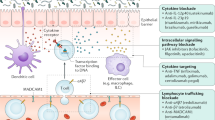
Abstract
Immune cell trafficking is a critical element of the intestinal immune response, both in homeostasis and in pathological conditions associated with inflammatory bowel disease (IBD). This process involves adhesion molecules, chemoattractants and receptors expressed on immune cell surfaces, blood vessels and stromal intestinal tissue as well as signalling pathways, including those modulated by sphingosine 1-phosphate (S1P). The complex biological processes of leukocyte recruitment, activation, adhesion and migration have been targeted by various monoclonal antibodies (vedolizumab, etrolizumab, ontamalimab). Promising preclinical and clinical data with several oral S1P modulators suggest that inhibition of lymphocyte egress from the lymph nodes to the bloodstream might be a safe and efficacious alternative mechanism for reducing inflammation in immune-mediated disorders, including Crohn’s disease and ulcerative colitis. Although various questions remain, including the potential positioning of S1P modulators in treatment algorithms and their long-term safety, this novel class of compounds holds great promise. This Review summarizes the critical mediators and mechanisms involved in immune cell trafficking in IBD and the available evidence for efficacy, safety and pharmacokinetics of S1P receptor modulators in IBD and other immune-mediated disorders. Further, it discusses potential future approaches to incorporate S1P modulators into the treatment of IBD.
Key points
-
Sphingosine 1-phosphate (S1P) is a pleiotropic and widely expressed bioactive molecule belonging to the sphingolipid family that binds to five receptors (found on numerous cell types) with varying affinities.
-
Oral S1P modulators have been tested in multiple immune-mediated disorders.
-
Cardiotoxicity of the non-selective S1P agonist fingolimod has led to the development of more selective compounds, including ozanimod and etrasimod.
-
Ozanimod and etrasimod have been shown to be safe and efficacious for the treatment of patients with inflammatory bowel disease.
-
Future treatment approaches including S1P modulators should be explored, such as combination therapy with approved biologic agents, as well as the identification of predictive biomarkers to facilitate personalized therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
07 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41575-022-00597-8
References
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Torres, J. et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J. Crohns Colitis 14, 4–22 (2020).
Sazonovs, A. et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 158, 189–199 (2020).
Kirchgesner, J. et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 155, 337–346 (2018).
Sandborn, W. J. New targets for small molecules in inflammatory bowel disease. Gastroenterol. Hepatol. 11, 338–340 (2015).
Olivera, P., Danese, S. & Peyrin-Biroulet, L. Next generation of small molecules in inflammatory bowel disease. Gut 66, 199–209 (2017).
Wiendl, M. et al. Targeting immune cell trafficking- insights from research models and implications for future IBD therapy. Front. Immunol. 12, 656452 (2021).
Panes, J. & Salas, A. Past, present and future of therapeutic interventions targeting leukocyte trafficking in inflammatory bowel disease. J. Crohns Colitis 12, S633–S640 (2018).
Pachynski, R. K., Wu, S. W., Gunn, M. D. & Erle, D. J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 161, 952–956 (1998).
Phan, U. T., Waldron, T. T. & Springer, T. A. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat. Immunol. 7, 883–889 (2006).
Salas, A. et al. Rolling adhesion through an extended conformation of integrin αLβ2 and relation to α I and β I-like domain interaction. Immunity 20, 393–406 (2004).
Sigal, A. et al. The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment. J. Immunol. 165, 442–452 (2000).
Trivedi, P. J. & Adams, D. H. Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; pitfalls and promise. J. Crohns Colitis 12, S641–S652 (2018).
Luster, A. D. Chemokines–chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338, 436–445 (1998).
Ajuebor, M. N. & Swain, M. G. Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology 105, 137–143 (2002).
Raab, Y., Gerdin, B., Ahlstedt, S. & Hallgren, R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut 34, 1203–1206 (1993).
Walana, W. et al. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed. Pharmacother. 103, 253–261 (2018).
Feng, N. et al. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J. Immunol. 176, 5749–5759 (2006).
Stenstad, H. et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 107, 3447–3454 (2006).
Svensson, M. et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121 (2002).
Wendland, M. et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl Acad. Sci. USA 104, 6347–6352 (2007).
