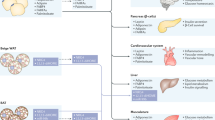
Abstract
Adipose tissue is an endocrine organ and a crucial regulator of energy storage and systemic metabolic homeostasis. Additionally, adipose tissue is a pivotal regulator of cardiovascular health and disease, mediated in part by the endocrine and paracrine secretion of several bioactive products, such as adipokines. Adipose vasculature has an instrumental role in the modulation of adipose tissue expansion, homeostasis and metabolism. The role of the adipose vasculature has been extensively explored in the context of obesity, which is recognized as a global health problem. Obesity-induced accumulation of fat, in combination with vascular rarefaction, promotes adipocyte dysfunction and induces oxidative stress, hypoxia and inflammation. It is now recognized that obesity-associated endothelial dysfunction often precedes the development of cardiovascular diseases. Investigations have revealed heterogeneity within the vascular niche and dynamic reciprocity between vascular and adipose cells, which can become dysregulated in obesity. Here we provide a comprehensive overview of the evolving functions of the vasculature in regulating adipose tissue biology in health and obesity.
Key points
-
Advances in technology have uncovered the heterogeneity of the adipose tissue endothelium, revealing that it consists of canonical (venous, capillary, arterial, lymphatic) and adipose tissue-specific (pre-adipocytes, early progenitor endothelial cells, and immunomodulatory endothelial cells) vascular subpopulations.
-
Adipose tissue endothelial phenotypes and gene transcription are governed by a plethora of transcription factors (and their post-translational modifications) and non-coding RNAs. Further investigation into transcriptional and epigenetic factors modulating adipose tissue endothelial phenotypes is warranted.
-
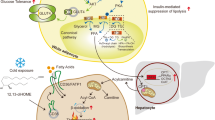
The vasculature of adipose tissue serves as a bidirectional channel for lipids, immune cells, and adipokines. Furthermore, endothelial cells within this tissue maintain active communication with adjacent adipocytes, particularly through paracrine signals such as extracellular vesicles.
-
Obesity correlates with disrupted blood flow in adipose tissue, endothelial dysfunction and reduced vascular density. These changes collectively lead to tissue hypoxia, inflammation and fibrosis.
-
Vascular ageing and obesity-associated vascular malfunction exhibit several common characteristics. Rejuvenating the vasculature might hold the potential to counteract the metabolic deterioration associated with obesity.
-
Obesity correlates with disturbances in the circadian rhythm of adipose tissue, which can trigger metabolic disorders; however, the chronobiology of adipose tissue endothelial cells is still largely unexplored.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wolfrum, C. & Gerhart-Hines, Z. Fueling the fire of adipose thermogenesis. Science 375, 1229–1231 (2022).
Chew, N. W. S. et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 35, 414–428.e3 (2023).
Herold, J. & Kalucka, J. Angiogenesis in adipose tissue: the interplay between adipose and endothelial cells. Front. Physiol. 11, 624903 (2020).
Li, M., Qian, M. & Xu, J. Vascular endothelial regulation of obesity-associated insulin resistance. Front. Cardiovasc. Med. 4, 51 (2017).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e20 (2020).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Strieder-Barboza, C. et al. Single-nuclei transcriptome of human AT reveals metabolically distinct depot-specific adipose progenitor subpopulations. Preprint at bioRxiv https://doi.org/10.1101/2022.06.29.496888 (2022).
Whytock, K. L. et al. Single cell full-length transcriptome of human subcutaneous adipose tissue reveals unique and heterogeneous cell populations. iScience 25, 104772 (2022).
Crewe, C. et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708.e13 (2018).
Bondareva, O. & Sheikh, B. N. Vascular homeostasis and inflammation in health and disease-lessons from single cell technologies. Int. J. Mol. Sci. 21, 4688 (2020).
Vijay, J. et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2, 97–109 (2020).
Bondareva, O. et al. Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity. Nat. Metab. 4, 1591–1610 (2022).
