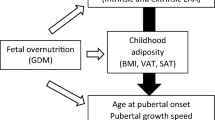
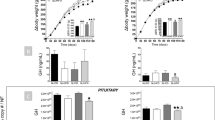
Abstract
An individual’s nutritional status has a powerful effect on sexual maturation. Puberty onset is delayed in response to chronic energy insufficiency and is advanced under energy abundance. The consequences of altered pubertal timing for human health are profound. Late puberty increases the chances of cardiometabolic, musculoskeletal and neurocognitive disorders, whereas early puberty is associated with increased risks of adult obesity, type 2 diabetes mellitus, cardiovascular diseases and various cancers, such as breast, endometrial and prostate cancer. Kennedy and Mitra’s trailblazing studies, published in 1963 and using experimental models, were the first to demonstrate that nutrition is a key factor in puberty onset. Building on this work, the field has advanced substantially in the past decade, which is largely due to the impressive development of molecular tools for experimentation and population genetics. In this Review, we discuss the latest advances in basic and translational sciences underlying the nutritional and metabolic control of pubertal development, with a focus on perspectives and future directions.
Key points
-
In 1963, Kennedy and Mitra published a seminal study in rats demonstrating that body weight is a major determinant of pubertal timing.
-
An increasing incidence of earlier ages at puberty has been documented; early pubertal timing favours the occurrence of type 2 diabetes mellitus, cardiovascular diseases and certain cancers in adulthood.
-
Macronutrients and hormones that modulate growth and/or signal adipose tissue mass serve as metabolic cues conveying the nutritional status and stored energy available for sexual maturation, differentiation and growth.
-
The effect of metabolic cues on puberty is mediated by neural targets upstream of GnRH neurons; considerable progress in defining the neuronal circuitry and glial components has been achieved.
-
A number of molecular pathways and epigenetic mechanisms have been identified as primary components in the modulation of pubertal timing by hormones and nutritional cues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Argente, J. et al. Molecular basis of normal and pathological puberty: from basic mechanisms to clinical implications. Lancet Diabetes Endocrinol. 11, 203–216 (2023).
Avendano, M. S., Vazquez, M. J. & Tena-Sempere, M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update 23, 737–763 (2017).
Plant, T. M. & Barker-Gibb, M. L. Neurobiological mechanisms of puberty in higher primates. Hum. Reprod. Update 10, 67–77 (2004).
Sisk, C. L. & Foster, D. L. The neural basis of puberty and adolescence. Nat. Neurosci. 7, 1040–1047 (2004).
Tena-Sempere, M. The roles of kisspeptins and G protein-coupled receptor-54 in pubertal development. Curr. Opin. Pediatr. 18, 442–447 (2006).
Kennedy, G. C. & Mitra, J. Body weight and food intake as initiating factors for puberty in the rat. J. Physiol. 166, 408–418 (1963).
Frisch, R. E. Fatness, menarche, and female fertility. Perspect. Biol. Med. 28, 611–633 (1985).
Frisch, R. E. The right weight: body fat, menarche and fertility. Proc. Nutr. Soc. 53, 113–129 (1994).
Schneider, J. E. Energy balance and reproduction. Physiol. Behav. 81, 289–317 (2004).
Kaplowitz, P. Pubertal development in girls: secular trends. Curr. Opin. Obstet. Gynecol. 18, 487–491 (2006).
Biro, F. M. et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126, e583–e590 (2010).
Herman-Giddens, M. E. et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99, 505–512 (1997).
Burt Solorzano, C. M. & McCartney, C. R. Obesity and the pubertal transition in girls and boys. Reproduction 140, 399–410 (2010).
Euling, S. Y. et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 121, S172–S191 (2008).
Ahmed, M. L., Ong, K. K. & Dunger, D. B. Childhood obesity and the timing of puberty. Trends Endocrinol. Metab. 20, 237–242 (2009).
Reinehr, T. & Roth, C. L. Is there a causal relationship between obesity and puberty? Lancet Child. Adolesc. Health 3, 44–54 (2019).
