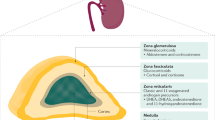
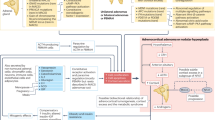
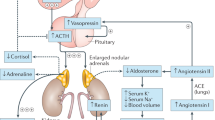
Abstract
Iatrogenic adrenal insufficiency (IAI) is the most common form of adrenal insufficiency in adult patients, although its overall exact prevalence remains unclear. IAI is associated with adverse clinical outcomes, including adrenal crisis, impaired quality of life and increased mortality; therefore, it is imperative that clinicians maintain a high index of suspicion in patients at risk of IAI to facilitate timely diagnosis and appropriate management. Herein, we review the major causes, clinical consequences, diagnosis and care of patients with IAI. The management of IAI, particularly glucocorticoid-induced (or tertiary) adrenal insufficiency, can be particularly challenging, and the provision of adequate glucocorticoid replacement must be balanced against minimizing the cardiometabolic effects of excess glucocorticoid exposure and optimizing recovery of the hypothalamic–pituitary–adrenal axis. We review current treatment strategies and their limitations and discuss developments in optimizing treatment of IAI. This comprehensive Review aims to aid clinicians in identifying who is at risk of IAI, how to approach screening of at-risk populations and how to treat patients with IAI, with a focus on emergency management and prevention of an adrenal crisis.
Key points
-
Iatrogenic adrenal insufficiency (IAI) is the most common cause of adrenal insufficiency. Exogenous glucocorticoid use is a major contributor to IAI, which can occur even with the use of relatively low doses of glucocorticoids.
-
Patients with glucocorticoid-induced adrenal insufficiency are at risk of concomitant Cushing syndrome and vice versa.
-
Patients with cancer are particularly at risk of adrenal insufficiency in the setting of radiation exposure, immune-checkpoint inhibitor therapy, opioid analgesia and glucocorticoid use. Clinical suspicion and input from clinical endocrinologists are vital for this vulnerable group of patients.
-
If adrenal insufficiency is suspected in an acutely unwell patient, emergency glucocorticoid treatment should be initiated without delay. A diagnosis can be confirmed once the patient is stabilized.
-
Prevention of adrenal crisis and patient education is a vital part of the management of adrenal insufficiency. All patients with confirmed adrenal insufficiency or at high risk of adrenal insufficiency should be educated on the steroid ‘sick day rules’ and should carry a steroid emergency card.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arlt, W. & Allolio, B. Adrenal insufficiency. Lancet 361, 1881–1893 (2003).
Bornstein, S. R. Predisposing factors for adrenal insufficiency. N. Engl. J. Med. 360, 2328–2339 (2009).
Björnsdottir, S. et al. Drug prescription patterns in patients with Addison’s disease: a Swedish population-based cohort study. J. Clin. Endocrinol. Metab. 98, 2009–2018 (2013).
Erichsen, M. M. et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J. Clin. Endocrinol. Metab. 94, 4882–4890 (2009).
Olafsson, A. S. & Sigurjonsdottir, H. A. Increasing prevalence of Addison disease: results from a nationwide study. Endocr. Pract. 22, 30–35 (2016).
Regal, M., Páramo, C., Sierra, J. M. & García-Mayor, R. V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. 55, 735–740 (2001).
Bancos, I., Hahner, S., Tomlinson, J. & Arlt, W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 3, 216–226 (2015).
Hannon, A. M. et al. Clinical features and autoimmune associations in patients presenting with idiopathic isolated ACTH deficiency. Clin. Endocrinol. 88, 491–497 (2018).
Martin-Grace, J., Dineen, R., Sherlock, M. & Thompson, C. J. Adrenal insufficiency: physiology, clinical presentation and diagnostic challenges. Clin. Chim. Acta 505, 78–91 (2020).
Raff, H., Sharma, S. T. & Nieman, L. K. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr. Physiol. 4, 739–769 (2014).
Crowley, R. K., Argese, N., Tomlinson, J. W. & Stewart, P. M. Central hypoadrenalism. J. Clin. Endocrinol. Metab. 99, 4027–4036 (2014).
Arlt, W., Society for Endocrinology Clinical Committee. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr. Connect. 5, G1–G3 (2016).
Hahner, S. et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur. J. Endocrinol. 162, 597–602 (2010).
Ho, W. & Druce, M. Quality of life in patients with adrenal disease: a systematic review. Clin. Endocrinol. 89, 119–128 (2018).
Aulinas, A. & Webb, S. M. Health-related quality of life in primary and secondary adrenal insufficiency. Expert Rev. Pharmacoecon. Outcomes Res. 14, 873–888 (2014).
Løvås, K., Loge, J. H. & Husebye, E. S. Subjective health status in Norwegian patients with Addison’s disease. Clin. Endocrinol. 56, 581–588 (2002).
Hahner, S. et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J. Clin. Endocrinol. Metab. 92, 3912–3922 (2007).
Li, D. et al. Determinants of self-reported health outcomes in adrenal insufficiency: a multisite survey study. J. Clin. Endocrinol. Metab. 106, e1408–e1419 (2021).
Sherlock, M. et al. ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J. Clin. Endocrinol. Metab. 94, 4216–4223 (2009).
O’Reilly, M. W. et al. ACTH and gonadotropin deficiencies predict mortality in patients treated for nonfunctioning pituitary adenoma: long-term follow-up of 519 patients in two large European centres. Clin. Endocrinol. 85, 748–756 (2016).
Burman, P. et al. Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J. Clin. Endocrinol. Metab. 98, 1466–1475 (2013).
Mebrahtu, T. F. et al. Dose dependency of iatrogenic glucocorticoid excess and adrenal insufficiency and mortality: a cohort study in England. J. Clin. Endocrinol. Metab. 104, 3757–3767 (2019).
Bergthorsdottir, R., Leonsson-Zachrisson, M., Oden, A. & Johannsson, G. Premature mortality in patients with Addison’s disease: a population-based study. J. Clin. Endocrinol. Metab. 91, 4849–4853 (2006).
Rushworth, R. L. & Torpy, D. J. The changing epidemiology of adrenal insufficiency: iatrogenic factors predominate. J. Endocr. Soc. 7, bvad017 (2023).
Aguilera, G., Kiss, A., Liu, Y. & Kamitakahara, A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress 10, 153–161 (2007).
Husebye, E. S., Pearce, S. H., Krone, N. P. & Kämpe, O. Adrenal insufficiency. Lancet 397, 613–629 (2021).
Rhodes, M. E. In: Stress: Neuroendocrinology and Neurobiology (ed. Fink, G.) 109-116 (Academic Press, 2017).
