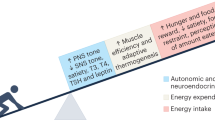
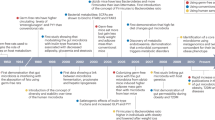
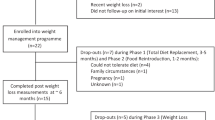
Abstract
Weight regain after successful weight loss resulting from lifestyle interventions is a major challenge in the management of overweight and obesity. Knowledge of the causal mechanisms for weight regain can help researchers and clinicians to find effective strategies to tackle weight regain and reduce obesity-associated metabolic and cardiovascular complications. This Review summarizes the current understanding of a number of potential physiological mechanisms underlying weight regain after weight loss, including: the role of adipose tissue immune cells; hormonal and neuronal factors affecting hunger, satiety and reward; resting energy expenditure and adaptive thermogenesis; and lipid metabolism (lipolysis and lipid oxidation). We describe and discuss obesity-associated changes in these mechanisms, their persistence during weight loss and weight regain and their association with weight regain. Interventions to prevent or limit weight regain based on these factors, such as diet, exercise, pharmacotherapy and biomedical strategies, and current knowledge on the effectiveness of these interventions are also reviewed.
Key points
-
Weight regain after diet-induced weight loss is a common phenomenon and probably involves physiological mechanisms.
-
Studies in the past decade suggest the existence of an obesity memory with respect to adipose tissue inflammatory cell populations and metabolic changes, which might have a role in weight regain.
-
The role of persistent changes in appetite-related factors in weight regain and the interaction of these factors with the brain needs further study.
-
Strategies to reduce weight regain after weigh loss (diet composition, exercise, pharmacotherapy and other biomedical applications) should be considered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van Baak, M. A. & Mariman, E. C. M. Mechanisms of weight regain after weight loss – the role of adipose tissue. Nat. Rev. Endocrinol. 15, 274–287 (2019).
Berthoud, H. R., Seeley, R. J. & Roberts, S. B. Physiology of energy intake in the weight-reduced state. Obesity 29, S25–S30 (2021).
Hill, D. A. et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl Acad. Sci. USA 115, E5096–E5105 (2018).
Hildreth, A. D. et al. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 22, 639–653 (2021).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Zeyda, M. et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. 31, 1420–1428 (2007).
Caslin, H. L., Bhanot, M., Bolus, W. R. & Hasty, A. H. Adipose tissue macrophages: unique polarization and bioenergetics in obesity. Immunol. Rev. 295, 101–113 (2020).
Cottam, M. A., Caslin, H. L., Winn, N. C. & Hasty, A. H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 13, 2950 (2022).
Clement, K. et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 18, 1657–1669 (2004).
Capel, F. et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 58, 1558–1567 (2009).
Vink, R. G. et al. Adipose tissue gene expression is differentially regulated with different rates of weight loss in overweight and obese humans. Int. J. Obes. 41, 309–316 (2017).
Caslin, H. L., Cottam, M. A., Pinon, J. M., Boney, L. Y. & Hasty, A. H. Weight cycling induces innate immune memory in adipose tissue macrophages. Front. Immunol. 13, 984859 (2022).
Schmitz, J. et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 5, 328–339 (2016).
Liu, P. S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Chavakis, T., Alexaki, V. I. & Ferrante, A. W. Jr. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat. Immunol. 24, 757–766 (2023).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Cao, Y. et al. TIDB: a comprehensive database of trained immunity. Database 2021, baab041 (2021).
Yi, J. S., Cox, M. A. & Zajac, A. J. T-cell exhaustion: characteristics, causes and conversion. Immunology 129, 474–481 (2010).
Porsche, C. E., Delproposto, J. B., Geletka, L., O’Rourke, R. & Lumeng, C. N. Obesity results in adipose tissue T cell exhaustion. JCI Insight 6, e139793 (2021).
