Abstract
Functional bowel disorders (FBDs) are a spectrum of disorders characterized by combinations of symptoms attributable to the lower gastrointestinal tract. Most current first-line therapies for IBS and other FBDs target the predominant symptom and mainly affect one symptom in the symptom complex. Additional broadly effective treatment alternatives targeting the entire symptom complex are needed. New drugs for FBDs (such as lubiprostone, linaclotide, plecanatide, prucalopride, eluxadoline and rifaximin) target key mechanisms in the pathophysiology of these disorders and improve both the abnormal bowel habit and other key symptoms, such as abdominal pain and bloating. The current development of new treatment alternatives is focusing on different aspects of the complex pathophysiology of IBS and other FBDs: gut microenvironment (via diet and modulation of gut microbiota), enterohepatic circulation of bile acids, gastrointestinal secretion, motility and sensation, gut–brain interactions, gut barrier function and the immune system within the gastrointestinal tract. Studies also suggest that personalized treatment of IBS and other FBDs is possible using various diagnostic markers.
Key points
-
Treatment options for functional bowel disorders (FBDs) target key pathophysiological factors along the gut–brain axis, including altered gastrointestinal motility, visceral hypersensitivity, increased intestinal permeability, immune activation and altered gut microbiota.
-
Current first-line therapies for IBS and other FBDs mainly affect one symptom in the symptom complex, which is an inherent limitation.
-
New drugs for FBDs target key pathophysiological mechanisms and differ from current therapies by improving the abnormal bowel habit as well as other symptoms, such as abdominal pain and bloating.
-
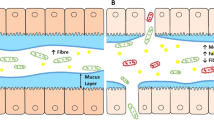
Gut luminal factors, such as food, microbiota and bile acids, and their interaction with each other and the host might be important for symptom generation in at least a subset of patients with FBDs.
-
Treatments affecting gastrointestinal motility and sensitivity, as well as gut barrier function, are promising; medical foods have also been tested in small trials in IBS, with a good safety profile and some efficacy.
-
Personalized treatment strategies for patients with FBDs, based on various diagnostic markers, seem possible in the not so distant future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lacy, B. E. et al. Bowel disorders. Gastroenterology 150, 1393–1407 (2016).
Ford, A. C. et al. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment. Pharmacol. Ther. 39, 312–321 (2014).
Wong, R. K. et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am. J. Gastroenterol. 105, 2228–2234 (2010).
Canavan, C., West, J. & Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 6, 71–80 (2014).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721 (2012).
Sperber, A. D. et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome foundation working team literature review. Gut 66, 1075–1082 (2017).
Locke, G. R. 3rd, Zinsmeister, A. R., Talley, N. J., Fett, S. L. & Melton, L. J. 3rd. Familial association in adults with functional gastrointestinal disorders. Mayo Clin. Proc. 75, 907–912 (2000).
Hungin, A. P., Whorwell, P. J., Tack, J. & Mearin, F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment. Pharmacol. Ther. 17, 643–650 (2003).
Peery, A. F. et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 149, 1731–1741 (2015).
Whitehead, W. E., Palsson, O. & Jones, K. R. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 122, 1140–1156 (2002).
Longstreth, G. F. & Yao, J. F. Irritable bowel syndrome and surgery: a multivariable analysis. Gastroenterology 126, 1665–1673 (2004).
Chang, J. Y. et al. Impact of functional gastrointestinal disorders on survival in the community. Am. J. Gastroenterol. 105, 822–832 (2010).
Simren, M., Tornblom, H., Palsson, O. S. & Whitehead, W. E. Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2, 112–122 (2017).
Manabe, N. et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 22, 293–e82 (2010).
Tornblom, H. et al. Colonic transit time and IBS symptoms: what’s the link? Am. J. Gastroenterol. 107, 754–760 (2012).
Simren, M. et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut 67, 255–262 (2018).
Bednarska, O. et al. VIP and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 153, 948–960 (2017).
Ohman, L., Tornblom, H. & Simren, M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat. Rev. Gastroenterol. Hepatol. 12, 36–49 (2015).
Tap, J. et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 152, 111–123 (2017).
Drossman, D. A. et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology 128, 580–589 (2005).
Engsbro, A. L., Simren, M. & Bytzer, P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment. Pharmacol. Ther. 35, 350–359 (2012).
Awouters, F. et al. Loperamide. survey of studies on mechanism of its antidiarrheal activity. Dig. Dis. Sci. 38, 977–995 (1993).
Cann, P. A., Read, N. W., Holdsworth, C. D. & Barends, D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig. Dis. Sci. 29, 239–247 (1984).
Ford, A. C. et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 109 (Suppl. 1), S2–26, quiz S27 (2014).
Bijkerk, C. J. et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 339, b3154 (2009).
McRorie, J. W. Jr & McKeown, N. M. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet 117, 251–264 (2017).
