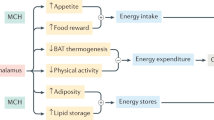
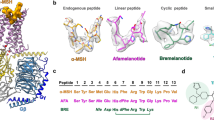
Abstract
A large body of preclinical and clinical data shows that the central melanocortin system is a promising therapeutic target for treating various metabolic disorders such as obesity and cachexia, as well as anorexia nervosa. Setmelanotide, which functions by engaging the central melanocortin circuitry, was approved by the FDA in 2020 for use in certain forms of syndromic obesity. Furthermore, the FDA approvals in 2019 of two peptide drugs targeting melanocortin receptors for the treatment of generalized hypoactive sexual desire disorder (bremelanotide) and erythropoietic protoporphyria-associated phototoxicity (afamelanotide) demonstrate the safety of this class of peptides. These approvals have also renewed excitement in the development of therapeutics targeting the melanocortin system. Here, we review the anatomy and function of the melanocortin system, discuss progress and challenges in developing melanocortin receptor-based therapeutics, and outline potential metabolic and behavioural disorders that could be addressed using pharmacological agents targeting these receptors.
Key points
-
Several monogenic and polygenic obesity syndromes involve defective leptin–melanocortin signalling.
-
The non-specific melanocortin peptide agonist setmelanotide, which acts via the MC4R to inhibit food intake and produce weight loss, has been approved by the FDA for the treatment of proopiomelanocortin deficiency, leptin receptor deficiency and Bardet–Biedl syndrome.
-
Two non-specific melanocortin agonist peptides, bremelanotide and afamelanotide, have been approved for the treatment of hypoactive sexual desire disorder and erythropoietic protoporphyria-associated phototoxicity, respectively, demonstrating the safety of this new drug class.
-
Melanocortin therapeutics might also have applications in eating disorders, such as cachexia and anorexia nervosa, and disorders of neuroendocrine function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gantz, I. et al. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 268, 8246–8250 (1993).
Gantz, I. et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 268, 15174–15179 (1993).
Roselli-Rehfuss, L. et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl Acad. Sci. USA 90, 8856–8860 (1993).
Mountjoy, K. G., Robbins, L. S., Mortrud, M. T. & Cone, R. D. The cloning of a family of genes that encode the melanocortin receptors. Science 257, 1248–1251 (1992).
Mountjoy, K. G. Distribution and function of melanocortin receptors within the brain. Adv. Exp. Med. Biol. 681, 29–48 (2010).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Shadiack, A. M., Sharma, S. D., Earle, D. C., Spana, C. & Hallam, T. J. Melanocortins in the treatment of male and female sexual dysfunction. Curr. Top. Med. Chem. 7, 1137–1144 (2007).
Butler, A. A. & Cone, R. D. The melanocortin receptors: lessons from knockout models. Neuropeptides 36, 77–84 (2002).
Cone, R. D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 27, 736–749 (2006).
Chen, W. et al. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 91, 789–798 (1997).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science 278, 135–138 (1997).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the Agouti obesity syndrome. Nature 385, 165–168 (1997).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Mountjoy, K. G. Pro-opiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: how understanding of this system has changed over the last decade. J. Neuroendocrinol. 27, 406–418 (2015).
Friedman, J. 20 years of leptin: leptin at 20: an overview. J. Endocrinol. 223, T1–T8 (2014).
Mercer, A. J., Hentges, S. T., Meshul, C. K. & Low, M. J. Unraveling the central proopiomelanocortin neural circuits. Front. Neurosci. 7, 19 (2013).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Baver, S. B. et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 34, 5486–5496 (2014).
Xu, J. et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509 (2018).
Cheung, C. C., Clifton, D. K. & Steiner, R. A. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138, 4489–4492 (1997).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Krashes, M. J., Lowell, B. B. & Garfield, A. S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 19, 206–219 (2016).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157 (1998).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20, 111–112 (1998).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, 271–279 (2000).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Tartaglia, L. A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40, 768–775 (2008).
Adan, R. A. & van Dijk, G. Melanocortin receptors as drug targets for disorders of energy balance. CNS Neurol. Disord. Drug Targets 5, 251–261 (2006).
Adan, R. A. & Gispen, W. H. Melanocortins and the brain: from effects via receptors to drug targets. Eur. J. Pharmacol. 405, 13–24 (2000).