Saito, T. et al. Vasopressin-dependent upregulation of aquaporin-2 gene expression in glucocorticoid-deficient rats. Am. J. Physiol. Ren. Physiol. 279, F502–508 (2000).
Sævik, Å. B. et al. Clues for early detection of autoimmune Addison’s disease – myths and realities. J. Intern. Med. 283, 190–199 (2018).
Taïeb, D. et al. European Association of Nuclear Medicine practice guideline/Society of Nuclear Medicine and Molecular Imaging procedure standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 46, 2112–2137 (2019).
Verbalis, J. G. et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am. J. Med. 126, S1–42 (2013).
Spasovski, G. et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 170, G1–47 (2014).
Li, D. et al. Quality of life and its determinants in patients with adrenal insufficiency: a survey study at three centers in the USA. J. Clin. Endocrinol. Metab. 107, e2851–e2861 (2022).
Stewart, P. M. et al. Exploring inpatient hospitalizations and morbidity in patients with adrenal insufficiency. J. Clin. Endocrinol. Metab. 101, 4843–4850 (2016).
Han, H.-S. et al. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support. Care Cancer 20, 1565–1572 (2012).
Li, T., Donegan, D., Hooten, W. M. & Bancos, I. Clinical presentation and outcomes of opioid-induced adrenal insufficiency. Endocr. Pract. 26, 1291–1297 (2020).
Dineen, R., Thompson, C. J. & Sherlock, M. Adrenal crisis: prevention and management in adult patients. Ther. Adv. Endocrinol. Metab. 10, 2042018819848218 (2019).
Hahner, S. et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J. Clin. Endocrinol. Metab. 100, 407–416 (2015).
Allolio, B. Extensive expertise in endocrinology. Adrenal crisis. Eur. J. Endocrinol. 172, R115–124 (2015).
Smans, L. C. C. J., Van der Valk, E. S., Hermus, A. R. M. M. & Zelissen, P. M. J. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin. Endocrinol. 84, 17–22 (2016).
Erichsen, M. M. et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur. J. Endocrinol. 160, 233–237 (2009).
Quinkler, M., Ekman, B., Zhang, P., Isidori, A. M. & Murray, R. D. Mortality data from the European Adrenal Insufficiency Registry — patient characterization and associations. Clin. Endocrinol. 89, 30–35 (2018).
Todd, G. R. G. Adrenal crisis due to inhaled steroids is underestimated. Arch. Dis. Child. 88, 554–555 (2003).
Todd, G. R. G. et al. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch. Dis. Child. 87, 457–461 (2002).
Fraser, C. G., Preuss, F. S. & Bigford, W. D. Adrenal atrophy and irreversible shock associated with cortisone therapy. J. Am. Med. Assoc. 149, 1542–1543 (1952).
Oltmanns, K. M., Fehm, H. L. & Peters, A. Chronic fentanyl application induces adrenocortical insufficiency. J. Intern. Med. 257, 478–480 (2005).
Wright, J. J. & Johnson, D. B. Approach to the patient with immune checkpoint inhibitor-associated endocrine dysfunction. J. Clin. Endocrinol. Metab. 108, 1514–1525 (2022).
Mizukoshi, T., Fukuoka, H. & Takahashi, Y. Immune checkpoint inhibitor-related hypophysitis. Best Pract. Res. Clin. Endocrinol. Metab. 36, 101668 (2022).
Oßwald, A. et al. Favorable long-term outcomes of bilateral adrenalectomy in Cushing’s disease. Eur. J. Endocrinol. 171, 209–215 (2014).
Ritzel, K. et al. Outcome of bilateral adrenalectomy in Cushing’s syndrome: a systematic review. J. Clin. Endocrinol. Metab. 98, 3939–3948 (2013).
Quinkler, M. et al. Characterization of patients with adrenal insufficiency and frequent adrenal crises. Eur. J. Endocrinol. 184, 761–771 (2021).
Driessens, N. et al. PAI-BEL: a Belgian multicentre survey of primary adrenal insufficiency. Endocr. Connect. 12, e230044 (2023).
Cuesta, M. et al. The contribution of undiagnosed adrenal insufficiency to euvolaemic hyponatraemia: results of a large prospective single-centre study. Clin. Endocrinol. 85, 836–844 (2016).
Laugesen, K., Petersen, I., Sørensen, H. T. & Jørgensen, J. O. L. Clinical indicators of adrenal insufficiency following discontinuation of oral glucocorticoid therapy: a Danish population-based self-controlled case series analysis. PLoS One 14, e0212259 (2019).
Bleecker, E. R. et al. Systematic literature review of systemic corticosteroid use for asthma management. Am. J. Respir. Crit. Care Med. 201, 276–293 (2020).
Bloechliger, M. et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir. Res. 19, 75 (2018).
Sarnes, E. et al. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin. Ther. 33, 1413–1432 (2011).
Savas, M. et al. Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J. Clin. Endocrinol. Metab. 102, 3765–3774 (2017).
Souverein, P. C. et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case–control study. Heart 90, 859–865 (2004).
Wei, L., MacDonald, T. M. & Walker, B. R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 141, 764–770 (2004).
Prete, A. & Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 374, n1380 (2021).
Filipsson, H., Monson, J. P., Koltowska-Häggström, M., Mattsson, A. & Johannsson, G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J. Clin. Endocrinol. Metab. 91, 3954–3961 (2006).
Sherlock, M. et al. Adrenal incidentaloma. Endocr. Rev. 41, 775–820 (2020).
Di Dalmazi, G., Berr, C. M., Fassnacht, M., Beuschlein, F. & Reincke, M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J. Clin. Endocrinol. Metab. 99, 2637–2645 (2014).
Fassnacht, M. et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016).
Arlt, W. et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2, e93196 (2017).
Heinrich, D. A. et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J. Clin. Endocrinol. Metab. 104, 5658–5664 (2019).
Fleseriu, M. et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 3888–3921 (2016).
Arafah, B. M. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 62, 1173–1179 (1986).
Al-Shamkhi, N. et al. Pituitary function before and after surgery for nonfunctioning pituitary adenomas — data from the Swedish Pituitary Register. Eur. J. Endocrinol. 189, 217–224 (2023).
Araujo-Castro, M., Mariño-Sánchez, F., García Fernández, A., Acitores Cancela, A. & Rodríguez Berrocal, V. Endoscopic endonasal approach to pituitary adenomas: impact on adenohypophyseal function. Study of 231 cases. Neurocirugia 33, 300–309 (2022).