Muller-Lissner, S. Pharmacokinetic and pharmacodynamic considerations for the current chronic constipation treatments. Expert Opin. Drug Metab. Toxicol. 9, 391–401 (2013).
Belsey, J. D., Geraint, M. & Dixon, T. A. Systematic review and meta analysis: polyethylene glycol in adults with non-organic constipation. Int. J. Clin. Pract. 64, 944–955 (2010).
Chapman, R. W., Stanghellini, V., Geraint, M. & Halphen, M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am. J. Gastroenterol. 108, 1508–1515 (2013).
Annahazi, A., Roka, R., Rosztoczy, A. & Wittmann, T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J. Gastroenterol. 20, 6031–6043 (2014).
Ford, A. C. et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 337, a2313 (2008).
Camilleri, M. & Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66, 966–974 (2017).
Tack, J., Fried, M., Houghton, L. A., Spicak, J. & Fisher, G. Systematic review: the efficacy of treatments for irritable bowel syndrome—a European perspective. Aliment. Pharmacol. Ther. 24, 183–205 (2006).
Hills, J. M. & Aaronson, P. I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 101, 55–65 (1991).
Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., Bartolini, A. & Ghelardini, C. Menthol: a natural analgesic compound. Neurosci. Lett. 322, 145–148 (2002).
Juergens, U. R., Stober, M. & Vetter, H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur. J. Med. Res. 3, 539–545 (1998).
Walstab, J. et al. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterol Motil. 26, 810–820 (2014).
Liu, B. et al. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 154, 2169–2177 (2013).
Khanna, R., MacDonald, J. K. & Levesque, B. G. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J. Clin. Gastroenterol. 48, 505–512 (2014).
Drossman, D. A. et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome foundation working team report. Gastroenterology 154, 1140–1171 e1141 (2018).
Ford, A. C. et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am. J. Gastroenterol. 109, 1350–1365 (2014).
Gorard, D. A., Libby, G. W. & Farthing, M. J. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment. Pharmacol. Ther. 8, 159–166 (1994).
Morgan, V., Pickens, D., Gautam, S., Kessler, R. & Mertz, H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 54, 601–607 (2005).
Poitras, P., Riberdy Poitras, M., Plourde, V., Boivin, M. & Verrier, P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Dig. Dis. Sci. 47, 914–920 (2002).
Van Oudenhove, L. et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology 150, 1355–1367 (2016).
Tornblom, H. & Drossman, D. A. Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil. 27, 455–467 (2015).
O’Leary, O. F. & Cryan, J. F. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol. Sci. 35, 675–687 (2014).
Laird, K. T., Tanner-Smith, E. E., Russell, A. C., Hollon, S. D. & Walker, L. S. Short-term and Long-term efficacy of psychological therapies for irritable bowel syndrome: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 14, 937–947 (2016).
Lackner, J. M. et al. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin. Gastroenterol. Hepatol. 6, 899–906 (2008).
Ljotsson, B. et al. Internet-delivered exposure-based treatment versus stress management for irritable bowel syndrome: a randomized trial. Am. J. Gastroenterol. 106, 1481–1491 (2011).
Moser, G. et al. Long-term success of GUT-directed group hypnosis for patients with refractory irritable bowel syndrome: a randomized controlled trial. Am. J. Gastroenterol. 108, 602–609 (2013).
Barbara, G. et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 150, 1305–1318 (2016).
Lacy, B. E. The science, evidence, and practice of dietary interventions in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 13, 1899–1906 (2015).
Simren, M. et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62, 159–176 (2013).
Bohn, L., Storsrud, S., Tornblom, H., Bengtsson, U. & Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 108, 634–641 (2013).
Le Neve, B. et al. A combined nutrient and lactulose challenge test allows symptom-based clustering of patients with irritable bowel syndrome. Am. J. Gastroenterol. 108, 786–795 (2013).
Posserud, I. et al. Symptom pattern following a meal challenge test in patients with irritable bowel syndrome and healthy controls. United European Gastroenterol. J. 1, 358–367 (2013).
Spencer, M., Chey, W. D. & Eswaran, S. Dietary renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr. Treat. Options Gastroenterol. 12, 424–440 (2014).
McKenzie, Y. A. et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet 29, 549–575 (2016).
Shepherd, S. J., Lomer, M. C. & Gibson, P. R. Short-chain carbohydrates and functional gastrointestinal disorders. Am. J. Gastroenterol. 108, 707–717 (2013).
Simren, M. Diet as a therapy for irritable bowel syndrome: progress at last. Gastroenterology 146, 10–12 (2014).
Staudacher, H. M., Irving, P. M., Lomer, M. C. & Whelan, K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 11, 256–266 (2014).
Shepherd, S. J., Parker, F. C., Muir, J. G. & Gibson, P. R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 6, 765–771 (2008).