Day, F. R., Elks, C. E., Murray, A., Ong, K. K. & Perry, J. R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci. Rep. 5, 11208 (2015).
Freedman, D. S. et al. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 3, 3 (2003).
Hollis, B. et al. Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat. Commun. 11, 1536 (2020).
Day, F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 49, 834 (2017).
Zhu, J. & Chan, Y. M. Adult consequences of self-limited delayed puberty. Pediatrics 139, e20163177 (2017).
Welt, C. K. et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 (2004).
Mountjoy, M. et al. The IOC consensus statement: beyond the Female Athlete Triad–Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 48, 491–497 (2014).
Papadimitriou, A. The evolution of the age at menarche from prehistorical to modern times. J. Pediatr. Adolesc. Gynecol. 29, 527–530 (2016).
Piras, G. N. et al. The levelling-off of the secular trend of age at menarche among Italian girls. Heliyon 6, e04222 (2020).
Parent, A. S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Jansen, E. C., Herran, O. F. & Villamor, E. Trends and correlates of age at menarche in Colombia: results from a nationally representative survey. Econ. Hum. Biol. 19, 138–144 (2015).
Pereira, A., Corvalan, C., Merino, P. M., Leiva, V. & Mericq, V. Age at pubertal development in a Hispanic-Latina female population: should the definitions be revisited? J. Pediatr. Adolesc. Gynecol. 32, 579–583 (2019).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. 174, e195881 (2020).
Rosenfield, R. L., Lipton, R. B. & Drum, M. L. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 123, 84–88 (2009).
Jasik, C. B. & Lustig, R. H. Adolescent obesity and puberty: the “perfect storm”. Ann. N. Y. Acad. Sci. 1135, 265–279 (2008).
Dunger, D. B., Ahmed, M. L. & Ong, K. K. Effects of obesity on growth and puberty. Best. Pract. Res. Clin. Endocrinol. Metab. 19, 375–390 (2005).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Hill, J. W. & Elias, C. F. Neuroanatomical framework of the metabolic control of reproduction. Physiol. Rev. 98, 2349–2380 (2018).
Evans, M. C., Campbell, R. E. & Anderson, G. M. Physiological regulation of leptin as an integrative signal of reproductive readiness. Curr. Opin. Pharmacol. 67, 102321 (2022).
Casado, M. E., Collado-Perez, R., Frago, L. M. & Barrios, V. Recent advances in the knowledge of the mechanisms of leptin physiology and actions in neurological and metabolic pathologies. Int. J. Mol. Sci. 24, 1422 (2023).
Chehab, F. F., Lim, M. E. & Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12, 318–320 (1996).
Farooqi, I. S. Leptin and the onset of puberty: insights from rodent and human genetics. Semin. Reprod. Med. 20, 139–144 (2002).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Ahima, R. S., Dushay, J., Flier, S. N., Prabakaran, D. & Flier, J. S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest. 99, 391–395 (1997).
Chehab, F. F., Mounzih, K., Lu, R. & Lim, M. E. Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88–90 (1997).
Matsubara, M., Maruoka, S. & Katayose, S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol. 147, 173–180 (2002).
Sitticharoon, C., Sukharomana, M., Likitmaskul, S., Churintaraphan, M. & Maikaew, P. Increased high molecular weight adiponectin, but decreased total adiponectin and kisspeptin, in central precocious puberty compared with aged-matched prepubertal girls. Reprod. Fertil. Dev. 29, 2466–2478 (2017).
Contreras, C. et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 9, 366–377 (2014).
Magnan, C., Levin, B. E. & Luquet, S. Brain lipid sensing and the neural control of energy balance. Mol. Cell Endocrinol. 418, 3–8 (2015).
Heras, V. et al. Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 32, 951–966 (2020).
Roa, J. et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc. Natl Acad. Sci. USA 115, E10758–E10767 (2018).
Torsoni, M. A. et al. AMPKɑ2 in Kiss1 neurons is required for reproductive adaptations to acute metabolic challenges in adult female mice. Endocrinology 157, 4803–4816 (2016).
Franssen, D. et al. AMP-activated protein kinase (AMPK) signaling in GnRH neurons links energy status and reproduction. Metabolism 115, 154460 (2021).