AlZaim, I., Festa, J. & Kalucka, J. Adipose tissue lymphatic endothelial cells: revisited functions in the modulation of adipose biology. Curr. Opin. Physiol. 34, 100675 (2023).
Corvera, S., Solivan-Rivera, J. & Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 25, 439–453 (2022).
Tabula Sapiens, C. et al. The tabula sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Abumrad, N. A. et al. Endothelial cell receptors in tissue lipid uptake and metabolism. Circ. Res. 128, 433–450 (2021).
Pi, X., Xie, L. & Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 123, 477–494 (2018).
Ioannidou, A., Fisher, R. M. & Hagberg, C. E. The multifaceted roles of the adipose tissue vasculature. Obes. Rev. 23, e13403 (2022).
Massier, L. et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat. Commun. 14, 1438 (2023).
Amersfoort, J., Eelen, G. & Carmeliet, P. Immunomodulation by endothelial cells – partnering up with the immune system? Nat. Rev. Immunol. 22, 576–588 (2022).
Haynes, B. A. et al. Endothelial-to-mesenchymal transition in human adipose tissue vasculature alters the particulate secretome and induces endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 39, 2168–2191 (2019).
Johnston, E. K. & Abbott, R. D. Adipose tissue paracrine-, autocrine-, and matrix-dependent signaling during the development and progression of obesity. Cells 12, 407 (2023).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Villarejo, A., Cortes-Cabrera, A., Molina-Ortiz, P., Portillo, F. & Cano, A. Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J. Biol. Chem. 289, 930–941 (2014).
Wilhelm, K. et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 529, 216–220 (2016).
Ibrahim, A. et al. Insulin-stimulated adipocytes secrete lactate to promote endothelial fatty acid uptake and transport. J. Cell Sci. 135, jcs258964 (2022).
Fan, M. et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 9, eadc9465 (2023).
Zhu, X. et al. Acetate controls endothelial-to-mesenchymal transition. Cell Metab. 35, 1163–1178.e10 (2023).
Zhang, H. et al. LncRNA MEG3 induces endothelial differentiation of mouse derived adipose-derived stem cells by targeting MiR-145-5p/KLF4. Mol. Biol. Rep. 49, 8495–8505 (2022).
Sun, X. et al. MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ. Res. 118, 810–821 (2016).
Liang, X. X. et al. Phosphorylation of Akt at Thr308 regulates p-eNOS Ser1177 during physiological conditions. FEBS Open Bio 11, 1953–1964 (2021).
Becker-Greene, D. et al. MiR-409-3p targets a MAP4K3-ZEB1-PLGF signaling axis and controls brown adipose tissue angiogenesis and insulin resistance. Cell Mol. Life Sci. 78, 7663–7679 (2021).
Tang, X. et al. Suppression of endothelial AGO1 promotes adipose tissue browning and improves metabolic dysfunction. Circulation 142, 365–379 (2020).
Mitić, T. & Caporali, A. Emerging roles of non-coding RNAs in endothelial cell function. Curr. Opin. Physiol. 34, 100672 (2023).
Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023).
Graupera, M. & Claret, M. Endothelial cells: new players in obesity and related metabolic disorders. Trends Endocrinol. Metab. 29, 781–794 (2018).
Vliora, M. et al. The impact of adipokines on vascular networks in adipose tissue. Cytokine Growth Factor Rev. 69, 61–72 (2023).
Vaiopoulos, A. G., Marinou, K., Christodoulides, C. & Koutsilieris, M. The role of adiponectin in human vascular physiology. Int. J. Cardiol. 155, 188–193 (2012).
Obata, Y. et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3, e99680 (2018).
Parker-Duffen, J. L. et al. T-cadherin is essential for adiponectin-mediated revascularization. J. Biol. Chem. 288, 24886–24897 (2013).
Rubina, K. A. et al. Revisiting the multiple roles of T-cadherin in health and disease. Eur. J. Cell Biol. 100, 151183 (2021).