Halmos, E. P., Power, V. A., Shepherd, S. J., Gibson, P. R. & Muir, J. G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75 (2014).
Staudacher, H. M. et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 142, 1510–1518 (2012).
Bohn, L. et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 149, 1399–1407 (2015).
Eswaran, S. L., Chey, W. D., Han-Markey, T., Ball, S. & Jackson, K. A. Randomized controlled trial comparing the low FODMAP diet versus modified NICE guidelines in US adults with IBS-D. Am. J. Gastroenterol. 111, 1824–1832 (2016).
Krogsgaard, L. R., Lyngesen, M. & Bytzer, P. Systematic review: quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment. Pharmacol. Ther. 45, 1506–1513 (2017).
Moayyedi, P. et al. The effect of dietary intervention on irritable bowel syndrome: a systematic review. Clin. Transl Gastroenterol. 6, e107 (2015).
Staudacher, H. M. et al. Diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology 153, 936–947 (2017).
O’Keeffe, M. et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 30, e13154 (2017).
Halmos, E. P. et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64, 93–100 (2015).
Biesiekierski, J. R. et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 106, 508–514; quiz 515 (2011).
Biesiekierski, J. R. et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145, 320–328 (2013).
Uhde, M. et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 65, 1930–1937 (2016).
Pimentel, M., Chow, E. J. & Lin, H. C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 95, 3503–3506 (2000).
Posserud, I., Stotzer, P. O., Bjornsson, E. S., Abrahamsson, H. & Simren, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56, 802–808 (2007).
Jalanka-Tuovinen, J. et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 63, 1737–1745 (2014).
Pimentel, M., Chow, E. J. & Lin, H. C. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 98, 412–419 (2003).
Koo, H. L. & DuPont, H. L. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr. Opin. Gastroenterol. 26, 17–25 (2010).
Pimentel, M., Park, S., Mirocha, J., Kane, S. V. & Kong, Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann. Intern. Med. 145, 557–563 (2006).
Sharara, A. I. et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am. J. Gastroenterol. 101, 326–333 (2006).
Pimentel, M. et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 364, 22–32 (2011).
Lembo, A. et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 151, 1113–1121 (2016).
Pimentel, M. et al. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig. Dis. Sci. 62, 2455–2463 (2017).
Xu, D. et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 146, 484–496 (2014).
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Ford, A. C. et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am. J. Gastroenterol. 109, 1547–1561; quiz 1546 (2014).
Hungin, A. P. S. et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms - an updated evidence-based international consensus. Aliment. Pharmacol. Ther. 47, 1054–1070 (2018).
Quigley, E. M. Probiotics in irritable bowel syndrome: the science and the evidence. J. Clin. Gastroenterol. 49 (Suppl. 1), S60–S64 (2015).
Tillisch, K. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401 (2013).
Pinto-Sanchez, M. I. et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459 (2017).
Drossman, D. A. Functional gastrointestinal disorders: history, pathophysiology, clinical features & Rome IV. Gastroenterology 150, 1262–1279 (2016).
Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Alexea, O., Bacarea, V. & Pique, N. The combination of oligo- and polysaccharides and reticulated protein for the control of symptoms in patients with irritable bowel syndrome: results of a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial. United European Gastroenterol. J. 4, 455–465 (2016).
Azpiroz, F. et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 9, e12911 (2017).
Dimidi, E., Rossi, M. & Whelan, K. Irritable bowel syndrome and diet: where are we in 2018? Curr. Opin. Clin. Nutr. Metab. Care 20, 456–463 (2017).
Niv, E. et al. Randomized clinical study: partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab. 13, 10 (2016).
Halkjaer, S. I., Boolsen, A. W., Gunther, S., Christensen, A. H. & Petersen, A. M. Can fecal microbiota transplantation cure irritable bowel syndrome? World J. Gastroenterol. 23, 4112–4120 (2017).
Pinn, D. M., Aroniadis, O. C. & Brandt, L. J. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 27, 19–29 (2015).
Cammarota, G. et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569–580 (2017).
Mizuno, S. et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 96, 29–38 (2017).
Pinn, D. M., Aroniadis, O. C. & Brandt, L. J. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am. J. Gastroenterol. 109, 1831–1832 (2014).
Tian, H. et al. Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J. Clin. Gastroenterol. 50, 865–870 (2016).
Johnsen, P. H. et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 3, 17–24 (2018).
Chang, B. W. & Rezaie, A. Irritable bowel syndrome-like symptoms following fecal microbiota transplantation: a possible donor-dependent complication. Am. J. Gastroenterol. 112, 186–187 (2017).
Mosinska, P., Storr, M. & Fichna, J. The role of AST-120 and protein-bound uremic toxins in irritable bowel syndrome: a therapeutic perspective. Therap Adv. Gastroenterol. 8, 278–284 (2015).