Hayashida, T. et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J. Endocrinol. 173, 239–245 (2002).
Aguilar, E., Tena-Sempere, M. & Pinilla, L. Role of excitatory amino acids in the control of growth hormone secretion. Endocrine 28, 295–302 (2005).
Torres, P. J. et al. The role of intragestational ghrelin on postnatal development and reproductive programming in mice. Reproduction 156, 331–341 (2018).
Velasquez, D. A. et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 60, 1177–1185 (2011).
Bergqvist, N. The gonadal function in female diabetics. Acta Endocrinol. Suppl. 19, 1–20 (1954).
Schriock, E. A., Winter, R. J. & Traisman, H. S. Diabetes mellitus and its effects on menarche. J. Adolesc. Health Care 5, 101–104 (1984).
Kjaer, K., Hagen, C., Sandø, S. H. & Eshøj, O. Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J. Clin. Endocrinol. Metab. 75, 524–529 (1992).
Codner, E., Merino, P. M. & Tena-Sempere, M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum. Reprod. Update 18, 568–585 (2012).
Gaete, X. et al. Earlier puberty in boys with type 1 diabetes mellitus compared to a simultaneously recruited group of control adolescents. Pediatr. Diabetes 20, 197–201 (2019).
Brüning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Evans, M. C., Hill, J. W. & Anderson, G. M. Role of insulin in the neuroendocrine control of reproduction. J. Neuroendocrinol. 33, e12930 (2021).
Saleh, F. L. et al. Hyperinsulinemia induces early and dyssynchronous puberty in lean female mice. J. Endocrinol. 254, 121–135 (2022).
Manaserh, I. H. et al. Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice. PLoS Biol. 17, e3000189 (2019).
Evans, M. C., Rizwan, M., Mayer, C., Boehm, U. & Anderson, G. M. Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J. Neuroendocrinol. 26, 468–479 (2014).
Xu, C. et al. KLB, encoding β‐Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol. Med. 9, 1379–1397 (2017).
Owen, B. M. et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 19, 1153–1156 (2013).
MacLusky, N. J. et al. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141, 752–762 (2000).
Outeirino-Iglesias, V., Romani-Perez, M., Gonzalez-Matias, L. C., Vigo, E. & Mallo, F. GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 156, 4226–4237 (2015).
Korpela, K. et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci. Rep. 11, 23297 (2021).
Sisk-Hackworth, L., Kelley, S. T. & Thackray, V. G. Sex, puberty, and the gut microbiome. Reproduction 165, R61–R74 (2023).
Ilyes, T., Silaghi, C. N. & Craciun, A. M. Diet-related changes of short-chain fatty acids in blood and feces in obesity and metabolic syndrome. Biology 11, 1556 (2022).
Wang, L. et al. Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol. 13, 1051797 (2022).
Yuan, X., Shangguan, H., Zhang, Y., Lin, X. & Chen, R. Intervention effect of probiotics on the early onset of puberty induced by daidzein in female mice. Mol. Nutr. Food Res. 67, e2200501 (2023).
Bo, T. et al. Effects of high-fat diet during childhood on precocious puberty and gut microbiota in mice. Front. Microbiol. 13, 930747 (2022).
Wang, M. et al. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology 161, bqz041 (2020).
Martha, P. M. Jr. et al. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J. Clin. Endocrinol. Metab. 69, 563–570 (1989).
Cemeroglu, A. P., Barkan, A. L., Kletter, G. B., Beitins, I. Z. & Foster, C. M. Changes in serum immunoreactive and bioactive growth hormone concentrations in boys with advancing puberty and in response to a 20-hour estradiol infusion. J. Clin. Endocrinol. Metab. 82, 2166–2171 (1997).
Batch, J. A. & Werther, G. A. Changes in growth hormone concentrations during puberty in adolescents with insulin dependent diabetes. Clin. Endocrinol. 36, 411–416 (1992).
Sabin, M. A. et al. Insulin and BMI as predictors of adult type 2 diabetes mellitus. Pediatrics 135, 144–151 (2015).