Varghese, M. et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology 160, 293–312 (2019).
Zou, J. et al. CD4+ T cells memorize obesity and promote weight regain. Cell Mol. Immunol. 15, 630–639 (2018).
Vink, R. G., Roumans, N. J., Arkenbosch, L. A., Mariman, E. C. & van Baak, M. A. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity 24, 321–327 (2016).
Roumans, N. J., Vink, R. G., Fazelzadeh, P., van Baak, M. A. & Mariman, E. C. A role for leukocyte integrins and extracellular matrix remodeling of adipose tissue in the risk of weight regain after weight loss. Am. J. Clin. Nutr. 105, 1054–1062 (2017).
Mariman, E. C. & Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol. Life Sci. 67, 1277–1292 (2010).
Lackey, D. E. et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol. Endocrinol. Metab. 306, E233–E246 (2014).
Ambeba, E. J. et al. Longitudinal effects of weight loss and regain on cytokine concentration of obese adults. Metabolism 62, 1218–1222 (2013).
Sougiannis, A. T. et al. Impact of weight loss and partial weight regain on immune cell and inflammatory markers in adipose tissue in male mice. J. Appl. Physiol. 129, 909–919 (2020).
Qiao, Q. et al. Plasma levels of triglycerides and IL-6 are associated with weight regain and fat mass expansion. J. Clin. Endocrinol. Metab. 107, 1920–1929 (2022).
Yadati, T., Houben, T., Bitorina, A. & Shiri-Sverdlov, R. The ins and outs of cathepsins: physiological function and role in disease management. Cells 9, 1679 (2020).
Smyth, P., Sasiwachirangkul, J., Williams, R. & Scott, C. J. Cathepsin S (CTSS) activity in health and disease – a treasure trove of untapped clinical potential. Mol. Asp. Med. 88, 101106 (2022).
Marques, A. R. A. et al. Enzyme replacement therapy with recombinant pro-CTSD (cathepsin D) corrects defective proteolysis and autophagy in neuronal ceroid lipofuscinosis. Autophagy 16, 811–825 (2020).
Bogardus, C. et al. Familial dependence of the resting metabolic rate. N. Engl. J. Med. 315, 96–100 (1986).
Ravussin, E. et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 318, 467–472 (1988).
Rimbach, R. et al. Total energy expenditure is repeatable in adults but not associated with short-term changes in body composition. Nat. Commun. 13, 99 (2022).
Vogels, N., Diepvens, K. & Westerterp-Plantenga, M. S. Predictors of long-term weight maintenance. Obes. Res. 13, 2162–2168 (2005).
Muller, M. J. & Bosy-Westphal, A. Adaptive thermogenesis with weight loss in humans. Obesity 21, 218–228 (2013).
Martins, C., Roekenes, J., Salamati, S., Gower, B. A. & Hunter, G. R. Metabolic adaptation is an illusion, only present when participants are in negative energy balance. Am. J. Clin. Nutr. 112, 1212–1218 (2020).
Westerterp, K. R. Adaptive thermogenesis during energy deficits: a different explanation. Eur. J. Clin. Nutr. 76, 1351–1352 (2022).
Muller, M. J., Heymsfield, S. B. & Bosy-Westphal, A. Are metabolic adaptations to weight changes an artefact. Am. J. Clin. Nutr. 114, 1386–1395 (2021).
Galgani, J. E. & Santos, J. L. Insights about weight loss-induced metabolic adaptation. Obesity 24, 277–278 (2016).
Leibel, R. L., Rosenbaum, M. & Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 (1995).
Rosenbaum, M. & Leibel, R. L. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity 24, 1620–1629 (2016).
Coutinho, S. R. et al. The impact of rate of weight loss on body composition and compensatory mechanisms during weight reduction: a randomized control trial. Clin. Nutr. 37, 1154–1162 (2018).
Most, J. & Redman, L. M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 133, 110875 (2020).