Staby, I. et al. Pituitary function after transsphenoidal surgery including measurement of basal morning cortisol as predictor of adrenal insufficiency. Endocr. Connect. 10, 750–757 (2021).
Nys, C. et al. Transnasal transsphenoidal pituitary surgery in a large tertiary hospital, a retrospective study. Acta Chir. Belg. 123, 272–280 (2021).
Prete, A., Corsello, S. M. & Salvatori, R. Current best practice in the management of patients after pituitary surgery. Ther. Adv. Endocrinol. Metab. 8, 33–48 (2017).
Ciric, I., Ragin, A., Baumgartner, C. & Pierce, D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40, 225–236 (1997).
Zamanipoor Najafabadi, A. H. et al. Starting point for benchmarking outcomes and reporting of pituitary adenoma surgery within the European Reference Network on Rare Endocrine Conditions (Endo-ERN): results from a meta-analysis and survey study. Endocr. Connect. 12, e220349 (2023).
Castle-Kirszbaum, M., Wang, Y. Y., King, J., Kam, J. & Goldschlager, T. Quality of life and surgical outcomes in incidental pituitary adenomas undergoing endoscopic endonasal resection. J. Neurosurg. 138, 567–573 (2023).
Tohti, M. et al. Is peri-operative steroid replacement therapy necessary for the pituitary adenomas treated with surgery? A systematic review and meta analysis. PLoS One 10, e0119621 (2015).
O’Sullivan, E. P. et al. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin. Endocrinol. 71, 709–714 (2009).
Karavitaki, N. et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 62, 397–409 (2005).
Crowley, R. K. et al. Morbidity and mortality in patients with craniopharyngioma after surgery. Clin. Endocrinol. 73, 516–521 (2010).
Yu, S. et al. Evolution of surgical outcomes in endoscopic endonasal resection of craniopharyngiomas. J. Neurol. Surg. Part. B Skull Base 84, 375–383 (2023).
Jung, T. Y. et al. Adult craniopharyngiomas: surgical results with a special focus on endocrinological outcomes and recurrence according to pituitary stalk preservation. J. Neurosurg. 111, 572–577 (2009).
Ordóñez-Rubiano, E. G. et al. Preserve or sacrifice the stalk? Endocrinological outcomes, extent of resection, and recurrence rates following endoscopic endonasal resection of craniopharyngiomas. J. Neurosurg. 131, 1163–1171 (2019).
Marko, N. F. et al. Use of morning serum cortisol level after transsphenoidal resection of pituitary adenoma to predict the need for long-term glucocorticoid supplementation. J. Neurosurg. 111, 540–544 (2009).
Filipsson, H. & Johannsson, G. GH replacement in adults: interactions with other pituitary hormone deficiencies and replacement therapies. Eur. J. Endocrinol. 161, S85–S95 (2009).
Toogood, A. A., Taylor, N. F., Shalet, S. M. & Monson, J. P. Modulation of cortisol metabolism by low-dose growth hormone replacement in elderly hypopituitary patients. J. Clin. Endocrinol. Metab. 85, 1727–1730 (2000).
Walker, B. R., Andrew, R., MacLeod, K. M. & Padfield, P. L. Growth hormone replacement inhibits renal and hepatic 11β-hydroxysteroid dehydrogenases in ACTH-deficient patients. Clin. Endocrinol. 49, 257–263 (1998).
Stewart, P. M., Toogood, A. A. & Tomlinson, J. W. Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle. Horm. Res. 56, 1–6 (2001).
Fonseca, V., Brown, R., Hochhauser, D., Ginsburg, J. & Havard, C. W. Acute adrenal crisis precipitated by thyroxine. Br. Med. J. 292, 1185–1186 (1986).
Follin, C., Wiebe, T., Moëll, C. & Erfurth, E. M. Moderate dose cranial radiotherapy causes central adrenal insufficiency in long-term survivors of childhood leukaemia. Pituitary 17, 7–12 (2014).
Appelman-Dijkstra, N. M. et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 96, 2330–2340 (2011).
Appelman-Dijkstra, N. M. et al. Pituitary dysfunction in adult patients after cranial irradiation for head and nasopharyngeal tumours. Radiother. Oncol. 113, 102–107 (2014).
Castinetti, F. et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J. Clin. Endocrinol. Metab. 94, 3400–3407 (2009).
Agha, A. et al. Hypothalamic-pituitary dysfunction after irradiation of nonpituitary brain tumors in adults. J. Clin. Endocrinol. Metab. 90, 6355–6360 (2005).
Kyriakakis, N. et al. Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumours. Clin. Endocrinol. 84, 372–379 (2016).
Patterson, B. C., Truxillo, L., Wasilewski-Masker, K., Mertens, A. C. & Meacham, L. R. Adrenal function testing in pediatric cancer survivors. Pediatr. Blood Cancer 53, 1302–1307 (2009).
Crowne, E., Gleeson, H., Benghiat, H., Sanghera, P. & Toogood, A. Effect of cancer treatment on hypothalamic-pituitary function. Lancet Diabetes Endocrinol. 3, 568–576 (2015).
Graffeo, C. S. et al. Biological effective dose as a predictor of hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis and cohort study of patients treated using contemporary techniques. Neurosurgery 88, E330–E335 (2021).
Schmiegelow, M. et al. Assessment of the hypothalamo-pituitary-adrenal axis in patients treated with radiotherapy and chemotherapy for childhood brain tumor. J. Clin. Endocrinol. Metab. 88, 3149–3154 (2003).
Sherlock, M. & Toogood, A. A. Sensitivity of anterior pituitary hormones to irradiation. Expert Rev. Endocrinol. Metab. 1, 633–649 (2006).
Kyriakakis, N. et al. Hypothalamic-pituitary axis irradiation dose thresholds for the development of hypopituitarism in adult-onset gliomas. Clin. Endocrinol. 91, 131–140 (2019).
Darzy, K. H. & Shalet, S. M. Hypopituitarism as a consequence of brain tumours and radiotherapy. Pituitary 8, 203–211 (2005).
Ratnasingam, J. et al. Hypothalamic pituitary dysfunction amongst nasopharyngeal cancer survivors. Pituitary 18, 448–455 (2015).
Lam, K. S. L., Tse, V. K. C., Wang, C., Yeung, R. T. T. & Ho, J. H. C. Effects of cranial irradiation on hypothalamic-pituitary function — a 5-year longitudinal-study in patients with nasopharyngeal carcinoma. Q. J. Med. 78, 165–176 (1991).
Pomeraniec, I. J. et al. Dose to neuroanatomical structures surrounding pituitary adenomas and the effect of stereotactic radiosurgery on neuroendocrine function: an international multicenter study. J. Neurosurg. 136, 813–821 (2022).