Cavarzere, P. et al. Growth hormone retesting during puberty: a cohort study. Eur. J. Endocrinol. 182, 559–567 (2020).
Juul, A. & Skakkebæk, N. E. Why do normal children have acromegalic levels of IGF-I during puberty? J. Clin. Endocrinol. Metab. 104, 2770–2776 (2019).
Orçun, A., Yildiz, Z. & Köroğlu Dağdelen, L. Pediatric reference intervals for free testosterone, 17-OH progesterone, androstenedione, and IGF-1 with chemiluminescence immunoassay. Steroids 186, 109078 (2022).
Baumgartner, M. et al. Plasma myostatin increases with age in male youth and negatively correlates with vitamin D in severe pediatric obesity. Nutrients 14, 2133 (2022).
Reinehr, T., Elfers, C., Lass, N. & Roth, C. L. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J. Clin. Endocrinol. Metab. 100, 2123–2130 (2015).
Chen, Y., Li, M., Liao, B., Zhong, J. & Lan, D. Serum irisin levels increase in girls with central precocious puberty not dependent on BMI: a pilot study. Endocr. Connect. 11, e220028 (2022).
Kutlu, E. et al. Serum irisin levels in central precocious puberty and its variants. J. Clin. Endocrinol. Metab. 106, e247–e254 (2021).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 (1997).
DiVall, S. A. et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Invest. 120, 2900–2909 (2010).
Decourt, C., Evans, M. C., Inglis, M. A. & Anderson, G. M. Central irisin signaling Is required for normal timing of puberty in female mice. Endocrinology 164, bqac208 (2023).
Bohlen, T. M. et al. Central growth hormone signaling is not required for the timing of puberty. J. Endocrinol. 243, 161–173 (2019).
Savage, M. O. et al. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J. Clin. Endocrinol. Metab. 77, 1465–1471 (1993).
Juul, A. et al. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 levels are increased in central precocious puberty: effects of two different treatment regimens with gonadotropin-releasing hormone agonists, without or in combination with an antiandrogen (cyproterone acetate). J. Clin. Endocrinol. Metab. 80, 3059–3067 (1995).
Baier, I., Pereira, A., Ferrer, P., Iniguez, G. & Mericq, V. Higher prepubertal IGF-1 concentrations associate to earlier pubertal tempo in both sexes. Horm. Res. Paediatr. 96, 404–411 (2023).
Hiney, J. K., Srivastava, V., Nyberg, C. L., Ojeda, S. R. & Dees, W. L. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology 137, 3717–3728 (1996).
Pazos Fanchez-Franco, F., Balsa, J., Lopez-Fernandez, J., Escalada, J. & Cacicedo, L. Regulation of gonadal and somatotropic axis by chronic intraventricular infusion of insulin-like growth factor 1 antibody at the initiation of puberty in male rats. Neuroendocrinology 69, 408–416 (1999).
Balint, F., Csillag, V., Vastagh, C., Liposits, Z. & Farkas, I. Insulin-like growth factor 1 increases GABAergic neurotransmission to GnRH neurons via suppressing the retrograde tonic endocannabinoid signaling pathway in mice. Neuroendocrinology 111, 1219–1230 (2021).
Gemelli, I. F. B., Farias, E. D. S. & Spritzer, P. M. Association of body composition and age at menarche in girls and adolescents in the Brazilian Legal Amazon. J. Pediatr. 96, 240–246 (2020).
Rosales Nieto, C. A. et al. Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Animal 7, 990–997 (2013).
Boyne, M. S. et al. Growth, body composition, and the onset of puberty: longitudinal observations in Afro-Caribbean children. J. Clin. Endocrinol. Metab. 95, 3194–3200 (2010).
de Ridder, C. M. et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab. 70, 888–893 (1990).
Han, S. Z. et al. Reproduction traits of heterozygous myostatin knockout sows crossbred with homozygous myostatin knockout boars. Reprod. Domest. Anim. 56, 26–33 (2021).