Parmo-Cabanas, M. et al. Intracellular signaling required for CCL25-stimulated T cell adhesion mediated by the integrin α4β1. J. Leukoc. Biol. 82, 380–391 (2007).
Miles, A., Liaskou, E., Eksteen, B., Lalor, P. F. & Adams, D. H. CCL25 and CCL28 promote α4β7-integrin-dependent adhesion of lymphocytes to MAdCAM-1 under shear flow. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1257–G1267 (2008).
Eksteen, B., Liaskou, E. & Adams, D. H. Lymphocyte homing and its role in the pathogenesis of IBD. Inflamm. Bowel Dis. 14, 1298–1312 (2008).
Ostvik, A. E. et al. Enhanced expression of CXCL10 in inflammatory bowel disease: potential role of mucosal Toll-like receptor 3 stimulation. Inflamm. Bowel Dis. 19, 265–274 (2013).
Uguccioni, M. et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am. J. Pathol. 155, 331–336 (1999).
Annunziato, F. et al. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J. Leukoc. Biol. 65, 691–699 (1999).
Shao, L., Serrano, D. & Mayer, L. The role of epithelial cells in immune regulation in the gut. Semin. Immunol. 13, 163–176 (2001).
Skovdahl, H. K. et al. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. PLoS ONE 10, e0141710 (2015).
Kaser, A. et al. Increased expression of CCL20 in human inflammatory bowel disease. J. Clin. Immunol. 24, 74–85 (2004).
Sugiura, Y. et al. TLR1-induced chemokine production is critical for mucosal immunity against Yersinia enterocolitica. Mucosal Immunol. 6, 1101–1109 (2013).
Yamazaki, T. et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 181, 8391–8401 (2008).
Calderon-Gomez, E. et al. Commensal-specific CD4+ cells from patients with Crohn’s disease have a T-helper 17 inflammatory profile. Gastroenterology 151, 489–500 (2016).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Sallusto, F. & Baggiolini, M. Chemokines and leukocyte traffic. Nat. Immunol. 9, 949–952 (2008).
Shattil, S. J., Kim, C. & Ginsberg, M. H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 (2010).
Arseneau, K. O. & Cominelli, F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin. Pharmacol. Ther. 97, 22–28 (2015).
Rivera-Nieves, J. Strategies that target leukocyte traffic in inflammatory bowel diseases: recent developments. Curr. Opin. Gastroenterol. 31, 441–448 (2015).
Wong, M. T. et al. A high-dimensional atlas of human T cell diversity reveals tissue-specific trafficking and cytokine signatures. Immunity 45, 442–456 (2016).
Cerf-Bensussan, N. et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur. J. Immunol. 17, 1279–1285 (1987).
Rott, L. S., Briskin, M. J., Andrew, D. P., Berg, E. L. & Butcher, E. C. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J. Immunol. 156, 3727–3736 (1996).
Meenan, J. et al. Altered expression of α4β7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut 40, 241–246 (1997).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Zundler, S. et al. The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm. Bowel Dis. 23, 379–391 (2017).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Panes, J., Perry, M. & Granger, D. N. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br. J. Pharmacol. 126, 537–550 (1999).
Hyun, Y. M., Choe, Y. H., Park, S. A. & Kim, M. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp. Mol. Med. 51, 1–13 (2019).
Yoshida, N. et al. Role of P-selectin and intercellular adhesion molecule-1 in TNB-induced colitis in rats. Digestion 63 (Suppl. 1), 81–86 (2001).
Soriano, A. et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab. Invest. 80, 1541–1551 (2000).
Briskin, M. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Souza, H. S., Elia, C. C., Spencer, J. & MacDonald, T. T. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut 45, 856–863 (1999).
Ala, A., Dhillon, A. P. & Hodgson, H. J. Role of cell adhesion molecules in leukocyte recruitment in the liver and gut. Int. J. Exp. Pathol. 84, 1–16 (2003).
Butcher, E. C., Williams, M., Youngman, K., Rott, L. & Briskin, M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72, 209–253 (1999).
Perez-Jeldres, T., Alvarez-Lobos, M. & Rivera-Nieves, J. Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs 81, 985–1002 (2021).
Kiuchi, M. et al. Synthesis and biological evaluation of 2,2-disubstituted 2-aminoethanols: analogues of FTY720. Bioorg. Med. Chem. Lett. 8, 101–106 (1998).