Palmisciano, P. et al. Endocrine disorders after primary gamma knife radiosurgery for pituitary adenomas: a systematic review and meta-analysis. Pituitary 25, 404–419 (2022).
Ironside, N. et al. Effect of distance from target on hypopituitarism after stereotactic radiosurgery for pituitary adenomas. J. Neurooncol. 158, 41–50 (2022).
Mathieu, D. et al. Stereotactic radiosurgery for secretory pituitary adenomas: systematic review and International Stereotactic Radiosurgery Society practice recommendations. J. Neurosurg. 136, 801–812 (2022).
Sims-Williams, H. P. et al. Long-term safety of gamma knife radiosurgery (SRS) for acromegaly. Pituitary 24, 724–736 (2021).
Abu Dabrh, A. M. et al. Radiotherapy versus radiosurgery in treating patients with acromegaly: a systematic review and meta-analysis. Endocr. Pract. 21, 943–956 (2015).
Keller-Wood, M. E. & Dallman, M. F. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 5, 1–24 (1984).
Ingle, D. J. & Kendall, E. C. Atrophy of the adrenal cortex of the rat produced by the administration of large amounts of cortin. Science 86, 245–245 (1937).
Broersen, L. H., Pereira, A. M., Jorgensen, J. O. & Dekkers, O. M. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 100, 2171–2180 (2015).
Güven, A. Different potent glucocorticoids, different routes of exposure but the same result: iatrogenic Cushing’s syndrome and adrenal insufficiency. J. Clin. Res. Pediatr. Endocrinol. 12, 383–392 (2020).
Borresen, S. W. et al. Approach to the patient with glucocorticoid-induced adrenal insufficiency. J. Clin. Endocrinol. Metab. 24, 2065–2076 (2022).
Hochberg, Z. E., Pacak, K. & Chrousos, G. P. Endocrine withdrawal syndromes. Endocr. Rev. 24, 523–538 (2003).
Zhang, C. D. et al. Glucocorticoid withdrawal syndrome following surgical remission of endogenous hypercortisolism: a longitudinal observational study. Eur. J. Endocrinol. 188, 592–602 (2023).
Hurtado, M. D., Cortes, T., Natt, N., Young, W. F. Jr & Bancos, I. Extensive clinical experience: hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin. Endocrinol. 89, 721–733 (2018).
Laugesen, K. et al. Management of endocrine disease: glucocorticoid-induced adrenal insufficiency: replace while we wait for evidence? Eur. J. Endocrinol. 184, R111–R122 (2021).
Theiler-Schwetz, V. & Prete, A. Glucocorticoid withdrawal syndrome: what to expect and how to manage. Curr. Opin. Endocrinol. Diabetes Obes. 30, 167–174 (2023).
He, X., Findling, J. W. & Auchus, R. J. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary 25, 393–403 (2022).
Fardet, L., Petersen, I. & Nazareth, I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology 50, 1982–1990 (2011).
Overman, R. A., Yeh, J. Y. & Deal, C. L. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 65, 294–298 (2013).
Gudbjornsson, B., Juliusson, U. I. & Gudjonsson, F. V. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann. Rheum. Dis. 61, 32–36 (2002).
van Staa, T. P. et al. Use of oral corticosteroids in the United Kingdom. QJM 93, 105–111 (2000).
Bénard-Laribière, A. et al. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open. 7, e015905 (2017).
Einarsdottir, M. J. et al. High prescription rate of oral glucocorticoids in children and adults: a retrospective cohort study from Western Sweden. Clin. Endocrinol. 92, 21–28 (2020).
Derendorf, H., Nave, R., Drollmann, A., Cerasoli, F. & Wurst, W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur. Respir. J. 28, 1042–1050 (2006).
Joseph, R. M., Hunter, A. L., Ray, D. W. & Dixon, W. G. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin. Arthritis Rheum. 46, 133–141 (2016).
Hawcutt, D. B. et al. Susceptibility to corticosteroid-induced adrenal suppression: a genome-wide association study. Lancet Respir. Med. 6, 442–450 (2018).
Brennan, V. et al. The contribution of oral and inhaled glucocorticoids to adrenal insufficiency in asthma. J. Allergy Clin. Immunol. Pract. 46, 2614–2623 (2022).
Kachroo, P. et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 28, 1723 (2022).
Simpson, H. L. & Erskine, D. Exogenous steroids treatment in adults. Adrenal insufficiency and adrenal crisis — who is at risk and how should they be managed safely. Society for Endocrinology https://www.endocrinology.org/media/4091/spssfe_supporting_sec_-final_10032021-1.pdf (2021).
Simpson, H., Tomlinson, J., Wass, J., Dean, J. & Arlt, W. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin. Med. 20, 371–378 (2020).
Tomkins, M. et al. Adrenal insufficiency is common amongst kidney transplant recipients receiving maintenance prednisolone and can be predicted using morning cortisol. Nephrol. Dial. Transpl. 38, 236–245 (2022).
Schlaghecke, R., Kornely, E., Santen, R. T. & Ridderskamp, P. The effect of long-term glucocorticoid therapy on pituitary–adrenal responses to exogenous corticotropin-releasing hormone. N. Engl. J. Med. 326, 226–230 (1992).
Dinsen, S. et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur. J. Intern. Med. 24, 714–720 (2013).
Skov, I. R. et al. Low-dose oral corticosteroids in asthma associates with increased morbidity and mortality. Eur. Respir. J. 60, 2103054 (2022).
Woods, C. P. et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur. J. Endocrinol. 173, 633–642 (2015).
Daley-Yates, P. et al. Therapeutic index of inhaled corticosteroids in asthma: a dose-response comparison on airway hyperresponsiveness and adrenal axis suppression. Br. J. Clin. Pharmacol. 87, 483–493 (2020).
Lapi, F., Kezouh, A., Suissa, S. & Ernst, P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur. Respir. J. 42, 79–86 (2013).
Woodcock, T. et al. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia 75, 654–663 (2020).
Wass, J., Simpson, H., Pearce, S. Arlt, W. Surgical Guidelines for Addison’s Disease and Other Forms of Adrenal Insufficiency https://www.addisonsdisease.org.uk/Handlers/Download.ashx?IDMF=26887766-029d-4728-9163-e4ce24eb34a7 (2021).
Martin-Grace, J., Costello, R. W. & Sherlock, M. Corticosteroid suppression in patients receiving inhaled glucocorticoids: time to reassess risk? J. Clin. Endocrinol. Metab. 107, e4256–e4258 (2022).