Cheng, H. L. et al. Impact of growth, gonadal hormones, adiposity and the sodium-to-potassium ratio on longitudinal adolescent measures of blood pressure at puberty. J. Hum. Hypertens. 37, 835–843 (2023).
Vanacker, C. et al. Neuropilin-1 expression in GnRH neurons regulates prepubertal weight gain and sexual attraction. EMBO J. 39, e104633 (2020).
Quennell, J. H. et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812 (2009).
Elias, C. F. & Purohit, D. Leptin signaling and circuits in puberty and fertility. Cell. Mol. life Sci. 70, 841–862 (2013).
Allison, M. B. & Myers, M. G. Jr. 20 years of leptin: connecting leptin signaling to biological function. J. Endocrinol. 223, T25–T35 (2014).
Balland, E. et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301 (2014).
Banks, W. A. The blood-brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 15, 444–455 (2019).
Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B. & Maness, L. M. Leptin enters the brain by a saturable system independent of insulin. Peptides 17, 305–311 (1996).
Popa, S. M., Clifton, D. K. & Steiner, R. A. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu. Rev. Physiol. 70, 213 (2008).
Seminara, S. B. & Crowley, W. F. Jr. Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J. Neuroendocrinol. 20, 727–731 (2008).
Pinilla, L., Aguilar, E., Dieguez, C., Millar, R. P. & Tena-Sempere, M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 92, 1235–1316 (2012).
Comninos, A. N., Jayasena, C. N. & Dhillo, W. S. The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 20, 153–174 (2014).
Manfredi-Lozano, M., Roa, J. & Tena-Sempere, M. Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 48, 37–49 (2018).
Navarro, V. M. et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866 (2009).
Cravo, R. M. et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS ONE 8, e58698 (2013).
Zuure, W. A., Roberts, A. L., Quennell, J. H. & Anderson, G. M. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci. 33, 17874–17883 (2013).
Martin, C. et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 34, 6047–6056 (2014).
Tritos, N. A., Elmquist, J. K., Mastaitis, J. W., Flier, J. S. & Maratos-Flier, E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology 139, 4634–4641 (1998).
Mizuno, T. M. et al. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140, 4551–4557 (1999).
Cone, R. D. et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 25, S63–S67 (2001).
Egan, O. K., Inglis, M. A. & Anderson, G. M. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J. Neurosci. 37, 3875–3886 (2017).
Padilla, S. L. et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl Acad. Sci. USA 114, 2413–2418 (2017).
Ellacott, K. L. & Cone, R. D. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent. Prog. Horm. Res. 59, 395–408 (2004).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
van de Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2007).
Hohmann, J. G. et al. Differential role of melanocortins in mediating leptin’s central effects on feeding and reproduction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R50–R59 (2000).
Manfredi-Lozano, M. et al. Defining a novel leptin–melanocortin–kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab. 5, 844–857 (2016).
Duckett, K. et al. Prevalence of deleterious variants in MC3R in patients with constitutional delay of growth and puberty. J. Clin. Endocrinol. Metab. 20, dgad373 (2023).
Lam, B. Y. H. et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature 599, 436–441 (2021).
Chachlaki, K. et al. NOS1 mutations cause hypogonadotropic hypogonadism with sensory and cognitive deficits that can be reversed in infantile mice. Sci. Transl. Med. 14, eabh2369 (2022).
Donato, J. Jr et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J. Neurosci. 29, 5240–5250 (2009).
Donato, J. Jr. et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest. 121, 355–368 (2011).
Williams, K. W. et al. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J. Neurosci. 31, 13147–13156 (2011).
Donato, J. Jr., Frazao, R., Fukuda, M., Vianna, C. R. & Elias, C. F. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology 151, 5415–5427 (2010).
Leshan, R. L. et al. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J. Neurosci. 29, 3138–3147 (2009).
Clasadonte, J., Poulain, P., Beauvillain, J. C. & Prevot, V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149, 587–596 (2008).
Chachlaki, K. et al. Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J. Comp. Neurol. 525, 3177–3189 (2017).
Delli, V. et al. Male minipuberty involves the gonad-independent activation of preoptic nNOS neurons. Free. Radic. Biol. Med. 194, 199–208 (2023).