Ericson, M. D. et al. Bench-top to clinical therapies: a review of melanocortin ligands from 1954 to 2016. Biochim. Biophys. Acta 1863, 2414–2435 (2017).
Yang, L. K. & Tao, Y. X. Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim. Biophys. Acta 1863, 2486–2495 (2017).
Kirwan, P. et al. Quantitative mass spectrometry for human melanocortin peptides in vitro and in vivo suggests prominent roles for β-MSH and desacetyl α-MSH in energy homeostasis. Mol. Metab. 17, 82–97 (2018).
Mountjoy, K. G. et al. Desacetyl-α-melanocyte stimulating hormone and α-melanocyte stimulating hormone are required to regulate energy balance. Mol. Metab. 9, 207–216 (2018).
Lee, Y. S. et al. A POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 3, 135–140 (2006).
Bumaschny, V. F. et al. Obesity-programmed mice are rescued by early genetic intervention. J. Clin. Invest. 122, 4203–4212 (2012).
Zhan, C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013).
D’Agostino, G. et al. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28, 619–630 (2018).
Wang, D. et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 9, 40 (2015).
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Low, M. J., Simerly, R. B. & Cone, R. D. Receptors for the melanocortin peptides in the central nervous system. Curr. Opin. Endocrinol. Diabetes Obes. 1, 79–88 (1994).
Bagnol, D. et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 19, RC26 (1999).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999).
Ghamari-Langroudi, M. et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520, 94–98 (2015).
Fenselau, H. et al. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci. 20, 42–51 (2017).
Skibicka, K. P. & Grill, H. J. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150, 5351–5361 (2009).
Li, G. et al. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am. J. Physiol. Endocrinol. Metab. 293, E252–E258 (2007).
Williams, D. L. Brainstem Melanocortin Receptor Contributions to Energy Balance. Dissertation, Univ. Pennsylvania https://repository.upenn.edu/dissertations/AAI3087483/ (2003).
Skibicka, K. P. & Grill, H. J. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149, 3605–3616 (2008).
Smith, S. R. et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363, 245–256 (2010).
Nigro, S. C., Luon, D. & Baker, W. L. Lorcaserin: a novel serotonin 2C agonist for the treatment of obesity. Curr. Med. Res. Opin. 29, 839–848 (2013).
Heisler, L. K. et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51, 239–249 (2006).
Xu, Y. et al. A serotonin and melanocortin circuit mediates d-fenfluramine anorexia. J. Neurosci. 30, 14630–14634 (2010).
Heisler, L. K. et al. Activation of central melanocortin pathways by fenfluramine. Science 297, 609–611 (2002).
Martin, C. K. et al. Lorcaserin, a 5-HT2C receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J. Clin. Endocrinol. Metab. 96, 837–845 (2011).
US Food and Drug Administration. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. FDA https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market (2020).
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314, 687–699 (2015).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Astrup, A. et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 (2009).
He, Z. et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons - implications for energy balance and glucose control. Mol. Metab. 28, 120–134 (2019).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Mehta, A., Marso, S. P. & Neeland, I. J. Liraglutide for weight management: a critical review of the evidence. Obes. Sci. Pract. 3, 3–14 (2017).
Christou, G. A., Katsiki, N., Blundell, J., Fruhbeck, G. & Kiortsis, D. N. Semaglutide as a promising antiobesity drug. Obes. Rev. 20, 805–815 (2019).
Blundell, J. et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 19, 1242–1251 (2017).
Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 5, e133429 (2020).
Drucker, D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018).
Kanoski, S. E., Hayes, M. R. & Skibicka, K. P. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R885–R895 (2016).
Fortin, S. M., Chen, J. & Hayes, M. R. Hindbrain melanocortin 3/4 receptors modulate the food intake and body weight suppressive effects of the GLP-1 receptor agonist, liraglutide. Physiol. Behav. 220, 112870 (2020).
Clemmensen, C. et al. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO Mol. Med. 7, 288–298 (2015).
Sweeney, P. et al. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med. 13, eabd6434 (2021).
Naltrexone/bupropion. Drugs R D 10, 25–32 (2010).
Hausenloy, D. Contrave™: novel treatment for obesity. Clin. Lipidol. 4, 279–285 (2017).
Naltrexone/bupropion for obesity. Drug. Ther. Bull. 55, 126–129 (2017).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Wu, Q., Boyle, M. P. & Palmiter, R. D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Burnett, C. J. et al. Need-based prioritization of behavior. eLife 8, e44527 (2019).