Reinhardt, M. et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes 64, 2859–2867 (2015).
Whytock, K. L. et al. Metabolic adaptation characterizes short-term resistance to weight loss induced by a low-calorie diet in overweight/obese individuals. Am. J. Clin. Nutr. 114, 267–280 (2021).
Martins, C., Gower, B. A., Hill, J. O. & Hunter, G. R. Metabolic adaptation is not a major barrier to weight-loss maintenance. Am. J. Clin. Nutr. 112, 558–565 (2020).
Martins, C., Roekenes, J., Gower, B. A. & Hunter, G. R. Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutr. Metab. 18, 60 (2021).
Ravussin, E. & Redman, L. M. Metabolic adaptation: is it really an illusion? Am. J. Clin. Nutr. 112, 1653–1654 (2020).
Westerterp, K. R. Absence of evidence is no evidence for absence of the phenomenon. Am. J. Clin. Nutr. 112, 501–502 (2020).
Martins, C., Roekenes, J., Salamati, S., Gower, B. A. & Hunter, G. R. Reply to E Ravussin and L Redman. Am. J. Clin. Nutr. 112, 1655–1656 (2020).
Rosenbaum, M., Hirsch, J., Gallagher, D. A. & Leibel, R. L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 88, 906–912 (2008).
Camps, S. G., Verhoef, S. P. & Westerterp, K. R. Weight loss, weight maintenance, and adaptive thermogenesis. Am. J. Clin. Nutr. 97, 990–994 (2013).
Fothergill, E. et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 24, 1612–1619 (2016).
Marlatt, K. L., Redman, L. M., Burton, J. H., Martin, C. K. & Ravussin, E. Persistence of weight loss and acquired behaviors 2 y after stopping a 2-y calorie restriction intervention. Am. J. Clin. Nutr. 105, 928–935 (2017).
Weyer, C. et al. Energy metabolism after 2 y of energy restriction: the Biosphere 2 experiment. Am. J. Clin. Nutr. 72, 946–953 (2000).
Weinsier, R. L. et al. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am. J. Clin. Nutr. 72, 1088–1094 (2000).
Nymo, S. et al. Physiological predictors of weight regain at 1-year follow-up in weight-reduced adults with obesity. Obesity 27, 925–931 (2019).
Thom, G. et al. The role of appetite-related hormones, adaptive thermogenesis, perceived hunger and stress in long-term weight-loss maintenance: a mixed-methods study. Eur. J. Clin. Nutr. 74, 622–632 (2020).
Abbott, W. G. et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am. J. Physiol. 255, E332–E337 (1988).
Flatt, J. P. Issues and misconceptions about obesity. Obesity 19, 676–686 (2011).
Schrauwen, P., Lichtenbelt, W. D., Saris, W. H. & Westerterp, K. R. Fat balance in obese subjects: role of glycogen stores. Am. J. Physiol. 274, E1027–E1033 (1998).
Zurlo, F. et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am. J. Physiol. 259, E650–E657 (1990).
Martins, C., Gower, B. A. & Hunter, G. R. Baseline metabolic variables do not predict weight regain in premenopausal women. Obesity 28, 902–906 (2020).
Nicklas, B. J., Rogus, E. M. & Goldberg, A. P. Exercise blunts declines in lipolysis and fat oxidation after dietary-induced weight loss in obese older women. Am. J. Physiol. 273, E149–E155 (1997).
van Aggel-Leijssen, D. P., Saris, W. H., Hul, G. B. & van Baak, M. A. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am. J. Clin. Nutr. 73, 523–531 (2001).
Larson, D. E., Ferraro, R. T., Robertson, D. S. & Ravussin, E. Energy metabolism in weight-stable postobese individuals. Am. J. Clin. Nutr. 62, 735–739 (1995).
Jackman, M. R. et al. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1117–R1129 (2008).