Tack, J. F., Miner, P. B. Jr., Fischer, L. & Harris, M. S. Randomised clinical trial: the safety and efficacy of AST-120 in non-constipating irritable bowel syndrome - a double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 34, 868–877 (2011).
Bharucha, A. E. & Waldman, S. A. Taking a lesson from microbial diarrheagenesis in the management of chronic constipation. Gastroenterology 138, 813–817 (2010).
Corsetti, M. & Tack, J. Linaclotide: a new drug for the treatment of chronic constipation and irritable bowel syndrome with constipation. United European Gastroenterol. J. 1, 7–20 (2013).
Andresen, V. et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133, 761–768 (2007).
Chey, W. D. et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 107, 1702–1712 (2012).
Lembo, A. J. et al. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med. 365, 527–536 (2011).
Rao, S. et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol. 107, 1714–1724 (2012).
Quigley, E. M. et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment. Pharmacol. Ther. 37, 49–61 (2013).
Castro, J. et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145, 1334–1346 (2013).
Bharucha, A. E., Locke, G. R. & Pemberton, J. H. Reply: To PMID 23261065. Gastroenterology 145, 488 (2013).
Johnston, J. M. et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 139, 1877–1886 (2010).
Fukudo, S. et al. Linaclotide is effective and safe for patients with irritable bowel syndrome with constipation in Japan: a phase III randomized, double-blind, and placebo-controlled and long-term extension study. Gastroenterology 152, S714 (2017).
Fukudo, S. et al. Determining an optimal dose of linaclotide for use in Japanese patients with irritable bowel syndrome with constipation: a phase II randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. 30, e13275 (2017).
Brenner, D. M. et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am. J. Gastroenterol. 113,735–745 (2018).
DeMicco, M., Barrow, L., Hickey, B., Shailubhai, K. & Griffin, P. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv. Gastroenterol. 10, 837–851 (2017).
Miner, P. B. Jr. et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am. J. Gastroenterol. 112, 613–621 (2017).
FDA. FDA approves trulance for chronic idiopathic constipation. FDA https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm537725.htm (2017).
Synergy Pharmaceuticals. Synergy Pharmaceuticals announces FDA approval of Trulance® (plecanatide) for the treatment of irritable bowel syndrome with constipation (IBS-C) in adults. Synergy Pharmaceuticals https://ir.synergypharma.com/press-releases/detail/1861/synergy-pharmaceuticals-announces-fda-approval-of (2018).
Rivkin, A. & Chagan, L. Lubiprostone: chloride channel activator for chronic constipation. Clin. Ther. 28, 2008–2021 (2006).
Barish, C. F., Drossman, D., Johanson, J. F. & Ueno, R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig. Dis. Sci. 55, 1090–1097 (2010).
Drossman, D. A. et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome — results of two randomized, placebo-controlled studies. Aliment. Pharmacol. Ther. 29, 329–341 (2009).
Chey, W. D. et al. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 35, 587–599 (2012).
Spencer, A. G. et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci. Transl. Med. 6, 227ra236 (2014).
Chey, W. D., Lembo, A. J. & Rosenbaum, D. P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am. J. Gastroenterol. 112, 763–774 (2017).
Ardelyx. Ardelyx reports successful Phase 3 T3MPO-1 trial of tenapanor in patients with IBS-C. Ardelyx http://ir.ardelyx.com/news-releases/news-release-details/ardelyx-reports-successful-phase-3-t3mpo-1-trial-tenapanor (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02686138?term=NCT02686138&rank=1 (2017).
Bagnol, D., Mansour, A., Akil, H. & Watson, S. J. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience 81, 579–591 (1997).
Bitar, K. N. & Makhlouf, G. M. Specific opiate receptors on isolated mammalian gastric smooth muscle cells. Nature 297, 72–74 (1982).
Lacy, B. E. Emerging treatments in neurogastroenterology: eluxadoline - a new therapeutic option for diarrhea-predominant IBS. Neurogastroenterol Motil. 28, 26–35 (2016).
Lembo, A. J. et al. Eluxadoline for irritable bowel syndrome with diarrhea. N. Engl. J. Med. 374, 242–253 (2016).
Chey, W. D., Dove, L. S., Andrae, D. A. & Covington, P. S. Early response predicts a sustained response to eluxadoline in patients with irritable bowel syndrome with diarrhoea in two phase 3 studies. Aliment. Pharmacol. Ther. 45, 1319–1328 (2017).
Lacy, B. E. et al. Eluxadoline efficacy in IBS-D patients who report prior loperamide use. Am. J. Gastroenterol. 112, 924–932 (2017).
Cash, B. D., Lacy, B. E., Schoenfeld, P. S., Dove, L. S. & Covington, P. S. Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am. J. Gastroenterol. 112, 365–374 (2017).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Ford, A. C. et al. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am. J. Gastroenterol. 104, 1831–1843 (2009).