Henning, R. J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 11, 504–529 (2021).
Bruder-Nascimento, T. et al. Leptin restores endothelial function via endothelial PPARγ-Nox1-mediated mechanisms in a mouse model of congenital generalized lipodystrophy. Hypertension 74, 1399–1408 (2019).
Sturzebecher, P. E. et al. Leptin treatment has vasculo-protective effects in lipodystrophic mice. Proc. Natl Acad. Sci. USA 119, e2110374119 (2022).
Fischer, A. W. et al. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metab. 33, 547–564.e7 (2021).
Thiemann, E. et al. Role of endothelial cell lipoprotein lipase for brown adipose tissue lipid and glucose handling. Front. Physiol. 13, 859671 (2022).
Davies, B. S. et al. The expression of GPIHBP1, an endothelial cell binding site for lipoprotein lipase and chylomicrons, is induced by peroxisome proliferator-activated receptor-γ. Mol. Endocrinol. 22, 2496–2504 (2008).
Surendran, R. P. et al. Decreased GPIHBP1 protein levels in visceral adipose tissue partly underlie the hypertriglyceridemic phenotype in insulin resistance. PLoS ONE 13, e0205858 (2018).
Son, N.-H. et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Invest. 128, 4329–4342 (2018).
Peche, V. et al. Endothelial cell CD36 regulates membrane ceramide formation, exosome fatty acid transfer and circulating fatty acid levels. Nat. Commun. 14, 4029 (2023).
Daquinag, A. C. et al. Fatty acid mobilization from adipose tissue is mediated by CD36 posttranslational modifications and intracellular trafficking. JCI Insight 6, e147057 (2021).
Salameh, A. et al. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 1, e86351 (2016).
Gao, Z., Daquinag, A. C., Yu, Y. & Kolonin, M. G. Endothelial prohibitin mediates bidirectional long-chain fatty acid transport in white and brown adipose tissues. Diabetes 71, 1400–1409 (2022).
Wyne, K. L., Pathak, R. K., Seabra, M. C. & Hobbs, H. H. Expression of the VLDL receptor in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 16, 407–415 (1996).
Goudriaan, J. R. et al. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lipid Res. 45, 1475–1481 (2004).
Kanda, T. et al. PPARγ in the endothelium regulates metabolic responses to high-fat diet in mice. J. Clin. Invest. 119, 110–124 (2008).
Gogg, S., Nerstedt, A., Boren, J. & Smith, U. Human adipose tissue microvascular endothelial cells secrete PPARγ ligands and regulate adipose tissue lipid uptake. JCI Insight 4, e125914 (2019).
Briot, A. et al. Senescence alters PPARγ (peroxisome proliferator-activated receptor gamma)-dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arterioscler. Thromb. Vasc. Biol. 38, 1134–1146 (2018).
Wang, C. et al. Smad4-mediated angiogenesis facilitates the beiging of white adipose tissue in mice. iScience 26, 106272 (2023).
Kalucka, J. et al. Quiescent endothelial cells upregulate fatty acid β-oxidation for vasculoprotection via redox homeostasis. Cell Metab. 28, 881–894.e13 (2018).
Monelli, E. et al. Angiocrine polyamine production regulates adiposity. Nat. Metab. 4, 327–343 (2022).
Aupetit, A. et al. Endothelial DLL4 is an adipose depot-specific fasting sensor regulating fatty acid fluxes. Arterioscler. Thromb. Vasc. Biol. 43, 684–696 (2023).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Furuuchi, R. et al. Endothelial SIRT-1 has a critical role in the maintenance of capillarization in brown adipose tissue. iScience 25, 105424 (2022).
Shimizu, I. et al. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 124, 2099–2112 (2014).
Schlein, C. et al. Endogenous fatty acid synthesis drives brown adipose tissue involution. Cell Rep. 34, 108624 (2021).