Mai, K. et al. An integrated understanding of the molecular mechanisms of how adipose tissue metabolism affects long-term body weight maintenance. Diabetes 68, 57–65 (2019).
Schiffelers, S. L., Saris, W. H. & van Baak, M. A. The effect of an increased free fatty acid concentration on thermogenesis and substrate oxidation in obese and lean men. Int. J. Obes. Relat. Metab. Disord. 25, 33–38 (2001).
Wolfe, R. R. et al. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am. J. Physiol. 252, E189–E196 (1987).
Bougneres, P. et al. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J. Clin. Invest. 99, 2568–2573 (1997).
Langin, D. et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 54, 3190–3197 (2005).
Schiffelers, S. L., Akkermans, J. A., Saris, W. H. & Blaak, E. E. Lipolytic and nutritive blood flow response to beta-adrenoceptor stimulation in situ in subcutaneous abdominal adipose tissue in obese men. Int. J. Obes. Relat. Metab. Disord. 27, 227–231 (2003).
Borsheim, E., Lonnroth, P., Knardahl, S. & Jansson, P. A. No difference in the lipolytic response to β-adrenoceptor stimulation in situ but a delayed increase in adipose tissue blood flow in moderately obese compared with lean men in the postexercise period. Metabolism 49, 579–587 (2000).
Jocken, J. W. et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 92, 2292–2299 (2007).
Jocken, J. W. et al. Association of a beta-2 adrenoceptor (ADRB2) gene variant with a blunted in vivo lipolysis and fat oxidation. Int. J. Obes. 31, 813–819 (2007).
Large, V. et al. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J. Clin. Invest. 100, 3005–3013 (1997).
Blaak, E. E., Schiffelers, S. L., Saris, W. H., Mensink, M. & Kooi, M. E. Impaired beta-adrenergically mediated lipolysis in skeletal muscle of obese subjects. Diabetologia 47, 1462–1468 (2004).
Bezaire, V., Mairal, A., Anesia, R., Lefort, C. & Langin, D. Chronic TNFα and cAMP pre-treatment of human adipocytes alter HSL, ATGL and perilipin to regulate basal and stimulated lipolysis. FEBS Lett. 583, 3045–3049 (2009).
Koppo, K. et al. Catecholamine and insulin control of lipolysis in subcutaneous adipose tissue during long-term diet-induced weight loss in obese women. Am. J. Physiol. Endocrinol. Metab. 302, E226–E232 (2012).
Kasher-Meron, M., Youn, D. Y., Zong, H. & Pessin, J. E. Lipolysis defect in white adipose tissue and rapid weight regain. Am. J. Physiol. Endocrinol. Metab. 317, E185–E193 (2019).
Verhoef, S. P., Camps, S. G., Bouwman, F. G., Mariman, E. C. & Westerterp, K. R. Physiological response of adipocytes to weight loss and maintenance. PLoS ONE 8, e58011 (2013).
Vink, R. G., Roumans, N. J., Mariman, E. C. & van Baak, M. A. Dietary weight loss-induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiol. Rep. 5, e13450 (2017).
Maclean, P. S., Bergouignan, A., Cornier, M. A. & Jackman, M. R. Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–R600 (2011).
Gibbons, C., Hopkins, M., Beaulieu, K., Oustric, P. & Blundell, J. E. Issues in measuring and interpreting human appetite (satiety/satiation) and its contribution to obesity. Curr. Obes. Rep. 8, 77–87 (2019).
Bessesen, D. H., Bull, S. & Cornier, M. A. Trafficking of dietary fat and resistance to obesity. Physiol. Behav. 94, 681–688 (2008).
van Galen, K. A. et al. Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat. Metab. 5, 1059–1072 (2023).
Aukan, M. I., Coutinho, S., Pedersen, S. A., Simpson, M. R. & Martins, C. Differences in gastrointestinal hormones and appetite ratings between individuals with and without obesity – a systematic review and meta-analysis. Obes. Rev. 2, e13531 (2022).