Yu, W. H., Walczewska, A., Karanth, S. & McCann, S. M. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 138, 5055–5058 (1997).
Bellefontaine, N. et al. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Invest. 124, 2550–2559 (2014).
Leshan, R. L., Greenwald-Yarnell, M., Patterson, C. M., Gonzalez, I. E. & Myers, M. G. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med. 18, 820–823 (2012).
Ross, R. A. et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 7, e35960 (2018).
Han, X. et al. Hypothalamic and cell-specific transcriptomes unravel a dynamic neuropil remodeling in leptin-induced and typical pubertal transition in female mice. iScience 23, 101563 (2020).
Prevot, V. Glial control of neuronal function. Nat. Rev. Endocrinol. 18, 195 (2022).
Nampoothiri, S., Nogueiras, R., Schwaninger, M. & Prevot, V. Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat. Metab. 4, 813–825 (2022).
Prevot, V. et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 39, 333–368 (2018).
Pellegrino, G. et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat. Neurosci. 24, 1660–1672 (2021).
Clasadonte, J. et al. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc. Natl Acad. Sci. USA 108, 16104–16109 (2011).
Vanacker, C., Defazio, R. A., Sykes, C. M. & Moenter, S. M. A role for glial fibrillary acidic protein (GFAP)-expressing cells in the regulation of gonadotropin-releasing hormone (GnRH) but not arcuate kisspeptin neuron output in male mice. eLife 10, e68205 (2021).
Prevot, V. et al. Normal female sexual development requires neuregulin–erbB receptor signaling in hypothalamic astrocytes. J. Neurosci. 23, 230–239 (2003).
Ma, Y. J., Junier, M. P., Costa, M. E. & Ojeda, S. R. Transforming growth factor-ɑ gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron 9, 657–670 (1992).
Moeller-Gnangra, H., Ernst, J., Pfeifer, M. & Heger, S. ErbB4 point mutation in CU3 inbred rats affects gonadotropin-releasing-hormone neuronal function via compromised neuregulin-stimulated prostaglandin E2 release from astrocytes. Glia 67, 309–320 (2019).
Verkhratsky, A. & Zorec, R. Astroglial signalling in health and disease. Neurosci. Lett. 689, 1–4 (2019).
Sloan, S. A. & Barres, B. A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 27, 75–81 (2014).
Pena-Leon, V. et al. Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21. Nat. Metab. 4, 901–917 (2022).
Rodriguez-Cortes, B. et al. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr. Biol. 32, 796–805 (2022).
García-Cáceres, C. et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880 (2016).
Duquenne, M. et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 3, 1071–1090 (2021).
Imbernon, M. et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 34, 1054–1063 (2022).
Porniece Kumar, M. et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 3, 1662–1679 (2021).
Clasadonte, J., Scemes, E., Wang, Z., Boison, D. & Haydon, P. G. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95, 1365–1380 (2017).
Lhomme, T. et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Invest. 131, e140521 (2021).
Banks, W. A., Owen, J. B. & Erickson, M. A. Insulin in the brain: there and back again. Pharmacol. Ther. 136, 82–93 (2012).
Collden, G. et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol. Metab. 4, 15–24 (2015).
Ogassawara, T. B. et al. Food deprivation in F0 generation and hypercaloric diet in F1 generation reduce F2 generation astrogliosis in several brain areas after immune challenge. Int. J. Dev. Neurosci. 64, 29–37 (2018).
Contu, L., Nizari, S., Heath, C. J. & Hawkes, C. A. Pre- and post-natal high fat feeding differentially affects the structure and integrity of the neurovascular unit of 16-month old male and female mice. Front. Neurosci. 13, 1045 (2019).
Evans, M. C., Lord, R. A. & Anderson, G. M. Multiple leptin signalling pathways in the control of metabolism and fertility: a means to different ends? Int. J. Mol. Sci. 22, 9210 (2021).
Singireddy, A. V., Inglis, M. A., Zuure, W. A., Kim, J. S. & Anderson, G. M. Neither signal transducer and activator of transcription 3 (STAT3) or STAT5 signaling pathways are required for leptin’s effects on fertility in mice. Endocrinology 154, 2434–2445 (2013).