Burnett, C. J. et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016).
Alhadeff, A. L. et al. A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152 (2018).
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Wu, Q., Clark, M. S. & Palmiter, R. D. Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597 (2012).
Padilla, S. L. et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl Acad. Sci. USA 114, 2413–2418 (2017).
Dietrich, M. O., Zimmer, M. R., Bober, J. & Horvath, T. L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Wu, Q., Howell, M. P., Cowley, M. A. & Palmiter, R. D. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl Acad. Sci. USA 105, 2687–2692 (2008).
Krashes, M. J., Shah, B. P., Koda, S. & Lowell, B. B. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 18, 588–595 (2013).
Engstrom Ruud, L., Pereira, M. M. A., de Solis, A. J., Fenselau, H. & Bruning, J. C. NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons. Nat. Commun. 11, 442 (2020).
Chen, Y. et al. Sustained NPY signaling enables AgRP neurons to drive feeding. eLife 8, e46348 (2019).
Hagan, M. M. et al. Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R47–R52 (2000).
Pritchard, L. E. & White, A. Agouti-related protein: more than a melanocortin-4 receptor antagonist? Peptides 26, 1759–1770 (2005).
Cheng, M. et al. Computational analyses of obesity associated loci generated by genome-wide association studies. PLoS One 13, e0199987 (2018).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15, e1007603 (2019).
Alkemade, A. et al. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in alphaMSH are related to type 2 diabetes. J. Clin. Endocrinol. Metab. 97, E925–E933 (2012).
Ilnytska, O. & Argyropoulos, G. The role of the Agouti-related protein in energy balance regulation. Cell Mol. Life Sci. 65, 2721–2731 (2008).
Song, Y. & Cone, R. D. Creation of a genetic model of obesity in a teleost. FASEB J. 21, 2042–2049 (2007).
Mequinion, M., Foldi, C. J. & Andrews, Z. B. The ghrelin-AgRP neuron nexus in anorexia nervosa: implications for metabolic and behavioral adaptations. Front. Nutr. 6, 190 (2019).
Muller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Muller, T. D., Perez-Tilve, D., Tong, J., Pfluger, P. T. & Tschop, M. H. Ghrelin and its potential in the treatment of eating/wasting disorders and cachexia. J. Cachexia Sarcopenia Muscle 1, 159–167 (2010).
Cummings, D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Tschop, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Romero, A. et al. GOAT: the master switch for the ghrelin system? Eur. J. Endocrinol. 163, 1–8 (2010).
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Fazeli, P. K. et al. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J. Clin. Psychiatry 79, 17m11585 (2018).
Fani, L., Bak, S., Delhanty, P., van Rossum, E. F. & van den Akker, E. L. The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int. J. Obes. 38, 163–169 (2014).
Gautron, L., Elmquist, J. K. & Williams, K. W. Neural control of energy balance: translating circuits to therapies. Cell 161, 133–145 (2015).
Nuffer, W. A. & Trujillo, J. M. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy 35, 926–934 (2015).
Garner, A. L. & Janda, K. D. A small molecule antagonist of ghrelin O-acyltransferase (GOAT). Chem. Commun. 47, 7512–7514 (2011).
Bedenbaugh, M. N. et al. Organization of neural systems expressing melanocortin-3 receptors in the mouse brain: evidence for sexual dimorphism. J. Comp. Neurol. 530, 2835–2851 (2022).
Lippert, R. N., Ellacott, K. L. & Cone, R. D. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 155, 1718–1727 (2014).
West, K. S., Lu, C., Olson, D. P. & Roseberry, A. G. Alpha-melanocyte stimulating hormone increases the activity of melanocortin-3 receptor-expressing neurons in the ventral tegmental area. J. Physiol. 597, 3217–3232 (2019).
Pandit, R. et al. Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology 41, 2241–2251 (2016).
Butler, A. A. et al. A life without hunger: the ups (and downs) to modulating melanocortin-3 receptor signaling. Front. Neurosci. 11, 128 (2017).
Ghamari-Langroudi, M. et al. Regulation of energy rheostasis by the melanocortin-3 receptor. Sci. Adv. 4, eaat0866 (2018).
You, P. et al. Effects of melanocortin 3 and 4 receptor deficiency on energy homeostasis in rats. Sci. Rep. 6, 34938 (2016).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 26, 97–102 (2000).
Renquist, B. J. et al. Melanocortin-3 receptor regulates the normal fasting response. Proc. Natl Acad. Sci. USA 109, E1489–E1498 (2012).