Lee, H. J. et al. Endothelial cell-derived stem cell factor promotes lipid accumulation through c-Kit-mediated increase of lipogenic enzymes in brown adipocytes. Nat. Commun. 14, 2754 (2023).
Sabaratnam, R. & Svenningsen, P. Adipocyte-endothelium crosstalk in obesity. Front. Endocrinol. 12, 681290 (2021).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017).
Crewe, C. et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 33, 1853–1868.e11 (2021).
Crewe, C. et al. Deficient caveolin-1 synthesis in adipocytes stimulates systemic insulin-independent glucose uptake via extracellular vesicles. Diabetes 71, 2496–2512 (2022).
Wadey, R. M. et al. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis 283, 19–27 (2019).
Lee, H.-J. et al. Vascular reactivity contributes to adipose tissue remodeling in obesity. J. Endocrinol. 251, 195–206 (2021).
Karpe, F. et al. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 51, 2467–2473 (2002).
Frayn, K. N. & Karpe, F. Regulation of human subcutaneous adipose tissue blood flow. Int. J. Obes. 38, 1019–1026 (2014).
Sotornik, R. et al. Update on adipose tissue blood flow regulation. Am. J. Physiol. Endocrinol. Metab. 302, E1157–E1170 (2012).
Goossens, G. H. et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124, 67–76 (2011).
Sakai, T. & Hosoyamada, Y. Are the precapillary sphincters and metarterioles universal components of the microcirculation? An historical review. J. Physiol. Sci. 63, 319–331 (2013).
Ardilouze, J. L., Fielding, B. A., Currie, J. M., Frayn, K. N. & Karpe, F. Nitric oxide and β-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation 109, 47–52 (2004).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Paavonsalo, S., Hariharan, S., Lackman, M. H. & Karaman, S. Capillary rarefaction in obesity and metabolic diseases – organ-specificity and possible mechanisms. Cells 9, 2683 (2020).
Belligoli, A. et al. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci. Rep. 9, 11333 (2019).
Rupnick, M. A. et al. Adipose tissue mass can be regulated through the vasculature. Proc. Natl Acad. Sci. USA 99, 10730–10735 (2002).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Seki, T. et al. Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. J. Exp. Med. 215, 611–626 (2018).
Robciuc, M. R. et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724 (2016).
Ngo, D. T. et al. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 130, 1072–1080 (2014).
Guzmán, A., Hernández‐Coronado, C. G., Gutiérrez, C. G. & Rosales‐Torres, A. M. The vascular endothelial growth factor (VEGF) system as a key regulator of ovarian follicle angiogenesis and growth. Mol. Reprod. Dev. 90, 201–217 (2023).
Karki, S. et al. WNT5A regulates adipose tissue angiogenesis via antiangiogenic VEGF-A165b in obese humans. Am. J. Physiol. Heart Circ. Physiol. 313, H200–H206 (2017).
AlZaim, I. et al. Adipose tissue immunomodulation: a novel therapeutic approach in cardiovascular and metabolic diseases. Front. Cardiovasc. Med. 7, 602088 (2020).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Elgazar-Carmon, V., Rudich, A., Hadad, N. & Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 (2008).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Preston, K. J. et al. Postprandial activation of leukocyte-endothelium interaction by fatty acids in the visceral adipose tissue microcirculation. FASEB J. 33, 11993 (2019).
Onogi, Y. et al. Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity. Sci. Rep. 10, 670 (2020).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2017).
Boden, G. et al. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 7, 304re307 (2015).
Kähler, J. et al. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J. Cardiovasc. Pharmacol. 38, 49–57 (2001).
Tripathy, D. et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52, 2882–2887 (2003).
Steinberg, H. O. et al. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49, 1231–1238 (2000).
Tampakakis, E. et al. Intravenous lipid infusion induces endoplasmic reticulum stress in endothelial cells and blood mononuclear cells of healthy adults. J. Am. Heart Assoc. 5, e002574 (2016).