Guaraldi, F. et al. Comparative assessment of hypothalamic-pituitary-adrenal axis suppression secondary to intrabursal injection of different glucocorticoids: a pilot study. J. Endocrinol. Invest. 42, 1117–1124 (2019).
Daley-Yates, P. T. & Baker, R. C. Systemic bioavailability of fluticasone propionate administered as nasal drops and aqueous nasal spray formulations. Br. J. Clin. Pharmacol. 51, 103–105 (2001).
Sastre, J. & Mosges, R. Local and systemic safety of intranasal corticosteroids. J. Investig. Allergol. Clin. Immunol. 22, 1–12 (2012).
Schuetz, P. et al. Prospective analysis of adrenal function in patients with acute exacerbations of COPD: the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial. Eur. J. Endocrinol. 173, 19–27 (2015).
Henzen, C. et al. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet 355, 542–545 (2000).
Debono, M., Chan, S., Rolfe, C. & Jones, T. H. Tramadol-induced adrenal insufficiency. Eur. J. Clin. Pharmacol. 67, 865–867 (2011).
Abs, R. et al. Endocrine consequences of long-term intrathecal administration of opioids. J. Clin. Endocrinol. Metab. 85, 2215–2222 (2000).
Vescovi, P. P. et al. Metyrapone effects on β-endorphin, ACTH and cortisol levels after chronic opiate receptor stimulation in man. Neuropeptides 15, 129–132 (1990).
Facchinetti, F. et al. Hypothalamus-pituitary-adrenal axis of heroin addicts. Drug Acohol Depend. 15, 361–366 (1985).
Valverde-Filho, J., da Cunha Neto, M. B., Fonoff, E. T., Meirelles Ede, S. & Teixeira, M. J. Chronic spinal and oral morphine-induced neuroendocrine and metabolic changes in noncancer pain patients. Pain Med. 16, 715–725 (2015).
Taylor, T., Dluhy, R. G. & Williams, G. H. β-Endorphin suppresses adrenocorticotropin and cortisol levels in normal human subjects. J. Clin. Endocrinol. Metab. 57, 592–596 (1983).
Palm, S., Moenig, H. & Maier, C. Effects of oral treatment with sustained release morphine tablets on hypothalamic-pituitary-adrenal axis. Methods Find. Exp. Clin. Pharmacol. 19, 269–273 (1997).
Fountas, A., Van Uum, S. & Karavitaki, N. Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol. 8, 68–80 (2020).
Glahn, A. et al. Atrial natriuretic peptide, arginine vasopressin peptide and cortisol serum levels in opiate-dependent patients. Neuropsychobiology 67, 111–115 (2013).
Daniell, H. W. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J. Pain 7, 901–907 (2006).
Facchinetti, F. et al. Impaired circadian rhythmicity of beta-lipotrophin, beta-endorphin and ACTH in heroin addicts. Acta Endocrinol. 105, 149–155 (1984).
Gadelha, M. R. et al. Opioids and pituitary function: expert opinion. Pituitary 25, 52–63 (2022).
Lamprecht, A., Sorbello, J., Jang, C., Torpy, D. J. & Inder, W. J. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur. J. Endocrinol. 179, 353–362 (2018).
Donegan, D. Opioid induced adrenal insufficiency: what is new? Curr. Opin. Endocrinol. Diabetes Obes. 26, 133–138 (2019).
Li, T. et al. Prevalence of opioid-induced adrenal insufficiency in patients taking chronic opioids. J. Clin. Endocrinol. Metab. 105, e3766–3775 (2020).
Saeed, Z. I., Bancos, I. & Donegan, D. Current knowledge and practices of heath care professionals on opioid-induced adrenal insufficiency. Endocr. Pract. 25, 1012–1021 (2019).
de Vries, F. et al. Opioids and their endocrine effects: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 105, 1020–1029 (2020).
Nenke, M. A. et al. Low-dose hydrocortisone replacement improves wellbeing and pain tolerance in chronic pain patients with opioid-induced hypocortisolemic responses. A pilot randomized, placebo-controlled trial. Psychoneuroendocrinology 56, 157–167 (2015).
Gibb, F. W., Stewart, A., Walker, B. R. & Strachan, M. W. Adrenal insufficiency in patients on long-term opioid analgesia. Clin. Endocrinol. 85, 831–835 (2016).
Shalaby, A. M., Aboregela, A. M., Alabiad, M. A. & El Shaer, D. F. Tramadol promotes oxidative stress, fibrosis, apoptosis, ultrastructural and biochemical alterations in the adrenal cortex of adult male rat with possible reversibility after withdrawal. Microsc. Microanal. 26, 509–523 (2020).
Müssig, K., Knaus-Dittmann, D., Schmidt, H., Mörike, K. & Häring, H.-U. Secondary adrenal failure and secondary amenorrhoea following hydromorphone treatment. Clin. Endocrinol. 66, 604–605 (2007).
Fountas, A., Chai, S. T., Kourkouti, C. & Karavitaki, N. MECHANISMS OF ENDOCRINOLOGY: endocrinology of opioids. Eur. J. Endocrinol. 179, R183–r196 (2018).
Pofi, R. et al. The short synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J. Clin. Endocrinol. Metab. 103, 3050–3059 (2018).
Byun, D. J., Wolchok, J. D., Rosenberg, L. M. & Girotra, M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 13, 195–207 (2017).
Husebye, E. S. et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur. J. Endocrinol. 187, G1–G21 (2022).
Tan, M. H. et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin. Diabetes Endocrinol. 5, 1 (2019).
Di Dalmazi, G., Ippolito, S., Lupi, I. & Caturegli, P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev. Endocrinol. Metab. 14, 381–398 (2019).
Johnson, J. et al. Hypophysitis and secondary adrenal insufficiency from immune checkpoint inhibitors: diagnostic challenges and link with survival. J. Natl Compr. Canc. Netw. 21, 281–287 (2023).
Kotwal, A. et al. Immune checkpoint inhibitor-induced hypophysitis: lessons learnt from a large cancer cohort. J. Investig. Med. 70, 939–946 (2022).
Jessel, S. et al. Immune checkpoint inhibitor-induced hypophysitis and patterns of loss of pituitary function. Front. Oncol. 12, 836859 (2022).
Nguyen, H. et al. Immune checkpoint inhibitor related hypophysitis: diagnostic criteria and recovery patterns. Endocr. Relat. Cancer 28, 419–431 (2021).
Albarel, F. et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 172, 195–204 (2015).
Faje, A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 19, 82–92 (2016).
Cooksley, T. et al. Immune checkpoint inhibitor-mediated hypophysitis: no place like home. Clin. Med. 23, 81–84 (2023).