Watterson, K. R. et al. Dual regulation of EDG1/S1P1 receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J. Biol. Chem. 277, 5767–5777 (2002).
Pham, T. H., Okada, T., Matloubian, M., Lo, C. G. & Cyster, J. G. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008).
Grigorova, I. L. et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 10, 58–65 (2009).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Holthuis, J. C., Pomorski, T., Raggers, R. J., Sprong, H. & Van Meer, G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 81, 1689–1723 (2001).
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 (2003).
Gault, C. R., Obeid, L. M. & Hannun, Y. A. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 688, 1–23 (2010).
Chalfant, C. E. & Spiegel, S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell Sci. 118, 4605–4612 (2005).
Mizugishi, K. et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 25, 11113–11121 (2005).
Serra, M. & Saba, J. D. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzym. Regul. 50, 349–362 (2010).
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Ito, K. et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 357, 212–217 (2007).
Venkataraman, K. et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676 (2008).
Vu, T. M. et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550, 524–528 (2017).
Spiegel, S. & Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 (2011).
Rodriguez, Y. I. et al. Sphingosine-1 phosphate: a new modulator of immune plasticity in the tumor microenvironment. Front. Oncol. 6, 218 (2016).
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Schwab, S. R. & Cyster, J. G. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8, 1295–1301 (2007).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Massberg, S. et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008 (2007).
Hla, T., Galvani, S., Rafii, S. & Nachman, R. S1P and the birth of platelets. J. Exp. Med. 209, 2137–2140 (2012).
Zhang, L. et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J. Exp. Med. 209, 2165–2181 (2012).
Murata, N. et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352, 809–815 (2000).
Christoffersen, C. et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl Acad. Sci. USA 108, 9613–9618 (2011).
Takabe, K. et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 285, 10477–10486 (2010).
Goetzl, E. J. & Rosen, H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J. Clin. Invest. 114, 1531–1537 (2004).
O’Sullivan, C. & Dev, K. K. The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 34, 401–412 (2013).
Jolly, P. S. et al. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 199, 959–970 (2004).
Hla, T. & Maciag, T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 265, 9308–9313 (1990).
Petti, L. et al. Unveiling role of sphingosine-1-phosphate receptor 2 as a brake of epithelial stem cell proliferation and a tumor suppressor in colorectal cancer. J. Exp. Clin. Cancer Res. 39, 253 (2020).
Lee, H. et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat. Med. 16, 1421–1428 (2010).
Li, C. et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 50, 1174–1183 (2009).
Jung, B. et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 (2012).
Lee, M. J. et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 (1999).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Lo, C. G., Xu, Y., Proia, R. L. & Cyster, J. G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 (2005).
Liu, C. H. et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 (1999).
Czeloth, N., Bernhardt, G., Hofmann, F., Genth, H. & Forster, R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175, 2960–2967 (2005).
Walzer, T. et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8, 1337–1344 (2007).
Carlson, C. M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Garris, C. S. et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14, 1166–1172 (2013).
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat. Immunol. 11, 1047–1056 (2010).
Kluk, M. J. & Hla, T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 1582, 72–80 (2002).
Duong, C. Q. et al. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim. Biophys. Acta 1682, 112–119 (2004).
Shatrov, V. A., Lehmann, V. & Chouaib, S. Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-κB in U937 cells. Biochem. Biophys. Res. Commun. 234, 121–124 (1997).
Gude, D. R. et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 22, 2629–2638 (2008).
Bryan, A. M. & Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell. Microbiol. 20, e12836 (2018).
Takabe, K., Paugh, S. W., Milstien, S. & Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60, 181–195 (2008).
Mendelson, K., Evans, T. & Hla, T. Sphingosine 1-phosphate signalling. Development 141, 5–9 (2014).
Kimura, T. et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 348, 71–76 (2000).
Rutherford, C. et al. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 4, e927 (2013).
Singleton, P. A., Dudek, S. M., Chiang, E. T. & Garcia, J. G. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and α-actinin. FASEB J. 19, 1646–1656 (2005).
Lee, M. J. et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8, 693–704 (2001).
Siehler, S. & Manning, D. R. Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim. Biophys. Acta 1582, 94–99 (2002).