Pellegrinelli, V., Rouault, C., Veyrie, N., Clément, K. & Lacasa, D. Endothelial cells from visceral adipose tissue disrupt adipocyte functions in a three-dimensional setting: partial rescue by angiopoietin-1. Diabetes 63, 535–549 (2014).
Hasegawa, Y. et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 125, 1122–1133 (2012).
Koenen, M., Hill, M. A., Cohen, P. & Sowers, J. R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 128, 951–968 (2021).
Virdis, A. et al. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur. Heart J. 36, 784–794 (2015).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Kuo, L. E. et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13, 803–811 (2007).
Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 93, 1–21 (2013).
Seo, J. B. et al. Knockdown of Ant2 reduces adipocyte hypoxia and improves insulin resistance in obesity. Nat. Metab. 1, 86–97 (2019).
Ye, J., Gao, Z., Yin, J. & He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 (2007).
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 29, 4467–4483 (2009).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007).
Rudnicki, M. et al. Transcriptomic profiling reveals sex-specific molecular signatures of adipose endothelial cells under obesogenic conditions. iScience 26, 105811 (2023).
Zhang, X. et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J. Biol. Chem. 285, 32869–32877 (2010).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 33, 904–917 (2013).
Kihira, Y., Fujimura, Y., Tomita, S., Tamaki, T. & Sato, E. Hypoxia-inducible factor-1α regulates Lipin1 differently in pre-adipocytes and mature adipocytes. Mol. Med. Rep. 22, 559–565 (2020).
Todorčević, M. et al. Markers of adipose tissue hypoxia are elevated in subcutaneous adipose tissue of severely obese patients with obesity hypoventilation syndrome but not in the moderately obese. Int. J. Obes. 45, 1618–1622 (2021).
Gozal, D. et al. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. Am. J. Respir. Cell Mol. Biol. 56, 477–487 (2017).
Borgeson, E., Boucher, J. & Hagberg, C. E. Of mice and men: pinpointing species differences in adipose tissue biology. Front. Cell Dev. Biol. 10, 1003118 (2022).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Cifarelli, V. et al. Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J. Clin. Invest. 130, 6688–6699 (2020).
ElShazly, M., Salama, M. & Elessawy, K. Changes in the macular vascular density after bariatric surgery measured by optical coherence tomography angiography. Clin. Ophthalmol. 15, 3131–3137 (2021).
Laiginhas, R., Guimarães, M., Nora, M., Chibante, J. & Falcão, M. Gastric bypass improves microvascular perfusion in patients with obesity. Obes. Surg. 31, 2080–2086 (2021).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Jiang, Y. et al. A PPARγ transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat. Commun. 8, 15926 (2017).
Donato, A. J. et al. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J. Physiol. 592, 4083–4096 (2014).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 373, eabc8479 (2021).
Sung, H.-K. et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 17, 61–72 (2013).
Elias, I. et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61, 1801–1813 (2012).
Wang, B. et al. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRα+ adipose progenitors. Cell Discov. 3, 17036 (2017).
Bagchi, M. et al. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J. 27, 3257 (2013).
Picchi, A. et al. Tumor necrosis factor-α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 99, 69–77 (2006).
Naessen, T., Einarsson, G., Henrohn, D. & Wikstrom, G. Peripheral vascular ageing in pulmonary arterial hypertension as assessed by common carotid artery intima thickness and intima/media thickness ratio: an investigation using non-invasive high-resolution ultrasound. Heart Lung Circ. 32, 338–347 (2022).
Dou, H. et al. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 37, 1180–1193 (2017).
Cartwright, M. J., Tchkonia, T. & Kirkland, J. L. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 42, 463–471 (2007).
Nguyen, H. P. et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev. Cell 56, 1437–1451.e3 (2021).
Tabula Muris Consortium A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Zhou, W. et al. High-resolution aging niche of human adipose tissues. Signal. Transduct. Target. Ther. 8, 105 (2023).
Barinda, A. J. et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 11, 481 (2020).