Sutton, G. M. et al. The melanocortin-3 receptor is required for entrainment to meal intake. J. Neurosci. 28, 12946–12955 (2008).
Begriche, K. et al. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav. 11, 291–302 (2012).
Lam, B. Y. H. et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature 599, 436–441 (2021).
Sohn, J. W., Elmquist, J. K. & Williams, K. W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512 (2013).
Marks, D. L., Butler, A. A., Turner, R., Brookhart, G. & Cone, R. D. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 144, 1513–1523 (2003).
Marks, D. L. in Energy Metabolism and Obesity: Research and Clinical Applications (ed P. A. Donohoue) 59–68 (Humana, 2008).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Shah, B. P. et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl Acad. Sci. USA 111, 13193–13198 (2014).
Li, Y. Q. et al. G(q/11)α and G(s)α mediate distinct physiological responses to central melanocortins. J. Clin. Invest. 126, 40–49 (2016).
Giraudo, S. Q., Billington, C. J. & Levine, A. S. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 809, 302–306 (1998).
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 (2018).
Sohn, J. W. et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152, 612–619 (2013).
Berglund, E. D. et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 17, 911–913 (2014).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Grill, H. J., Ginsberg, A. B., Seeley, R. J. & Kaplan, J. M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 (1998).
Yu, J. et al. Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science 368, 428–433 (2020).
Anderson, E. J. P. et al. Late onset obesity in mice with targeted deletion of potassium inward rectifier Kir7.1 from cells expressing the melanocortin-4 receptor. J. Neuroendocrinol. 31, e12670 (2019).
Srisai, D. et al. MRAP2 regulates ghrelin receptor signaling and hunger sensing. Nat. Commun. 8, 713 (2017).
Rouault, A. A. J., Lee, A. A. & Sebag, J. A. Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim. Biophys. Acta 1864, 2322–2329 (2017).
Sebag, J. A., Zhang, C., Hinkle, P. M., Bradshaw, A. M. & Cone, R. D. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science 341, 278–281 (2013).
Asai, M. et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278 (2013).
Lotta, L. A. et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 177, 597–607 (2019).
Brouwers, B. et al. Human MC4R variants affect endocytosis, trafficking and dimerization revealing multiple cellular mechanisms involved in weight regulation. Cell Rep. 34, 108862 (2021).
Gillyard, T., Fowler, K., Williams, S. Y. & Cone, R. D. Obesity-associated mutant melanocortin-4 receptors with normal Gαs coupling frequently exhibit other discoverable pharmacological and biochemical defects. J. Neuroendocrinol. 31, e12795 (2019).
He, S. & Tao, Y. X. Defect in MAPK signaling as a cause for monogenic obesity caused by inactivating mutations in the melanocortin-4 receptor gene. Int. J. Biol. Sci. 10, 1128–1137 (2014).
Van der Ploeg, L. H. et al. A role for the melanocortin 4 receptor in sexual function. Proc. Natl Acad. Sci. USA 99, 11381–11386 (2002).
King, S. H. et al. Melanocortin receptors, melanotropic peptides and penile erection. Curr. Top. Med. Chem. 7, 1098–1106 (2007).
Hadley, M. E. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides 26, 1687–1689 (2005).
Hadley, M. E. & Dorr, R. T. Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides 27, 921–930 (2006).
Dhillon, S. & Keam, S. J. Bremelanotide: first approval. Drugs 79, 1599–1606 (2019).
Bremelanotide (Vyleesi) for hypoactive sexual desire disorder. Med. Lett. Drugs Ther. 61, 114–116 (2019).
Huang, S. A. & Lie, J. D. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T 38, 407–419 (2013).
Pfaus, J., Giuliano, F. & Gelez, H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J. Sex. Med. 4, 269–279 (2007).
Kingsberg, S. A. et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet. Gynecol. 134, 899–908 (2019).
Mayer, D. & Lynch, S. E. Bremelanotide: new drug approved for treating hypoactive sexual desire disorder. Ann. Pharmacother. 54, 684–690 (2020).
Tao, Y. X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr. Rev. 31, 506–543 (2010).
Clement, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24, 551–555 (2018).
Kuhnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375, 240–246 (2016).
Haqq, A. M. et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alstrom syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 10, 859–868 (2022).
Haws, R. M. et al. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp. Clin. Trials Commun. 22, 100780 (2021).