Piper, M. L., Unger, E. K., Myers, M. G. & Xu, A. W. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol. 22, 751–759 (2008).
Bates, S. H. et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Gao, Q. et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl Acad. Sci. USA 101, 4661–4666 (2004).
Lee, J. Y. et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS ONE 3, e1639 (2008).
Patterson, C. M. et al. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol. Metab. 1, 61–69 (2012).
Zhang, S. Q. et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341–355 (2004).
Garcia-Galiano, D. et al. PI3Kɑ inactivation in leptin receptor cells increases leptin sensitivity but disrupts growth and reproduction. JCI Insight 2, e96728 (2017).
Kitamura, T. et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 12, 534–540 (2006).
Yang, G. et al. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J. Biol. Chem. 284, 3719–3727 (2009).
Kim, M. S. et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat. Neurosci. 9, 901–906 (2006).
Xu, J., Ji, J. & Yan, X. H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 52, 373–381 (2012).
Roa, J. et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150, 5016–5026 (2009).
Lomniczi, A. et al. Epigenetic control of female puberty. Nat. Neurosci. 16, 281–289 (2013).
Wright, H., Aylwin, C. F., Toro, C. A., Ojeda, S. R. & Lomniczi, A. Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression. Sci. Rep. 11, 1996 (2021).
Vazquez, M. J. et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 9, 4194 (2018).
Gaytan, F. et al. Distinct expression patterns predict differential roles of the miRNA-binding proteins, Lin28 and Lin28b, in the mouse testis: studies during postnatal development and in a model of hypogonadotropic hypogonadism. Endocrinology 154, 1321–1336 (2013).
Wang, J. M. & Zhang, K. Microarray analysis of microRNA expression in bone marrow-derived progenitor cells from mice with type 2 diabetes. Genom. Data 7, 86–87 (2016).
Messina, A. et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci. 19, 835–844 (2016).
Manfredi-Lozano, M. et al. GnRH replacement rescues cognition in Down syndrome. Science 377, eabq4515 (2022).
Roa, J. et al. Dicer ablation in Kiss1 neurons impairs puberty and fertility preferentially in female mice. Nat. Commun. 13, 4663 (2022).
Heras, V. et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 17, e3000532 (2019).
Abreu, A. P. et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 368, 2467–2475 (2013).
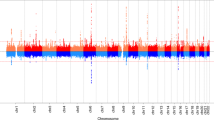
Elks, C. E. et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 42, 1077–1085 (2010).
Mumby, H. S. et al. Mendelian randomisation study of childhood BMI and early menarche. J. Obes. 2011, 180729 (2011).
Cousminer, D. L. et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 23, 4452–4464 (2014).
Katherine, A. K. et al. Understanding the genetic complexity of puberty timing across the allele frequency spectrum. Preprint at medRxiv www.medrxiv.org/content/10.1101/2023.06.14.23291322v1 (2023).
Kelsey, M. M. & Zeitler, P. S. Insulin resistance of puberty. Curr. Diabetes Rep. 16, 64 (2016).
Abreu, A. P. et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J. Clin. Invest. 130, 4486–4500 (2020).
Naule, L. et al. MKRN3 inhibits puberty onset via interaction with IGF2BP1 and regulation of hypothalamic plasticity. JCI Insight 8, e164178 (2023).
Roberts, S. A. et al. Hypothalamic overexpression of makorin ring finger protein 3 results in delayed puberty in female mice. Endocrinology 163, bqac132 (2022).
Roberts, S. A. et al. The peripubertal decline in makorin ring finger protein 3 expression is independent of leptin action. J. Endocr. Soc. 4, bvaa059 (2020).
Eren, S. E. & Simsek, E. Comparison of makorin ring finger protein 3 levels between obese and normal weight patients with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 15, 182–189 (2023).