Tennis, P. et al. The relationship between dosing of alosetron and discontinuation patterns reported by patients participating in a follow-up programme. Aliment. Pharmacol. Ther. 25, 317–322 (2007).
Fukudo, S., Ida, M., Akiho, H., Nakashima, Y. & Matsueda, K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 12, 953–959 (2014).
Fukudo, S. et al. Ramosetron reduces symptoms of irritable bowel syndrome with diarrhea and improves quality of life in women. Gastroenterology 150, 358–366 (2016).
Billio, A., Morello, E. & Clarke, M. J. Serotonin receptor antagonists for highly emetogenic chemotherapy in adults. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006272.pub2 (2010).
Garsed, K. et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63, 1617–1625 (2014).
Holzer, P. & Holzer-Petsche, U. Tachykinins in the gut. Part I. expression, release and motor function. Pharmacol. Ther. 73, 173–217 (1997).
Corsetti, M., Akyuz, F. & Tack, J. Targeting tachykinin receptors for the treatment of functional gastrointestinal disorders with a focus on irritable bowel syndrome. Neurogastroenterol Motil. 27, 1354–1370 (2015).
Tack, J. et al. The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 66, 1403–1413 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02107196?term=NCT02107196&rank=1 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02120027?term=NCT02120027&rank=1 (2017).
Brown, P. M. et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology 141, 507–516 (2011).
Kulke, M. H. et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of Carcinoid Syndrome. J. Clin. Oncol. 35, 14–23 (2017).
Mangel, A. W. & Hicks, G. A. Asimadoline and its potential for the treatment of diarrhea-predominant irritable bowel syndrome: a review. Clin. Exp. Gastroenterol. 5, 1–10 (2012).
Mangel, A. W. et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 28, 239–249 (2008).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01100684?term=asimadoline&cond=IBS+-+Irritable+Bowel+Syndrome&rank=1 (2013).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02475447?term=NCT02475447&rank=1 (2017).
Yamaguchi, O. et al. Randomized, double-blind, placebo- and propiverine-controlled trial of the once-daily antimuscarinic agent solifenacin in Japanese patients with overactive bladder. BJU Int. 100, 579–587 (2007).
Fukushima, Y., Suzuki, H., Matsuzaki, J., Kiyosue, A. & Hibi, T. Efficacy of solifenacin on irritable bowel syndrome with diarrhea: open-label prospective pilot trial. J. Neurogastroenterol Motil. 18, 317–323 (2012).
Clave, P. & Tack, J. Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis. Therap Adv. Gastroenterol. 10, 311–322 (2017).
Lee, K. N. et al. Efficacy and safety of tiropramide in the treatment of patients with irritable bowel syndrome: a multicenter, randomized, double-blind, non-inferiority trial, compared with octylonium. J. Neurogastroenterol Motil. 20, 113–121 (2014).
Aziz, I. et al. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on rome III criteria. Clin. Gastroenterol. Hepatol. 13, 1650–1655 (2015).
Bajor, A., Tornblom, H., Rudling, M., Ung, K. A. & Simren, M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 64, 84–92 (2015).
Mekjian, H. S., Phillips, S. F. & Hofmann, A. F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J. Clin. Invest. 50, 1569–1577 (1971).
Bampton, P. A., Dinning, P. G., Kennedy, M. L., Lubowski, D. Z. & Cook, I. J. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G443–449 (2002).
Mottacki, N., Simren, M. & Bajor, A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment. Pharmacol. Ther. 43, 884–898 (2016).
Fernandez-Banares, F. et al. Randomised clinical trial: colestyramine versus hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment. Pharmacol. Ther. 41, 1132–1140 (2015).
Orekoya, O. et al. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin. Med. 15, 252–257 (2015).
Wilcox, C., Turner, J. & Green, J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment. Pharmacol. Ther. 39, 923–939 (2014).
Camilleri, M. et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 41, 438–448 (2015).
Appleby, R. N. et al. Effects of conventional and a novel colonic-release bile acid sequestrant, A3384, on fibroblast growth factor 19 and bile acid metabolism in healthy volunteers and patients with bile acid diarrhoea. United Eurpoean Gastroenterol. J. 5, 380–388 (2017).
Walters, J. R. et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin. Gastroenterol. Hepatol. 7, 1189–1194 (2009).
Keely, S. J. & Walters, J. R. The farnesoid X receptor: good for bad. Cell. Mol. Gastroenterol. Hepatol. 2, 725–732 (2016).
Alawad, A. S. & Levy, C. FXR agonists: from bench to bedside, a guide for clinicians. Dig. Dis. Sci. 61, 3395–3404 (2016).
Walters, J. R. et al. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment. Pharmacol. Ther. 41, 54–64 (2015).