Sugimoto, N., Takuwa, N., Okamoto, H., Sakurada, S. & Takuwa, Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol. Cell Biol. 23, 1534–1545 (2003).
Sato, K. et al. Activation of phospholipase C-Ca2+ system by sphingosine 1-phosphate in CHO cells transfected with Edg-3, a putative lipid receptor. FEBS Lett. 443, 25–30 (1999).
Gonda, K. et al. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 337, 67–75 (1999).
Graler, M. H. et al. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 89, 507–519 (2003).
Van Brocklyn, J. R. et al. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood 95, 2624–2629 (2000).
Novgorodov, A. S., El-Alwani, M., Bielawski, J., Obeid, L. M. & Gudz, T. I. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 21, 1503–1514 (2007).
Yamazaki, Y. et al. Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca2+ signaling pathway. Biochem. Biophys. Res. Commun. 268, 583–589 (2000).
Sit, S. T. & Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 124, 679–683 (2011).
Olesch, C., Ringel, C., Brune, B. & Weigert, A. Beyond immune cell migration: the emerging role of the sphingosine-1-phosphate receptor S1PR4 as a modulator of innate immune cell activation. Mediators Inflamm. https://doi.org/10.1155/2017/6059203 (2017).
Olesch, C. et al. S1PR4 ablation reduces tumor growth and improves chemotherapy via CD8+ T cell expansion. J. Clin. Invest. 130, 5461–5476 (2020).
Chun, J. & Hartung, H. P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 (2010).
Cohen, J. A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Foster, C. A. et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J. Pharmacol. Exp. Ther. 323, 469–475 (2007).
Coelho, R. P., Payne, S. G., Bittman, R., Spiegel, S. & Sato-Bigbee, C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J. Pharmacol. Exp. Ther. 323, 626–635 (2007).
Cohen, J. A. & Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 69, 759–777 (2011).
Choi, J. W. et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl Acad. Sci. USA 108, 751–756 (2011).
Vargas, W. S. & Perumal, J. S. Fingolimod and cardiac risk: latest findings and clinical implications. Ther. Adv. Drug Saf. 4, 119–124 (2013).
Derfuss, T. et al. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 19, 336–347 (2020).
Selmaj, K. et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 12, 756–767 (2013).
Kappos, L. et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391, 1263–1273 (2018).
Cohen, J. A. et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 15, 373–381 (2016).
Cohen, J. A. et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 18, 1021–1033 (2019).
Comi, G. et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 18, 1009–1020 (2019).
Kappos, L. et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol. 78, 558–567 (2021).
Vaclavkova, A. et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 384, 2036–2045 (2014).
Karuppuchamy, T. et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 10, 162–171 (2017).
Mizushima, T. et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm. Bowel Dis. 10, 182–192 (2004).
Daniel, C. et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 178, 2458–2468 (2007).
Deguchi, Y. et al. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol. Rep. 16, 699–703 (2006).
Scott, F. L. et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 173, 1778–1792 (2016).
Al-Shamma, H. et al. The selective sphingosine 1-phosphate receptor modulator etrasimod regulates lymphocyte trafficking and alleviates experimental colitis. J. Pharmacol. Exp. Ther. 369, 311–317 (2019).
Shimano, K. et al. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 functional antagonist, inhibits progress of chronic colitis induced by transfer of CD4+CD45RBhigh T cells. PLoS ONE 14, e0226154 (2019).
Sandborn, W. J. et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N. Engl. J. Med. 374, 1754–1762 (2016).
Sandborn, W. J. et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 385, 1280–1291 (2021).
Feagan, B. G. et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 5, 819–828 (2020).
Tran, J. Q. et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor agonist. Mult. Scler. J. 25, 524–525 (2019).
Surapaneni, S. et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor modulator. Drug Metab. Dispos. 49, 405–419 (2021).
Tran, J. Q. et al. Single-dose pharmacokinetics of ozanimod and its major active metabolites alone and in combination with gemfibrozil, itraconazole, or rifampin in healthy subjects: a randomized, parallel-group, open-label study. Adv. Ther. 37, 4381–4395 (2020).
Tran, J. Q. et al. Multiple-dose pharmacokinetics of ozanimod and its major active metabolites and the pharmacodynamic and pharmacokinetic interactions with pseudoephedrine, a sympathomimetic agent, in healthy subjects. Adv. Ther. 37, 4944–4958 (2020).