Wang, Y. et al. Aging affects KV7 channels and perivascular adipose tissue-mediated vascular tone. Front. Physiol. 12, 749709 (2021).
Trim, W. V. et al. Divergent immunometabolic changes in adipose tissue and skeletal muscle with ageing in healthy humans. J. Physiol. 600, 921–947 (2022).
Agabiti-Rosei, C. et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 40, 41–50 (2017).
Bailey-Downs, L. C. et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol.: Ser. A 68, 780–792 (2013).
Sena, C. M., Pereira, A., Fernandes, R., Letra, L. & Seiça, R. M. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br. J. Pharmacol. 174, 3514–3526 (2017).
Mahabala, C., Kamath, P., Bhaskaran, U., Pai, N. D. & Pai, A. U. Antihypertensive therapy: nocturnal dippers and nondippers. Do we treat them differently? Vasc. Health Risk Manag. 9, 125–133 (2013).
Viswambharan, H. et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 115, 2188–2195 (2007).
Nernpermpisooth, N. et al. Obesity alters the peripheral circadian clock in the aorta and microcirculation. Microcirculation 22, 257–266 (2015).
Padgett, C. A. et al. Obesity induces disruption of microvascular endothelial circadian rhythm. Front. Physiol. 13, 887559 (2022).
Moczulska, B., Zechowicz, M., Lesniewska, S., Osowiecka, K. & Gromadzinski, L. The impact of obesity on nighttime blood pressure dipping. Medicina 56, 700 (2020).
Stenvers, D. J., Scheer, F., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Manoogian, E. N. et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the Healthy Heroes randomized control trial. Cell Metab. 34, 1442–1456.e7 (2022).
Ribas-Latre, A. et al. Cellular and physiological circadian mechanisms drive diurnal cell proliferation and expansion of white adipose tissue. Nat. Commun. 12, 3482 (2021).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 (2005).
Paschos, G. K. et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777 (2012).
Yue, K., Rensen, P. C. N. & Kooijman, S. Circadian control of white and brown adipose tissues. Curr. Opin. Genet. Dev. 80, 102056 (2023).
Poirier, P. et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113, 898–918 (2006).
Maniyadath, B., Zhang, Q., Gupta, R. K. & Mandrup, S. Adipose tissue at single-cell resolution. Cell Metab. 35, 386–413 (2023).
Jiang, Y., Berry, D. C., Tang, W. & Graff, J. M. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 9, 1007–1022 (2014).
Cawthorn, W. P., Scheller, E. L. & MacDougald, O. A. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 53, 227–246 (2012).
Min, S. Y. et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 22, 312–318 (2016).
Tang, W. et al. White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 (2008).
Tran, K.-V. et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 (2012).
Gupta, R. K. et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15, 230–239 (2012).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Cattaneo, P. et al. Parallel lineage-tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Rep. 30, 571–582.e2 (2020).
Han, X. et al. A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell 28, 1160–1176.e7 (2021).
Medici, D. et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 16, 1400–1406 (2010).
Yang Loureiro, Z. et al. Wnt signaling preserves progenitor cell multipotency during adipose tissue development. Nat. Metab. 5, 1014–1028 (2023).
Palani, N. P. et al. Adipogenic and SWAT cells separate from a common progenitor in human brown and white adipose depots. Nat. Metab. 5, 996–1013 (2023).
Park, K. et al. Endothelial cells induced progenitors into brown fat to reduce atherosclerosis. Circ. Res. 131, 168–183 (2022).
Seki, T. et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat. Commun. 7, 12152 (2016).
Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997).
Wang, Z. et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40, 897–903 (2008).
Zhao, Y. & Garcia, B. A. Comprehensive catalog of currently documented histone modifications. Cold Spring Harb. Perspect. Biol. 7, a025064 (2015).
Sheikh, B. N. & Akhtar, A. The many lives of KATs – detectors, integrators and modulators of the cellular environment. Nat. Rev. Genet. 20, 7–23 (2019).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Simithy, J. et al. Characterization of histone acylations links chromatin modifications with metabolism. Nat. Commun. 8, 1141 (2017).