Odunsi-Shiyanbade, S. T. et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin. Gastroenterol. Hepatol. 8, 159–165 (2010).
Simren, M., Bajor, A., Gillberg, P. G., Rudling, M. & Abrahamsson, H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 versus placebo in patients with chronic idiopathic constipation — a double-blind study. Aliment. Pharmacol. Ther. 34, 41–50 (2011).
Rao, A. S. et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 139, 1549–1558 (2010).
Chey, W. D., Camilleri, M., Chang, L., Rikner, L. & Graffner, H. A randomized placebo-controlled phase IIb trial ofa3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am. J. Gastroenterol. 106, 1803–1812 (2011).
Nakajima, A., Seki, M. & Taniguchi, S. Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: a phase II, multicenter, double-blind, placebo-controlled randomized clinical trial. J. Gastroenterol. 53, 525–534 (2017).
Tack, J. et al. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment. Pharmacol. Ther. 35, 745–767 (2012).
Tack, J. & Corsetti, M. Prucalopride: evaluation of the pharmacokinetics, pharmacodynamics, efficacy and safety in the treatment of chronic constipation. Expert Opin. Drug Metab. Toxicol. 8, 1327–1335 (2012).
Tack, J., Quigley, E., Camilleri, M., Vandeplassche, L. & Kerstens, R. Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis. United European Gastroenterol. J. 1, 48–59 (2013).
Tack, J. et al. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 26, 21–27 (2014).
Sanger, G. J. & Furness, J. B. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat. Rev. Gastroenterol. Hepatol. 13, 38–48 (2016).
Camilleri, M. et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology 153, 1240–1250 (2017).
Van der Ploeg, L. et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sci. 109, 20–29 (2014).
Acosta, A. et al. Relamorelin relieves constipation and accelerates colonic transit in a phase 2, placebo-controlled, randomized trial. Clin. Gastroenterol. Hepatol. 13, 2312–2319 (2015).
Acosta, A. et al. Short-term effects of relamorelin on descending colon motility in chronic constipation: a randomized, controlled trial. Dig. Dis. Sci. 61, 852–860 (2016).
Parkinson Study Group. Electronic address, p. o. e. A randomized trial of relamorelin for constipation in Parkinson’s disease (MOVE-PD): trial results and lessons learned. Parkinsonism Relat. Disord. 37, 101–105 (2017).
Barbara, G. et al. Randomised controlled trial of mesalazine in IBS. Gut 65, 82–90 (2016).
Lam, C. et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 65, 91–99 (2016).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Wouters, M. M. et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150, 875–887 (2016).
Klooker, T. K. et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59, 1213–1221 (2010).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01908465?term=ebastine&rank=2 (2017).
Ciampa, B. P., Reyes Ramos, E., Borum, M. & Doman, D. B. The emerging therapeutic role of medical foods for gastrointestinal disorders. Gastroenterol. Hepatol. 13, 104–115 (2017).
Cash, B. D., Epstein, M. S. & Shah, S. M. A. Novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig. Dis. Sci. 61, 560–571 (2016).
Petschow, B. W., Burnett, B., Shaw, A. L., Weaver, E. M. & Klein, G. L. Serum-derived bovine immunoglobulin/protein isolate: postulated mechanism of action for management of enteropathy. Clin. Exp. Gastroenterol. 7, 181–190 (2014).
Good, L., Rosario, R. & Panas, R. New therapeutic option for irritable bowel syndrome: serum-derived bovine immunoglobulin. World J. Gastroenterol. 21, 3361–3366 (2015).
Wilson, D., Evans, M., Weaver, E., Shaw, A. L. & Klein, G. L. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin. Med. Insights Gastroenterol. 6, 49–60 (2013).
Souba, W. W. et al. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 48, 383–391 (1990).
Camilleri, M., Madsen, K., Spiller, R., Greenwood-Van Meerveld, B. & Verne, G. N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 24, 503–512 (2012).
Zhou, Q., Souba, W. W., Croce, C. M. & Verne, G. N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59, 775–784 (2010).
Bertrand, J. et al. Glutamine restores tight junction protein Claudin-1 expression in colonic mucosa of patients with diarrhea-predominant irritable bowel syndrome. JPEN J. Parenter. Enteral. Nutr. 40, 1170–1176 (2016).
Zhou, Q. & Verne, G. N. Reply: to PMID 25277410. Gastroenterology 148, 1080–1081 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01414244?term=glutamine&cond=Irritable+Bowel+Syndrome&rank=1 (2016).
Hesselink, J. M. Evolution in pharmacologic thinking around the natural analgesic palmitoylethanolamide: from nonspecific resistance to PPAR-alpha agonist and effective nutraceutical. J. Pain Res. 6, 625–634 (2013).