Lubrano-Berthelier, C. et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum. Mol. Genet. 12, 145–153 (2003).
Pierroz, D. D. et al. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51, 1337–1345 (2002).
Bischof, J. M., Van Der Ploeg, L. H., Colmers, W. F. & Wevrick, R. Magel2-null mice are hyper-responsive to setmelanotide, a melanocortin 4 receptor agonist. Br. J. Pharmacol. 173, 2614–2621 (2016).
Stephenson, E., Stayton, A. & Han, J. Setmelanotide (RM-493) reduces food intake and rapidly induces weight loss in a mouse model of Alström syndrome [abstract]. J. Endocr. Soc. 3(Suppl. 1), MON-LB019 (2019).
Center for Drug Evaluation & Research. FDA approves treatment for weight management in patients with Bardet-Biedl syndrome aged 6 or older. FDA https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-weight-management-patients-bardet-biedl-syndrome-aged-6-or-older (2022).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 6, 1321–1329 (2017).
Sharma, S. et al. Current mechanistic and pharmacodynamic understanding of melanocortin-4 receptor activation. Molecules 24, 1892 (2019).
Kanti, V. et al. A Melanocortin-4 receptor agonist induces skin and hair pigmentation in patients with monogenic mutations in the leptin-melanocortin pathway. Skin. Pharmacol. Physiol. 34, 307–316 (2021).
Sutton, A. K. & Krashes, M. J. Integrating hunger with rival motivations. Trends Endocrinol. Metab. 31, 495–507 (2020).
Marks, D. L., Hruby, V., Brookhart, G. & Cone, R. D. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R). Peptides 27, 259–264 (2006).
Lee, M. et al. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides 29, 440–447 (2008).
Herpertz-Dahlmann, B., Holtkamp, K. & Konrad, K. Eating disorders: anorexia and bulimia nervosa. Handb. Clin. Neurol. 106, 447–462 (2012).
Klawonn, A. M. et al. Motivational valence is determined by striatal melanocortin 4 receptors. J. Clin. Invest. 128, 3160–3170 (2018).
Lim, B. K., Huang, K. W., Grueter, B. A., Rothwell, P. E. & Malenka, R. C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Chaki, S. & Okuyama, S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides 26, 1952–1964 (2005).
Garnsey, M. R. et al. Discovery of the potent and selective MC4R antagonist PF-07258669 for the potential treatment of appetite loss. J. Med. Chem. 66, 3195–3211 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05113940 (2022).
Gruber, K. A. et al. Development of a therapeutic peptide for cachexia suggests a platform approach for drug-like peptides. ACS Pharmacol. Transl. Sci. 5, 344–361 (2022).
Israeli, H. et al. Structure reveals the activation mechanism of the MC4 receptor to initiate satiation signaling. Science 372, 808–814 (2021).
Heyder, N. A. et al. Structures of active melanocortin-4 receptor-Gs-protein complexes with NDP-α-MSH and setmelanotide. Cell Res. 31, 1176–1189 (2021).
Langendonk, J. G. et al. Afamelanotide for erythropoietic protoporphyria. N. Engl. J. Med. 373, 48–59 (2015).
Acknowledgements
The authors acknowledge the support of NIH grants DK126715 (R.D.C.), F32HD095620 (P.S.), K99DK127065 (P.S.), R00 DK127065 (P.S.), Brain and Behaviour Research Foundation Young Investigator Award (P.S.), Foundation for Prader Willi Research (P.S.), Courage Therapeutics (R.D.C.) and The Klarman Family Foundation (R.D.C.). The authors thank R. Arora (Life Sciences Institute, University of Michigan) for assistance in preparing the first drafts of the figures.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
P.S., L.E.G., R.D.C. and the University of Michigan hold equity in Courage Therapeutics Inc. and are inventors of intellectual property optioned to Courage Therapeutics Inc.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Roger Adan, Ya-Xiong Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sweeney, P., Gimenez, L.E., Hernandez, C.C. et al. Targeting the central melanocortin system for the treatment of metabolic disorders. Nat Rev Endocrinol 19, 507–519 (2023). https://doi.org/10.1038/s41574-023-00855-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00855-y
This article is cited by
-
The role of central neurotransmitters in appetite regulation of broilers and layers: similarities and differences
Veterinary Research Communications (2024)
-
Neuroactive steroids in the neuroendocrine control of food intake, metabolism, and reproduction
Endocrine (2024)