Simeni Njonnou, S. R. et al. Isolated adrenocorticotropic hormone deficiency and sialadenitis associated with nivolumab: a case report. J. Med. Case Rep. 16, 456 (2022).
Lin, S. H. et al. Isolated adrenocorticotropic hormone deficiency associated with sintilimab therapy in a patient with advanced lung adenocarcinoma: a case report and literature review. BMC Endocr. Disord. 22, 239 (2022).
Yamauchi, I. et al. Clinical features and thyroid dysfunction in adverse events involving the pituitary gland during PD-1 blockade therapy. Clin. Endocrinol. 94, 258–268 (2021).
Iglesias, P., Sánchez, J. C. & Díez, J. J. Isolated ACTH deficiency induced by cancer immunotherapy: a systematic review. Pituitary 24, 630–643 (2021).
Heck, A. & Winge-Main, A. K. Silent, isolated ACTH deficiency in malignant melanoma patients treated with immune checkpoint inhibitors. BMJ Case Rep. 14, e241981 (2021).
Suzuki, K. et al. Nivolumab-induced adrenal insufficiency in patients with renal cell carcinoma. J. Immunother. 43, 38–42 (2020).
Faje, A. et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 181, 211–219 (2019).
Barroso-Sousa, R. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 4, 173–182 (2018).
Grouthier, V. et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: who vigibase report analysis. Oncologist 25, 696–701 (2020).
Bischoff, J. et al. It’s not always SIAD: immunotherapy-triggered endocrinopathies enter the field of cancer-related hyponatremia. J. Endocr. Soc. 6, bvac036 (2022).
Hanna, R. M. et al. Acute kidney injury after pembrolizumab-induced adrenalitis and adrenal insufficiency. Case Rep. Nephrol. Dial. 8, 171–177 (2018).
Paepegaey, A. C. et al. Polyendocrinopathy resulting from pembrolizumab in a patient with a malignant melanoma. J. Endocr. Soc. 1, 646–649 (2017).
Ruggeri, R. M. et al. Endocrine and metabolic adverse effects of immune checkpoint inhibitors: an overview (what endocrinologists should know). J. Endocrinol. Invest. 42, 745–756 (2019).
Atkinson, M. & Lansdown, A. J. Endocrine immune-related adverse events: adrenal, parathyroid, diabetes insipidus, and lipoatrophy. Best Pract. Res. Clin. Endocrinol. Metab. 36, 101635 (2022).
Bacanovic, S., Burger, I. A., Stolzmann, P., Hafner, J. & Huellner, M. W. Ipilimumab-induced adrenalitis: a possible pitfall in 18F-FDG-PET/CT. Clin. Nucl. Med. 40, e518–519 (2015).
Shi, Y. et al. ICPis-induced autoimmune polyendocrine syndrome type 2: a review of the literature and a protocol for optimal management. J. Clin. Endocrinol. Metab. 105, dgaa553 (2020).
Higham, C. E. et al. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr. Connect. 7, G1–G7 (2018).
Nieman, L. K. et al. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 2807–2831 (2015).
Daniel, E. et al. Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients. J. Clin. Endocrinol. Metab. 100, 4146–4154 (2015).
Nieman, L. K. et al. Metyrapone treatment in endogenous Cushing’s syndrome: results at week 12 from PROMPT, a prospective international multicenter, open-label, phase III/IV study. J. Endocr. Soc. 5, A515 (2021).
Pivonello, R. et al. Levoketoconazole in the treatment of patients with endogenous Cushing’s syndrome: a double-blind, placebo-controlled, randomized withdrawal study (LOGICS). Pituitary 25, 911–926 (2022).
Fleseriu, M. et al. Levoketoconazole treatment in endogenous Cushing’s syndrome: extended evaluation of clinical, biochemical, and radiologic outcomes. Eur. J. Endocrinol. 187, 859–871 (2022).
Pivonello, R., Simeoli, C., Di Paola, N. & Colao, A. Cushing’s disease: adrenal steroidogenesis inhibitors. Pituitary 25, 726–732 (2022).
Bonnet-Serrano, F. et al. Differences in the spectrum of steroidogenic enzyme inhibition between osilodrostat and metyrapone in ACTH-dependent Cushing syndrome patients. Eur. J. Endocrinol. 187, 315–322 (2022).
Hahner, S. et al. Etomidate unmasks intraadrenal regulation of steroidogenesis and proliferation in adrenal cortical cell lines. Horm. Metab. Res. 42, 528–534 (2010).
Preda, V. A., Sen, J., Karavitaki, N. & Grossman, A. B. THERAPY IN ENDOCRINE DISEASE: etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur. J. Endocrinol. 167, 137–143 (2012).
Fleseriu, M. et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 9, 847–875 (2021).
Castinetti, F., Nieman, L. K., Reincke, M. & Newell-Price, J. Approach to the patient treated with steroidogenesis inhibitors. J. Clin. Endocrinol. Metab. 106, 2114–2123 (2021).
Fleseriu, M. & Biller, B. M. K. Treatment of Cushing’s syndrome with osilodrostat: practical applications of recent studies with case examples. Pituitary 25, 795–809 (2022).
Tritos, N. A. Adrenally directed medical therapies for Cushing syndrome. J. Clin. Endocrinol. Metab. 106, 16–25 (2020).
Poli, G. et al. Morphofunctional effects of mitotane on mitochondria in human adrenocortical cancer cells. Endocr. Relat. Cancer 20, 537–550 (2013).
Chortis, V. et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5alpha-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J. Clin. Endocrinol. Metab. 98, 161–171 (2013).
Fassnacht, M. et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 179, G1–G46 (2018).
Fleseriu, M. et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 97, 2039–2049 (2012).
Molitch, M. E. Glucocorticoid receptor blockers. Pituitary 25, 733–736 (2022).
Hopkins, S. & Fleseriu, M. In: Edward R. Laws (ed.) Cushing’s Disease 103-123 (Academic Press, 2017).
Sai, K., Lal, A., Lakshmi Maradana, J., Velamala, P. R. & Nitin, T. Hypokalemia associated with mifepristone use in the treatment of Cushing’s syndrome. Endocrinol. Diabetes Metab. Case Rep. 2019, https://doi.org/10.1530/EDM-19-0064 (2019).
Arosemena, M. A., Rodriguez, A. & Ediriweera, H. Bilateral adrenal haemorrhage secondary to rivaroxaban in a patient with antiphospholipid syndrome. BMJ Case Rep. 13, e234947 (2020).