Marino, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014).
Zheng, Y., Thomas, P. M. & Kelleher, N. L. Measurement of acetylation turnover at distinct lysines in human histones identifies long-lived acetylation sites. Nat. Commun. 4, 2203 (2013).
Allfrey, V. G., Faulkner, R. & Mirsky, A. E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl Acad. Sci. USA 51, 786–794 (1964).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Chakraborty, A. A. et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science 363, 1217–1222 (2019).
Batie, M. et al. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science 363, 1222–1226 (2019).
Chen, Y. et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell Proteom. 6, 812–819 (2007).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Tan, M. et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028 (2011).
Zou, C. et al. Acyl-CoA:lysophosphatidylcholine acyltransferase I (Lpcat1) catalyzes histone protein O-palmitoylation to regulate mRNA synthesis. J. Biol. Chem. 286, 28019–28025 (2011).
Xie, Z. et al. Lysine succinylation and lysine malonylation in histones. Mol. Cell Proteom. 11, 100–107 (2012).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Andrade, J. et al. Control of endothelial quiescence by FOXO-regulated metabolites. Nat. Cell Biol. 23, 413–423 (2021).
Rudnicki, M. et al. Endothelial-specific FoxO1 depletion prevents obesity-related disorders by increasing vascular metabolism and growth. Elife 7, e39780 (2018).
Gan, F. et al. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 17, e0261498 (2022).
Miranville, A. et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110, 349–355 (2004).
Nakagami, H. et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 25, 2542–2547 (2005).
Planat-Benard, V. et al. Plasticity of human adipose lineage cells toward endothelial cells. Circulation 109, 656–663 (2004).
Arderiu, G. et al. MicroRNA-145 regulates the differentiation of adipose stem cells toward microvascular endothelial cells and promotes angiogenesis. Circ. Res. 125, 74–89 (2019).
Shang, T. et al. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 10, 133 (2019).
Cheng, F. et al. Conversion of human adipose-derived stem cells into functional and expandable endothelial-like cells for cell-based therapies. Stem Cell Res. Ther. 9, 350 (2018).
Jumabay, M. et al. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J. Mol. Cell Cardiol. 53, 790–800 (2012).
Poloni, A. et al. Plasticity of human dedifferentiated adipocytes toward endothelial cells. Exp. Hematol. 43, 137–146 (2015).
Watanabe, H. et al. The neovascularization effect of dedifferentiated fat cells. Sci. Rep. 10, 9211 (2020).
Acknowledgements
The work of B.N.S. is funded by the DFG (grants 45720345, 511049882), the German Diabetes Foundation, the free-state of Saxony, and the Helmholtz Centre Munich. E.B. receives support from the European Research Council (ERC-StG no. 804418), Aarhus University Research Foundation (AUFF-E-2022-7-8), Novo Nordisk Foundation (NNF22OC0079363), the Swedish state’s ALF-agreement (ALFGBG-978978), and Regionala FoU-medel, Västra Götalandsregionen (OLG-2023-02-22). The work of J.K. is funded by Lundbeckfonden (R307-2018-3667), Carlsberg Fonden (CF19-0687), Kræftens Bekæmpelse (R302-A17296), Novo Nordisk Fonden (0073440), Riisfort Fonden. J.K and I.A. are supported by Steno Diabetes Center Aarhus (SDCA).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Jian Xu and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AlZaim, I., de Rooij, L.P., Sheikh, B.N. et al. The evolving functions of the vasculature in regulating adipose tissue biology in health and obesity. Nat Rev Endocrinol 19, 691–707 (2023). https://doi.org/10.1038/s41574-023-00893-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00893-6
This article is cited by
-
Precision net ultrafiltration dosing in continuous kidney replacement therapy: a practical approach
Intensive Care Medicine Experimental (2023)