Yang, B. et al. Polydatin attenuated food allergy via store-operated calcium channels in mast cell. World J. Gastroenterol. 19, 3980–3989 (2013).
Camilleri, M. et al. Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G553–G560 (2013).
Cremon, C. et al. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment. Pharmacol. Ther. 45, 909–922 (2017).
Ottillinger, B., Storr, M., Malfertheiner, P. & Allescher, H. D. STW 5 (Iberogast(R)) — a safe and effective standard in the treatment of functional gastrointestinal disorders. Wien Med. Wochenschr. 163, 65–72 (2013).
Krueger, D. et al. The multi-herbal drug STW 5 (Iberogast) has prosecretory action in the human intestine. Neurogastroenterol Motil. 21, 1203–e1110 (2009).
Wegener, T. & Wagner, H. The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine 13 (Suppl. 5), 20–35 (2006).
Madisch, A., Holtmann, G., Plein, K. & Hotz, J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment. Pharmacol. Ther. 19, 271–279 (2004).
Teschke, R., Wolff, A., Frenzel, C., Eickhoff, A. & Schulze, J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J. Gastroenterol. 21, 4466–4490 (2015).
Fan, H. et al. Tongxie formula reduces symptoms of irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 15, 1724–1732 (2017).
Ahluwalia, B., Magnusson, M. K., Isaksson, S., Larsson, F. & Ohman, L. Effects of aloe barbadensis Mill. extract (AVH200(R)) on human blood T cell activity in vitro. J. Ethnopharmacol 179, 301–309 (2016).
Langmead, L., Makins, R. J. & Rampton, D. S. Anti-inflammatory effects of aloe vera gel in human colorectal mucosa in vitro. Aliment. Pharmacol. Ther. 19, 521–527 (2004).
Storsrud, S., Ponten, I. & Simren, M. A. Pilot study of the effect of aloe barbadensis Mill. Extract (AVH200(R)) in patients with irritable bowel syndrome: a randomized, double-blind, placebo-controlled study. J. Gastrointestin Liver Dis. 24, 275–280 (2015).
Davis, K., Philpott, S., Kumar, D. & Mendall, M. Randomised double-blind placebo-controlled trial of aloe vera for irritable bowel syndrome. Int. J. Clin. Pract. 60, 1080–1086 (2006).
Hutchings, H. A. et al. A randomised, cross-over, placebo-controlled study of aloe vera in patients with irritable bowel syndrome: effects on patient quality of life. ISRN Gastroenterol. 2011, 206103 (2011).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01400048?term=aloe+vera&cond=IBS+-+Irritable+Bowel+Syndrome&rank=1 (2016).
Shishodia, S., Sethi, G. & Aggarwal, B. B. Curcumin: getting back to the roots. Ann. NY Acad. Sci. 1056, 206–217 (2005).
Ostad, S. N., Soodi, M., Shariffzadeh, M., Khorshidi, N. & Marzban, H. The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J. Ethnopharmacol 76, 299–304 (2001).
Portincasa, P. et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointestin Liver Dis. 25, 151–157 (2016).
Chang, L., Tong, K. & Ameen, V. Ischemic colitis and complications of constipation associated with the use of alosetron under a risk management plan: clinical characteristics, outcomes, and incidences. Am. J. Gastroenterol. 105, 866–875 (2010).
Pasricha, P. J. Desperately seeking serotonin… A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders. Gastroenterology 132, 2287–2290 (2007).
Anderson, J. L. et al. Lack of association of tegaserod with adverse cardiovascular outcomes in a matched case-control study. J. Cardiovasc. Pharmacol. Ther. 14, 170–175 (2009).
Loughlin, J. et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study. J. Cardiovasc. Pharmacol. Ther. 15, 151–157 (2010).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 (2015).
Bennet, S. M. P. et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 67, 872–881 (2017).
Annahazi, A. et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am. J. Gastroenterol. 108, 1322–1331 (2013).
Cenac, N. et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 (2007).
Vergnolle, N. Protease inhibition as new therapeutic strategy for GI diseases. Gut 65, 1215–1224 (2016).
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Khaleghi, S., Ju, J. M., Lamba, A. & Murray, J. A. The potential utility of tight junction regulation in celiac disease: focus on larazotide acetate. Therap Adv. Gastroenterol. 9, 37–49 (2016).
Vanheel, H. et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 63, 262–271 (2014).
Sweetser, S. et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1299–G1306 (2009).
Lobo, B. et al. Down-regulation of mucosal mast cell activation and immune response in diarrhea-irritable bowel syndrome by oral disodium cromoglicate. United European Gastroenterol. J. 5, 887–897 (2017).
Wouters, M. M., Vicario, M. & Santos, J. The role of mast cells in functional GI disorders. Gut 65, 155–168 (2016).