Dauber, A. et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J. Clin. Endocrinol. Metab. 102, 1557–1567 (2017).
da Silva, C., Durandt, C., Kallmeyer, K., Ambele, M. A. & Pepper, M. S. The role of pref-1 during adipogenic differentiation: an overview of suggested mechanisms. Int. J. Mol. Sci. 21, 4104 (2020).
Gomes, L. G. et al. DLK1 is a novel link between reproduction and metabolism. J. Clin. Endocrinol. Metab. 104, 2112–2120 (2019).
Frisch, R. E. & McArthur, J. W. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185, 949–951 (1974).
Bessa, D. S. et al. Methylome profiling of healthy and central precocious puberty girls. Clin. Epigenetics 10, 146 (2018).
Toro, C. A., Wright, H., Aylwin, C. F., Ojeda, S. R. & Lomniczi, A. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat. Commun. 9, 57 (2018).
Lopez-Rodriguez, D. et al. Multi- and transgenerational outcomes of an exposure to a mixture of endocrine-disrupting chemicals (EDCs) on puberty and maternal behavior in the female rat. Env. Health Perspect. 129, 87003 (2021).
Perry, J. R. et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat. Genet. 41, 648–650 (2009).
He, C. et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat. Genet. 41, 724–728 (2009).
Sulem, P. et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat. Genet. 41, 734–738 (2009).
Ong, K. K. et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 41, 729–733 (2009).
Osinubi, A., Lewis-de los Angeles, C. P., Poitevien, P. & Topor, L. S. Are black girls exhibiting puberty earlier? Examining implications of race-based guidelines. Pediatrics 150, e2021055595 (2022).
Parnell, W., Scragg, R., Wilson, N., Schaaf, D. & Fitzgerald, E. NZ food NZ children. Key results of the 2002 National Children’s Nutrition Survey. Ministry of Health https://www.health.govt.nz/system/files/documents/publications/nzfoodnzchildren.pdf (2003).
Cabrera, S. M., Bright, G. M., Frane, J. W., Blethen, S. L. & Lee, P. A. Age of thelarche and menarche in contemporary US females: a cross-sectional analysis. J. Pediatr. Endocrinol. Metab. 27, 47–51 (2014).
Staiano, A. E., Broyles, S. T., Gupta, A. K. & Katzmarzyk, P. T. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity 21, 1251–1255 (2013).
Goran, M. I. et al. Visceral fat in white and African American prepubertal children. Am. J. Clin. Nutr. 65, 1703–1708 (1997).
Rush, E. C., Plank, L. D., Davies, P. S., Watson, P. & Wall, C. R. Body composition and physical activity in New Zealand Maori, Pacific and European children aged 5-14 years. Br. J. Nutr. 90, 1133–1139 (2003).
Acknowledgements
The authors acknowledge the support of the Royal Society of New Zealand #UOO1706 (G.M.A.), of NIH grants R01HD104418 (J.W.H.), R37HD019938, R01HD082314, R21HD098684 (U.B.K.) R01HD090151, R01HD099084, R01DK133760 (V.M.N.), U54AG062322 (V.M.N. and U.B.K.), R01HD069702, R01HD096324 (C.F.E.), from Agencia Estatal de Investigación, Spain PID2020-118660GB-I00; co-funded with EU funds from FEDER Program (M.T.-S.), from the European Commission, Program Horizon Europe HE-ERC-2022-ADG-101096793 (M.T.-S.), the European Union Horizon 2020 research and innovation programme no. 847941 miniNO (V.P.) and no. 810331 WATCH ERC Synergy (V.P.) and the Medical Research Council unit programmes MC_UU_12015/2, MC_UU_00006/2 (J.R.B.P. and K.K.O.).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of this review.
Corresponding authors
Ethics declarations
Competing interests
J.R.B.P. is an employee of Insmed Innovation UK, holds stock/stock options in Insmed, and receives research funding from GSK. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Ei Terasawa, Veronica Mericq and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anderson, G.M., Hill, J.W., Kaiser, U.B. et al. Metabolic control of puberty: 60 years in the footsteps of Kennedy and Mitra’s seminal work. Nat Rev Endocrinol 20, 111–123 (2024). https://doi.org/10.1038/s41574-023-00919-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00919-z