Cui, H., Lopez, M. & Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 13, 338–351 (2017).
Hintze, L. J., Mahmoodianfard, S., Auguste, C. B. & Doucet, E. Weight loss and appetite control in women. Curr. Obes. Rep. 6, 334–351 (2017).
Zhao, X. et al. The role of gut hormones in diet-induced weight change: a systematic review. Horm. Metab. Res. 49, 816–825 (2017).
Koliaki, C., Liatis, S., Dalamaga, M. & Kokkinos, A. The implication of gut hormones in the regulation of energy homeostasis and their role in the pathophysiology of obesity. Curr. Obes. Rep. 9, 255–271 (2020).
Wadden, T. A. et al. Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss. J. Clin. Endocrinol. Metab. 83, 214–218 (1998).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Strohacker, K., McCaffery, J. M., MacLean, P. S. & Wing, R. R. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int. J. Obes. 38, 388–396 (2014).
Thom, G. et al. Weight loss-induced increase in fasting ghrelin concentration is a predictor of weight regain: evidence from the diabetes remission clinical trial (DiRECT). Diabetes Obes. Metab. 23, 711–719 (2021).
Buso, M. E. C. et al. Can a higher protein/low glycemic index vs. a conventional diet attenuate changes in appetite and gut hormones following weight loss? a 3-year PREVIEW sub-study. Front. Nutr. 8, 640538 (2021).
Rejeski, J. J., Fanning, J., Nicklas, B. J. & Rejeski, W. J. Six-month changes in ghrelin and glucagon-like peptide-1 with weight loss are unrelated to long-term weight regain in obese older adults. Int. J. Obes. 45, 888–894 (2021).
Simon, J. J. et al. Neural food reward processing in successful and unsuccessful weight maintenance. Obesity 26, 895–902 (2018).
van Baak, M. A. Dietary carbohydrates and weight loss maintenance. Curr. Opin. Clin. Nutr. Metab. Care 24, 354–358 (2021).
van Baak, M. A. & Mariman, E. C. M. Dietary strategies for weight loss maintenance. Nutrients 11, 1916 (2019).
Ramos-Lopez, O., Martinez-Urbistondo, D., Vargas-Nunez, J. A. & Martinez, J. A. The role of nutrition on meta-inflammation: insights and potential targets in communicable and chronic disease management. Curr. Obes. Rep. 11, 305–335 (2022).
Naatanen, M. et al. Post-weight loss changes in fasting appetite- and energy balance-related hormone concentrations and the effect of the macronutrient content of a weight maintenance diet: a randomised controlled trial. Eur. J. Nutr. 60, 2603–2616 (2021).
Muhammad, H. F. L. et al. Dietary intake after weight loss and the risk of weight regain: macronutrient composition and inflammatory properties of the diet. Nutrients 9, 1205 (2017).
Foright, R. M. et al. Is regular exercise an effective strategy for weight loss maintenance? Physiol. Behav. 188, 86–93 (2018).
Gonzalo-Encabo, P., Maldonado, G., Valades, D., Ferragut, C. & Perez-Lopez, A. The role of exercise training on low-grade systemic inflammation in adults with overweight and obesity: a systematic review. Int. J. Env. Res. Public. Health 18, 13258 (2021).
Patterson, C. M., Bouret, S. G., Dunn-Meynell, A. A. & Levin, B. E. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R537–R548 (2009).
Martelli, D. & Brooks, V. L. Leptin increases: physiological roles in the control of sympathetic nerve activity, energy balance, and the hypothalamic-pituitary-thyroid axis. Int. J. Mol. Sci. 24, 2684 (2023).
Della Guardia, L. & Codella, R. Exercise restores hypothalamic health in obesity by reshaping the inflammatory network. Antioxidants 12, 297 (2023).