Fabisiak, A., Wlodarczyk, J., Fabisiak, N., Storr, M. & Fichna, J. Targeting histamine receptors in irritable bowel syndrome: a critical appraisal. J. Neurogastroenterol Motil. 23, 341–348 (2017).
Balemans, D., Boeckxstaens, G. E., Talavera, K. & Wouters, M. M. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G635–G648 (2017).
Zhou, Q. et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 65, 797–805 (2016).
Grinsvall, C., Tornblom, H., Tack, J., Van Oudenhove, L. & Simren, M. Psychological factors selectively upregulate rectal pain perception in hypersensitive patients with irritable bowel syndrome. Neurogastroenterol Motil. 27, 1772–1782 (2015).
Van Oudenhove, L., Tornblom, H., Storsrud, S., Tack, J. & Simren, M. Depression and somatization are associated with increased postprandial symptoms in patients with irritable bowel syndrome. Gastroenterology 150, 866–874 (2016).
Wilpart, K. et al. Coping skills are associated with gastrointestinal symptom severity and somatization in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 15, 1565–1571 (2017).
Jones, M. P. et al. A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Aliment. Pharmacol. Ther. 39, 426–437 (2014).
Lembo, A. J. et al. Use of serum biomarkers in a diagnostic test for irritable bowel syndrome. Aliment. Pharmacol. Ther. 29, 834–842 (2009).
Pimentel, M. et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS ONE 10, e0126438 (2015).
Camilleri, M. et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am. J. Gastroenterol. 109, 1621–1630 (2014).
Peleman, C. et al. Colonic transit and bile acid synthesis or excretion in patients with irritable bowel syndrome-diarrhea without bile acid malabsorption. Clin. Gastroenterol. Hepatol. 15, 720–727 e721 (2017).
Camilleri, M. et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology 123, 425–432 (2002).
Enck, P. et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2, 16014 (2016).
Gazouli, M. et al. Lessons learned—resolving the enigma of genetic factors in IBS. Nat. Rev. Gastroenterol. Hepatol. 13, 77–87 (2016).
Henstrom, M. et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 76, 263–270 (2016).
Wong, B. S. et al. Pharmacogenetics of the effects of colesevelam on colonic transit in irritable bowel syndrome with diarrhea. Dig. Dis. Sci. 57, 1222–1226 (2012).
Boeckxstaens, G. E. et al. Phenotyping of subjects for large scale studies on patients with IBS. Neurogastroenterol Motil. 28, 1134–1147 (2016).
Review criteria
This Review is based on literature searches performed in the PubMed database in August 2017 using the search terms “irritable bowel syndrome”, “functional bowel disorder”, “constipation”, “diarrhea”, “bloating”, “clinical trial”, “treatment”, “diet”, “probiotics”, “antibiotics”, “prebiotics”, “FMT”, “inflammation”, “prokinetics”, “microbiota”, “5-hydroxytryptamine”, “serotonin”, “secretagogue”, “herb” and “medical foods”. The reference lists of identified articles or linked articles were searched for further papers. English-language original research and review articles were considered. No publication date restrictions were applied. The Review is also based on the authors’ personal knowledge of ongoing clinical trials as well as on a search of Clinicaltrials.gov using the terms “irritable bowel syndrome”, “constipation” and “diarrhea”.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks B. Niesler, E. Quigley and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
M.S. has received unrestricted research grants from Danone and Ferring Pharmaceuticals; he has served as a consultant and/or advisory board member for Albireo, Allergan, Almirall, AstraZeneca, Danone, Glycom, Menarini, Nestlé and Shire and as a speaker for Allergan, Almirall, Menarini, Shire, Takeda and Tillotts. J.T. has given scientific advice to Almirall, AstraZeneca, Danone, Menarini, Novartis, Nycomed, Ocera, Ono pharma, Shire, SK Life Sciences, Theravance, Tranzyme, XenoPort and Zeria and has been a member of the Speaker Bureau for Abbott, Alfa Wasserman, Almirall, AstraZeneca, Janssen, Menarini, Novartis, Nycomed, Shire and Zeria.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simrén, M., Tack, J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat Rev Gastroenterol Hepatol 15, 589–605 (2018). https://doi.org/10.1038/s41575-018-0034-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0034-5
This article is cited by
-
Targeting the gut microenvironment in IBS to improve symptoms
Nature Reviews Gastroenterology & Hepatology (2023)
-
Targeting the endocannabinoid system for the treatment of abdominal pain in irritable bowel syndrome
Nature Reviews Gastroenterology & Hepatology (2023)
-
An Update of Pharmacological Management in Children with Functional Constipation
Pediatric Drugs (2023)
-
The efficacy of vitamin D supplementation for irritable bowel syndrome: a systematic review with meta-analysis
Nutrition Journal (2022)
-
Probiotics-loaded nanoparticles attenuated colon inflammation, oxidative stress, and apoptosis in colitis
Scientific Reports (2022)