Tran, J. Q., Hartung, J. P., Tompkins, C. A. & Frohna, P. A. Effects of high- and low-fat meals on the pharmacokinetics of ozanimod, a novel sphingosine-1-phosphate receptor modulator. Clin. Pharmacol. Drug Dev. 7, 634–640 (2018).
Sandborn, W. J. et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology 158, 550–561 (2020).
Lee, C. A. et al. Mass balance, metabolic disposition, and pharmacokinetics of [14C] etrasimod following oral administration to healthy male volunteers (American Association of Pharmaceutical Scientists, 2019).
Lee, C. A. et al. P396 Pharmacokinetics and circulating total lymphocyte count pharmacodynamic response from single and multiple oral doses of etrasimod in Japanese and Caucasian healthy male subjects. J. Crohns Colitis 14, S368–S368 (2020).
Peyrin-Biroulet, L., Christopher, R., Behan, D. & Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 16, 495–503 (2017).
D’Haens, G. R., Danese, S., Davies, M., Watanabe, M. & Hibi, T. DOP48 Amiselimod, a selective S1P receptor modulator in Crohn’s disease patients: a proof-of-concept study. J. Crohns Colitis 14, S055–S056 (2019).
Sugahara, K. et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br. J. Pharmacol. 174, 15–27 (2017).
Kifuji, T. et al. Absorption, disposition and metabolic pathway of amiselimod (MT-1303) in healthy volunteers in a mass balance study. Xenobiotica 49, 1033–1043 (2019).
Radeke, H. H. et al. A multicentre, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy, safety, and tolerability of the S1P receptor agonist KRP203 in patients with moderately active refractory ulcerative colitis. Inflamm. Intest. Dis. 5, 180–190 (2020).
Forrest, M. et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J. Pharmacol. Exp. Ther. 309, 758–768 (2004).
Mazurais, D. et al. Cell type-specific localization of human cardiac S1P receptors. J. Histochem. Cytochem. 50, 661–670 (2002).
Siarey, R. Pharmacology/toxicology NDA review and evaluation Gilenya (fingolimod HCl). FDA https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022527Orig1s000pharmr.pdf (2009).
Toscano, C. D. Pharmacology/toxicology NDA review and evaluation Zeposia (ozanimod). FDA https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/209899Orig1s000PharmR.pdf (2019).
Christopher, R. et al. P-250 Preclinical safety assessment of etrasimod (APD334), an oral sphingosine-1-phosphate receptor (S1P) modulator with a favorable profile. Inflamm. Bowel Dis. 23, S82 (2017).
Shimizu, H. et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation 111, 222–229 (2005).
Song, J. et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J. Pharmacol. Exp. Ther. 324, 276–283 (2008).
Fryer, R. M. et al. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS ONE 7, e52985 (2012).
Tran, J. Q. et al. Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc Study. Clin. Pharmacol. Drug Dev. 7, 263–276 (2018).
Tran, J. Q. et al. Results from the first-in-human study with ozanimod, a novel, selective sphingosine-1-phosphate receptor modulator. J. Clin. Pharmacol. 57, 988–996 (2017).
Kovarik, J. M., Schmouder, R., Barilla, D., Wang, Y. & Kraus, G. Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. Br. J. Clin. Pharmacol. 57, 586–591 (2004).
Cohen, J. A. et al. Efficacy and safety of ozanimod in multiple sclerosis: dose-blinded extension of a randomized phase II study. Mult. Scler. 25, 1255–1262 (2019).
Sandborn, W. J. et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J. Crohns Colitis 15, 1120–1129 (2021).
Swallow, E. et al. Comparative safety and efficacy of ozanimod versus fingolimod for relapsing multiple sclerosis. J. Comp. Eff. Res. 9, 275–285 (2020).
Timony, G. et al. Pharmacokinetics and pharmacodynamics of RPC1063 and its metabolites in healthy adult volunteers. Neurology 82, P1.211 (2014).
Harada, T. et al. Cardiac effects of amiselimod compared with fingolimod and placebo: results of a randomised, parallel-group, phase I study in healthy subjects. Br. J. Clin. Pharmacol. 83, 1011–1027 (2017).
Kappos, L. et al. Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 15, 1148–1159 (2016).