Fanning, J. et al. Intervening on exercise and daylong movement for weight loss maintenance in older adults: a randomized, clinical trial. Obesity 30, 85–95 (2022).
Kaikkonen, K. M., Korpelainen, R., Vanhala, M. L., Keinanen-Kiukaanniemi, S. M. & Korpelainen, J. T. Long-term effects on weight loss and maintenance by intensive start with diet and exercise. Scand. J. Med. Sci. Sports 33, 246–256 (2022).
Pavlou, K. N., Krey, S. & Steffee, W. P. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am. J. Clin. Nutr. 49, 1115–1123 (1989).
Jeffery, R. W., Wing, R. R., Sherwood, N. E. & Tate, D. F. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome. Am. J. Clin. Nutr. 78, 684–689 (2003).
Fogelholm, M., Kukkonen-Harjula, K., Nenonen, A. & Pasanen, M. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch. Intern. Med. 160, 2177–2184 (2000).
Borg, P., Kukkonen-Harjula, K., Fogelholm, M. & Pasanen, M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int. J. Obes. Relat. Metab. Disord. 26, 676–683 (2002).
Hintze, L. J. et al. A one-year resistance training program following weight loss has no significant impact on body composition and energy expenditure in postmenopausal women living with overweight and obesity. Physiol. Behav. 189, 99–106 (2018).
Lundgren, J. R. et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N. Engl. J. Med. 384, 1719–1730 (2021).
Washburn, R. A. et al. A randomized trial evaluating exercise for the prevention of weight regain. Obesity 29, 62–70 (2021).
Jakicic, J. M., Winters, C., Lang, W. & Wing, R. R. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA 282, 1554–1560 (1999).
Jakicic, J. M., Marcus, B. H., Lang, W. & Janney, C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch. Intern. Med. 168, 1550–1559 (2008).
Jakicic, J. M. et al. Objective physical activity and weight loss in adults: the step-up randomized clinical trial. Obesity 22, 2284–2292 (2014).
Tate, D. F., Jeffery, R. W., Sherwood, N. E. & Wing, R. R. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am. J. Clin. Nutr. 85, 954–959 (2007).
Swift, D. L. et al. The effects of exercise and physical activity on weight loss and maintenance. Prog. Cardiovasc. Dis. 61, 206–213 (2018).
Schoeller, D. A., Shay, K. & Kushner, R. F. How much physical activity is needed to minimize weight gain in previously obese women? Am. J. Clin. Nutr. 66, 551–556 (1997).
van Baak, M. A. et al. Leisure-time activity is an important determinant of long-term weight maintenance after weight loss in the Sibutramine Trial on Obesity Reduction and Maintenance (STORM trial). Am. J. Clin. Nutr. 78, 209–214 (2003).
van Baak, M. A., Hul, G., Astrup, A. & Saris, W. H. Physical activity, weight loss, and weight maintenance in the diogenes multicenter trial. Front. Nutr. 8, 683369 (2021).
Catenacci, V. A. et al. Physical activity patterns in the National Weight Control Registry. Obesity 16, 153–161 (2008).
Ostendorf, D. M. et al. Physical activity energy expenditure and total daily energy expenditure in successful weight loss maintainers. Obesity 27, 496–504 (2019).
Thomas, J. G., Bond, D. S., Phelan, S., Hill, J. O. & Wing, R. R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am. J. Prev. Med. 46, 17–23 (2014).
Finer, N. Future directions in obesity pharmacotherapy. Eur. J. Intern. Med. 93, 13–20 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Muller, T. D., Bluher, M., Tschop, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug. Discov. 21, 201–223 (2022).
Wadden, T. A. et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int. J. Obes. 37, 1443–1451 (2013).
Iepsen, E. W. et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int. J. Obes. 39, 834–841 (2015).
Rubino, D. et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 325, 1414–1425 (2021).
Rosenbaum, M. et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586 (2005).
Baldwin, K. M. et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1259–R1266 (2011).