Saleem, N., Khan, M., Parveen, S. & Balavenkatraman, A. Bilateral adrenal haemorrhage: a cause of haemodynamic collapse in heparin-induced thrombocytopaenia. BMJ Case Rep. 2016, bcr2016214679 (2016).
Patousis, A., Patousis, P., Barbakis, G. & Sachinis, N. P. Bilateral adrenal hemorrhage following femoral hip hemiarthroplasty: a case report. Cureus 14, e27748 (2022).
VanderVeer, E. A., Torbiak, R. P., Prebtani, A. P. & Warkentin, T. E. Spontaneous heparin-induced thrombocytopenia syndrome presenting as bilateral adrenal infarction after knee arthroplasty. BMJ Case Rep. 12, e232769 (2019).
Elhassan, Y. S., Ronchi, C. L., Wijewickrama, P. & Baldeweg, S. E. Approach to the patient with adrenal hemorrhage. J. Clin. Endocrinol. Metab. 108, 995–1006 (2022).
Ali, A., Singh, G. & Balasubramanian, S. P. Acute non-traumatic adrenal haemorrhage-management, pathology and clinical outcomes. Gland. Surg. 7, 428–432 (2018).
Dunlop, D. Eighty-six cases of Addison’s disease. Br. Med. J. 2, 887–891 (1963).
Dineen, R. et al. Outcomes of the short synacthen test: what is the role of the 60 min sample in clinical practice? Postgrad. Med. J. 96, 67–72 (2020).
Sbardella, E. et al. Baseline morning cortisol level as a predictor of pituitary-adrenal reserve: a comparison across three assays. Clin. Endocrinol. 86, 177–184 (2017).
Sagar, R., Mackie, S., Morgan, A. W., Stewart, P. & Abbas, A. Evaluating tertiary adrenal insufficiency in rheumatology patients on long-term systemic glucocorticoid treatment. Clin. Endocrinol. 94, 361–370 (2021).
Bornstein, S. R. et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 364–389 (2016).
El-Farhan, N. et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin. Endocrinol. 78, 673–680 (2013).
Prete, A. et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J. Clin. Endocrinol. Metab. 105, 2262–2274 (2020).
Elecsys® Cortisol II https://labogids.sintmaria.be/sites/default/files/files/cortisol_ii_2020-03_v6.pdf (Roche Diagnostics, 2020).
Stokes, F. J., Bailey, L. M., Ganguli, A. & Davison, A. S. Assessment of endogenous, oral and inhaled steroid cross-reactivity in the Roche cortisol immunoassay. Ann. Clin. Biochem. 51, 503–506 (2014).
Taylor, A. E., Keevil, B. & Huhtaniemi, I. T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 173, D1–12 (2015).
Monaghan, P. J. et al. Comparison of serum cortisol measurement by immunoassay and liquid chromatography-tandem mass spectrometry in patients receiving the 11β-hydroxylase inhibitor metyrapone. Ann. Clin. Biochem. 48, 441–446 (2011).
Klose, M. et al. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J. Clin. Endocrinol. Metab. 92, 1326–1333 (2007).
Edo, N. et al. Diagnostic value of standard deviation score of log-transformed serum dehydroepiandrosterone sulfate in patients with hypothalamic-pituitary-adrenal axis insufficiency. Endocr. J. 68, 1337–1345 (2021).
Blair, J., Adaway, J., Keevil, B. & Ross, R. Salivary cortisol and cortisone in the clinical setting. Curr. Opin. Endocrinol. Diabetes Obes. 24, 161–168 (2017).
Debono, M. et al. Home waking salivary cortisone to screen for adrenal insufficiency. NEJM Evid. 2, EVIDoa2200182 (2023).
Debono, M. et al. Waking salivary cortisone as screening test for adrenal insufficiency. Endocr. Abstr. 81, P7 (2022).
El-Farhan, N., Rees, D. A. & Evans, C. Measuring cortisol in serum, urine and saliva — are our assays good enough? Ann. Clin. Biochem. 54, 308–322 (2017).
Agha, A., Tomlinson, J. W., Clark, P. M., Holder, G. & Stewart, P. M. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J. Clin. Endocrinol. Metab. 91, 43–47 (2006).
Stewart, P. M., Corrie, J., Seckl, J. R., Edwards, C. R. & Padfield, P. L. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet 1, 1208–1210 (1988).
Bangar, V. & Clayton, R. N. How reliable is the short synacthen test for the investigation of the hypothalamic-pituitary-adrenal axis? Eur. J. Endocrinol. 139, 580–583 (1998).
Dekkers, O. M., Timmermans, J. M., Smit, J. W., Romijn, J. A. & Pereira, A. M. Comparison of the cortisol responses to testing with two doses of ACTH in patients with suspected adrenal insufficiency. Eur. J. Endocrinol. 164, 83–87 (2011).
Ospina, N. S. et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 101, 427–434 (2016).
Mukherjee, J. J. et al. A comparison of the insulin tolerance/glucagon test with the short ACTH stimulation test in the assessment of the hypothalamo-pituitary-adrenal axis in the early post-operative period after hypophysectomy. Clin. Endocrinol. 47, 51–60 (1997).
Sherlock, M. & Stewart, P. M. The short synacthen test and its utility in assessing recovery of adrenal function in patients with central adrenal insufficiency. J. Clin. Endocrinol. Metab. 104, 17–20 (2019).
Lawrence, N. R. et al. Multivariable model to predict an ACTH stimulation test to diagnose adrenal insufficiency using previous test results. J. Endocr. Soc. 7, bvad127 (2023).
Plumpton, F. S. & Besser, G. M. The adrenocortical response to surgery and insulin-induced hypoglycaemia in corticosteroid-treated and normal subjects. Br. J. Surg. 56, 216–219 (1969).
Agha, A. et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J. Clin. Endocrinol. Metab. 89, 4929–4936 (2004).
Garrahy, A., Sherlock, M. & Thompson, C. J. MANAGEMENT OF ENDOCRINE DISEASE: neuroendocrine surveillance and management of neurosurgical patients. Eur. J. Endocrinol. 176, R217–R233 (2017).
Fiad, T. M., Kirby, J. M., Cunningham, S. K. & McKenna, T. J. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic-pituitary-adrenal axis. Clin. Endocrinol. 40, 603–609 (1994).
Cegla, J. et al. Comparison of the overnight metyrapone and glucagon stimulation tests in the assessment of secondary hypoadrenalism. Clin. Endocrinol. 78, 738–742 (2013).
Johannsson, G., Skrtic, S., Lennernäs, H., Quinkler, M. & Stewart, P. M. Improving outcomes in patients with adrenal insufficiency: a review of current and future treatments. Curr. Med. Res. Opin. 30, 1833–1847 (2014).