Kappos, L. et al. Two-year results from a phase 2 extension study of oral amiselimod in relapsing multiple sclerosis. Mult. Scler. 24, 1605–1616 (2018).
Van Assche, G. et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 353, 362–368 (2005).
Berger, J. R. et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 90, e1815–e1821 (2018).
Arvin, A. M. et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 72, 31–39 (2015).
Brossard, P. et al. Pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator, in the first-in-human study. Br. J. Clin. Pharmacol. 76, 888–896 (2013).
Jain, N. & Bhatti, M. T. Fingolimod-associated macular edema: incidence, detection, and management. Neurology 78, 672–680 (2012).
Cugati, S., Chen, C. S., Lake, S. & Lee, A. W. Fingolimod and macular edema: pathophysiology, diagnosis, and management. Neurol. Clin. Pract. 4, 402–409 (2014).
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J. Hepatol. 69, 461–511 (2018).
Yang, E. et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment. Pharmacol. Ther. 51, 1031–1038 (2020).
Liang, J. et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 (2013).
Alsoud, D., Verstockt, B., Fiocchi, C. & Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 6, 589–595 (2021).
Verstockt, B. et al. Results of the Seventh Scientific Workshop of ECCO: Precision medicine in IBD-disease outcome and response to therapy. J. Crohns Colitis 15, 1431–1442 (2021).
Acknowledgements
The authors would like to thank L. Petti for granting permission to re-use her figure in our manuscript (Fig. 2 Overview of S1P metabolism), and L. M. Shackelton for critical technical review and editing.
Author information
Authors and Affiliations
Consortia
Contributions
The authors contributed equally to all aspects of the article. All authors approved the final version of the manuscript and agreed to submission.
Corresponding author
Ethics declarations
Competing interests
B.V. reports financial support for research from Pfizer; lecture fees from Abbvie, Biogen, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda and Truvion; and consultancy fees from Janssen, Guidepont and Sandoz; these activities were all outside of the submitted work. A.S. reports research grants from Roche-Genentech, Abbvie, GSK, Scipher Medicine, Alimentiv (formerly Robarts Clinical Trials) and Boehringer Ingelheim and consulting fees from Genentech, GSK, Pfizer, HotSpot Therapeutics, Surrozen, and Morphic Therapeutic. M.D. reports advisory fees from Echo Pharma and Alimentiv (formerly Robarts Clinical Trials); speaker fees from Janssen, Merck & Co., Pfizer, Takeda and Tillotts Pharma; and non-financial support from Dr. Falk Pharm; these activities were all outside of the submitted work. N.V.C. reports research grants and personal fees from R-Biopharm, Takeda, and UCB and personal fees from Alimentiv (formerly Robarts Clinical Trials), Celltrion and Prometheus; these activities were all outside of the submitted work. N.V.C. holds a Research Scholar Award from the American Gastroenterological Association and this project was, in part, supported by the Digestive Diseases Research Center grant NIH DK120515. Alimentiv (formerly Robarts Clinical Trials) is an academic gastrointestinal contract research organization (CRO), operating under the Alimentiv Health Trust. Alimentiv provides centralized imaging management solutions in clinical trials, including endoscopy, histopathology and magnetic resonance imaging. Alimentiv provides full service CRO capabilities as well as precision medicine services. A.S., M.D. and N.V.C. as well as Alimentiv Translational Research Consortium Member Authors listed in Supplementary Box 1, including G.D’H., B.G.F., V.J., C.M. and W.J.S., are consultants, and have neither equity positions nor shares in the corporation. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Julie Saba and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Verstockt, B., Vetrano, S., Salas, A. et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 19, 351–366 (2022). https://doi.org/10.1038/s41575-021-00574-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00574-7
This article is cited by
-
IL-10 constrains sphingolipid metabolism to limit inflammation
Nature (2024)
-
Group 3 innate lymphoid cells in intestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
Pathogenic sphingosine 1-phosphate pathway in psoriasis: a critical review of its pathogenic significance and potential as a therapeutic target
Lipids in Health and Disease (2023)
-
A CCL2+DPP4+ subset of mesenchymal stem cells expedites aberrant formation of creeping fat in humans
Nature Communications (2023)
-
Inflammation responsive tofacitinib loaded albumin nanomedicine for targeted synergistic therapy in ulcerative colitis
Nano Research (2023)