Conroy, R. et al. Leptin administration does not prevent the bone mineral metabolism changes induced by weight loss. Metabolism 60, 1222–1226 (2011).
Kissileff, H. R. et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am. J. Clin. Nutr. 95, 309–317 (2012).
Reidelberger, R. et al. Effects of leptin replacement alone and with exendin-4 on food intake and weight regain in weight-reduced diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 302, E1576–E1585 (2012).
Christoffersen, B. O. et al. Beyond appetite regulation: targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity 30, 841–857 (2022).
Hostrup, M. & Onslev, J. The beta2-adrenergic receptor – a re-emerging target to combat obesity and induce leanness? J. Physiol. 600, 1209–1227 (2022).
Zapata, R. C. et al. Adipocytes control food intake and weight regain via vacuolar-type H+ ATPase. Nat. Commun. 13, 5092 (2022).
Gounarides, J. S. et al. Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity mouse model. Endocrinology 149, 758–766 (2008).
Kuo, T., Harris, C. A. & Wang, J. C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell Endocrinol. 380, 79–88 (2013).
Prabhu, S. et al. Nanocarriers targeting adipose macrophages increase glucocorticoid anti-inflammatory potency to ameliorate metabolic dysfunction. Biomater. Sci. 9, 506–518 (2021).
Xu, C. et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 23, 1161–1170 (2009).
Joffin, N. et al. Adipose tissue macrophages exert systemic metabolic control by manipulating local iron concentrations. Nat. Metab. 4, 1474–1494 (2022).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Kolonin, M. G., Saha, P. K., Chan, L., Pasqualini, R. & Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 10, 625–632 (2004).
Yan, J. et al. Gold nanobipyramid-mediated apoptotic camouflage of adipocytes for obesity immunotherapy. Adv. Mater. 35, e2207686 (2022).
Garcia, J. M. et al. Rise of plasma ghrelin with weight loss is not sustained during weight maintenance. Obesity 14, 1716–1723 (2006).
Adam, T. C., Lejeune, M. P. & Westerterp-Plantenga, M. S. Nutrient-stimulated glucagon-like peptide 1 release after body-weight loss and weight maintenance in human subjects. Br. J. Nutr. 95, 160–167 (2006).
Iepsen, E. W., Lundgren, J., Holst, J. J., Madsbad, S. & Torekov, S. S. Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3-36. Eur. J. Endocrinol. 174, 775–784 (2016).
DeBenedictis, J. N. et al. Changes in the homeostatic appetite system after weight loss reflect a normalization toward a lower body weight. J. Clin. Endocrinol. Metab. 105, e2538–e2546 (2020).
Anderson, J. W., Konz, E. C., Frederich, R. C. & Wood, C. L. Long-term weight-loss maintenance: a meta-analysis of US studies. Am. J. Clin. Nutr. 74, 579–584 (2001).
Hartmann-Boyce, J. et al. Weight regain after behavioural weight management programmes and its impact on quality of life and cost effectiveness: evidence synthesis and health economic analyses. Diabetes Obes. Metab. 25, 526–535 (2022).
Cambi, M. P. C. et al. Multidisciplinary approach for weight regain – how to manage this challenging condition: an expert review. Obes. Surg. 31, 1290–1303 (2021).
Wilding, J. P. H. et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes. Metab. 24, 1553–1564 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Jose Galgani; Amanda Sainsbury; Alyssa Hasty, who co-reviewed with Jamie Garcia; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
The Human Protein Atlas summary of cathepsin S expression: https://www.proteinatlas.org/ENSG00000163131-CTSS
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Baak, M.A., Mariman, E.C.M. Obesity-induced and weight-loss-induced physiological factors affecting weight regain. Nat Rev Endocrinol 19, 655–670 (2023). https://doi.org/10.1038/s41574-023-00887-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00887-4
This article is cited by
-
Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
Nature Communications (2024)