Walker, J. J. et al. The origin of glucocorticoid hormone oscillations. PLoS Biol. 10, e1001341 (2012).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 (2010).
Gjerstad, J. K., Lightman, S. L. & Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21, 403–416 (2018).
Krieger, D. T., Allen, W., Rizzo, F. & Krieger, H. P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J. Clin. Endocrinol. Metab. 32, 266–284 (1971).
Behan, L.-A. et al. Optimizing glucocorticoid replacement therapy in severely adrenocorticotropin-deficient hypopituitary male patients. Clin. Endocrinol. 75, 505–513 (2011).
Upton, T. J. et al. High-resolution daily profiles of tissue adrenal steroids by portable automated collection. Sci. Transl. Med. 15, eadg8464 (2023).
Dineen, R., Martin-Grace, J., Thompson, C. J. & Sherlock, M. The management of glucocorticoid deficiency: current and future perspectives. Clin. Chim. Acta 505, 148–159 (2020).
Murray, R. D. et al. Management of glucocorticoid replacement in adrenal insufficiency shows notable heterogeneity — data from the EU-AIR. Clin. Endocrinol. 86, 340–346 (2017).
Quinkler, M. et al. Prednisolone is associated with a worse lipid profile than hydrocortisone in patients with adrenal insufficiency. Endocr. Connect. 6, 1–8 (2017).
Husebye, E. S. et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 275, 104–115 (2014).
Plat, L. et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J. Clin. Endocrinol. Metab. 84, 3082–3092 (1999).
Esteban, N. V. et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J. Clin. Endocrinol. Metab. 72, 39–45 (1991).
Vulto, A. et al. Residual endogenous corticosteroid production in patients with adrenal insufficiency. Clin. Endocrinol. 91, 383–390 (2019).
Werumeus Buning, J. et al. Effects of hydrocortisone on the regulation of blood pressure: results from a randomized controlled trial. J. Clin. Endocrinol. Metab. 101, 3691–3699 (2016).
Mohammedi, K. et al. Evidence of persistent mild hypercortisolism in patients medically treated for Cushing disease: the Haircush study. J. Clin. Endocrinol. Metab. 108, e963–e970 (2023).
Simon, N. et al. Pharmacokinetic evidence for suboptimal treatment of adrenal insufficiency with currently available hydrocortisone tablets. Clin. Pharmacokinet. 49, 455–463 (2010).
Gagliardi, L. et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison’s disease: a randomized, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 99, 4149–4157 (2014).
Isidori, A. M. et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 6, 173–185 (2018).
Mallappa, A. et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 100, 1137–1145 (2015).
Øksnes, M. et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison’s disease: a randomized clinical trial. J. Clin. Endocrinol. Metab. 99, 1665–1674 (2014).
Dineen, R. et al. Cardiometabolic and psychological effects of dual-release hydrocortisone: a cross-over study. Eur. J. Endocrinol. 184, 253–265 (2021).
Quinkler, M., Miodini Nilsen, R., Zopf, K., Ventz, M. & Øksnes, M. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur. J. Endocrinol. 172, 619–626 (2015).
Oprea, A., Bonnet, N. C. G., Pollé, O. & Lysy, P. A. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther. Adv. Endocrinol. Metab. 10, 2042018818821294 (2019).
Grossman, A. B. Clinical review#: the diagnosis and management of central hypoadrenalism. J. Clin. Endocrinol. Metab. 95, 4855–4863 (2010).
Hahner, S. et al. Adrenal insufficiency. Nat. Rev. Dis. Prim. 7, 19 (2021).
Rushworth, R. L., Torpy, D. J. & Falhammar, H. Adrenal crises: perspectives and research directions. Endocrine 55, 336–345 (2017).
Rushworth, R. L., Torpy, D. J. & Falhammar, H. Adrenal crisis. N. Engl. J. Med. 381, 852–861 (2019).
Puar, T. H. K., Stikkelbroeck, N. M. M. L., Smans, L. C. C. J., Zelissen, P. M. J. & Hermus, A. R. M. M. Adrenal crisis: still a deadly event in the 21st century. Am. J. Med. 129, 339.e1-9 (2016).
Allolio, B. EXTENSIVE EXPERTISE IN ENDOCRINOLOGY: adrenal crisis. Eur. J. Endocrinol. 172, R115–R124 (2015).
Arlt, W., Baldeweg, S. E., Pearce, S. H. S. & Simpson, H. L. ENDOCRINOLOGY IN THE TIME OF COVID-19: management of adrenal insufficiency. Eur. J. Endocrinol. 183, G25 (2020).
Roberts, A., James, J. & Dhatariya, K. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet. Med. 35, 1011–1017 (2018).
Othonos, N. et al. 11β-HSD1 inhibition in men mitigates prednisolone-induced adverse effects in a proof-of-concept randomised double-blind placebo-controlled trial. Nat. Commun. 14, 1025 (2023).
Betterle, C., Dal Pra, C., Mantero, F. & Zanchetta, R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr. Rev. 23, 327–364 (2002).
Nerup, J. Addison’s disease — clinical studies. A report fo 108 cases. Acta Endocrinol. 76, 127–141 (1974).
Martin-Grace, J., Tomkins, M., O’Reilly, M. W., Thompson, C. J. & Sherlock, M. Approach to the patient: hyponatremia and the syndrome of inappropriate antidiuresis (SIAD). J. Clin. Endocrinol. Metab. 35, 2362–2376 (2022).
Acknowledgements
J.M.-G. has received funding from the Irish Endocrine Society Clinical Science Award. M.T. is an Irish Clinical Academic Training programme fellow funded by the Health Research Board (HRB) and Wellcome Trust (grant 203930/B/16/Z). M.W.O'.R. is funded by a HRB Emerging Clinician Scientist Award (ECSA2020-001).
Author information
Authors and Affiliations
Contributions
M.S. and M.W.O’R. made a substantial contribution to the discussion of content and wrote, reviewed and edited the manuscript before submission. J.M.-G. and M.T. researched data for the article, made a substantial contribution to the discussion of content, and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.S. receives research grant funding from Shire Ltd. The remaining authors declare no competing interests.
Peer review
Peer-review information
Nature Reviews Endocrinology thanks Olle Kämpe, Charlotte Elder, Frederic Castinetti and Eystein Husebye for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martin-Grace, J., Tomkins, M., O’Reilly, M.W. et al. Iatrogenic adrenal insufficiency in adults. Nat Rev Endocrinol 20, 209–227 (2024). https://doi.org/10.1038/s41574-023-00929-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00929-x