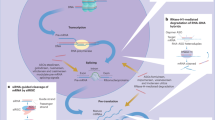
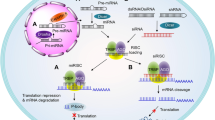
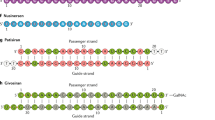
Abstract
More than 25 years after its discovery, the post-transcriptional gene regulation mechanism termed RNAi is now transforming pharmaceutical development, proved by the recent FDA approval of multiple small interfering RNA (siRNA) drugs that target the liver. Synthetic siRNAs that trigger RNAi have the potential to specifically silence virtually any therapeutic target with unprecedented potency and durability. Bringing this innovative class of medicines to patients, however, has been riddled with substantial challenges, with delivery issues at the forefront. Several classes of siRNA drug are under clinical evaluation, but their utility in treating extrahepatic diseases remains limited, demanding continued innovation. In this Review, we discuss principal considerations and future directions in the design of therapeutic siRNAs, with a particular emphasis on chemistry, the application of informatics, delivery strategies and the importance of careful target selection, which together influence therapeutic success.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
Zimmermann, T. S. et al. RNAi-mediated gene silencing in non-human primates. Nature 441, 111–114 (2006).
Frank-Kamenetsky, M. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl Acad. Sci. USA 105, 11915–11920 (2008).
Akinc, A. et al. Development of lipidoid–siRNA formulations for systemic delivery to the liver. Mol. Ther. 17, 872–879 (2009).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug. Discov. 8, 129–138 (2009).
Tao, W. et al. Noninvasive imaging of lipid nanoparticle–mediated systemic delivery of small-interfering RNA to the liver. Mol. Ther. 18, 1657–1666 (2010).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819–829 (2013).
Hoy, S. M. Patisiran: first global approval. Drugs 78, 1625–1631 (2018).
Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014). This article reports the use of GalNAc conjugates for targeted delivery of siRNA to hepatocytes.
Zimmermann, T. S. et al. Clinical proof of concept for a novel hepatocyte-targeting GalNAc-siRNA conjugate. Mol. Ther. 25, 71–78 (2017).
Garber, K. Alnylam terminates revusiran program, stock plunges. Nat. Biotechnol. 34, 1213–1214 (2016).
Maraganore, J. Reflections on Alnylam. Nat. Biotechnol. 40, 641–650 (2022).
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023). This article provides a comprehensive review on the chemistry and function of approved oligonucleotide-based drugs by early 2023.
Setten, R. L., Rossi, J. J. & Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18, 421–446 (2019). This review provides an excellent resource for understanding the basic biology of RNAi therapeutics.
Dowdy, S. F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 35, 222–229 (2017).
Dowdy, S. F., Setten, R. L., Cui, X.-S. & Jadhav, S. G. Delivery of RNA therapeutics: the great endosomal escape! Nucleic Acid. Ther. 32, 361–368 (2022). References 16 and 17 discuss the fundamental challenges of cellular uptake and endosomal escape of siRNAs.
Layzer, J. M. et al. In vivo activity of nuclease-resistant siRNAs. RNA 10, 766–771 (2004).
Gantier, M. P. & Williams, B. R. G. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 18, 363–371 (2007).
Scacheri, P. C. et al. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl Acad. Sci. USA 101, 1892–1897 (2004).
Khvorova, A. & Watts, J. K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 35, 238–248 (2017). This review discusses chemical modifications that evolve ASO and siRNA therapeutics towards clinical application.
De Smet, M. D., Meenken, C. & Van Den Horn, G. J. Fomivirsen—a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul. Immunol. Inflamm. 7, 189–198 (1999).
Kurreck, J. Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem. 270, 1628–1644 (2003).
Lee, J.-H. et al. A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc. Natl Acad. Sci. USA 102, 18902–18907 (2005).
Brown, C. R. et al. Investigating the pharmacodynamic durability of GalNAc–siRNA conjugates. Nucleic Acids Res. 48, 11827–11844 (2020).
Hassler, M. R. et al. Comparison of partially and fully chemically-modified siRNA in conjugate-mediated delivery in vivo. Nucleic Acids Res. 46, 2185–2196 (2018). This article demonstrates that full chemical modification is essential for improving siRNA in vivo efficacy.
Allerson, C. R. et al. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 48, 901–904 (2005). This paper compares the potency of fully 2′-modified siRNA with unmodified siRNA.
Manoharan, M. et al. Unique gene-silencing and structural properties of 2′-fluoro-modified siRNAs. Angew. Chem. Int. Ed. 50, 2284–2288 (2011).
Jahns, H. et al. Stereochemical bias introduced during RNA synthesis modulates the activity of phosphorothioate siRNAs. Nat. Commun. 6, 6317 (2015).
Schirle, N. T., Sheu-Gruttadauria, J. & MacRae, I. J. Structural basis for microRNA targeting. Science 346, 608–613 (2014).
Manoharan, M. 2′-Carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim. Biophys. Acta Gene Struct. Expr. 1489, 117–130 (1999).
Foster, D. J. et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol. Ther. 26, 708–717 (2018).
Blidner, R. A., Hammer, R. P., Lopez, M. J., Robinson, S. O. & Monroe, W. T. Fully 2′-deoxy-2′-fluoro substituted nucleic acids induce RNA interference in mammalian cell culture. Chem. Biol. Drug Des. 70, 113–122 (2007).
Janas, M. M. et al. Safety evaluation of 2′-deoxy-2′-fluoro nucleotides in GalNAc–siRNA conjugates. Nucleic Acids Res. 47, 3306–3320 (2019). This article investigates the safety of 2′-F nucleotides in GalNAc–siRNAs.
Schirle, N. T. & MacRae, I. J. The crystal structure of human argonaute2. Science 336, 1037–1040 (2012).
Davis, S. M. et al. Chemical optimization of siRNA for safe and efficient silencing of placental sFLT1. Mol. Ther. Nucleic Acids 29, 135–149 (2022).
Grimm, D. et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441, 537–541 (2006).
Nair, J. K. et al. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res. 45, 10969–10977 (2017).
Judge, D. P. et al. Phase 3 multicenter study of revusiran in patients with hereditary transthyretin-mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR). Cardiovasc. Drugs Ther. 34, 357–370 (2020).
Biscans, A. et al. The chemical structure and phosphorothioate content of hydrophobically modified siRNAs impact extrahepatic distribution and efficacy. Nucleic Acids Res. 48, 7665–7680 (2020).
Ly, S., Echeverria, D., Sousa, J. & Khvorova, A. Single-stranded phosphorothioated regions enhance cellular uptake of cholesterol-conjugated siRNA but not silencing efficacy. Mol. Ther. Nucleic Acids 21, 991–1005 (2020).
Wang, Y., Sheng, G., Juranek, S., Tuschl, T. & Patel, D. J. Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 (2008).
Frank, F., Sonenberg, N. & Nagar, B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822 (2010).
Parmar, R. et al. 5′-(E)-vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. ChemBioChem 17, 985–989 (2016).
Haraszti, R. A. et al. 5΄-Vinylphosphonate improves tissue accumulation and efficacy of conjugated siRNAs in vivo. Nucleic Acids Res. 45, 7581–7592 (2017).
Lima, W. F. et al. Single-stranded siRNAs activate RNAi in animals. Cell 150, 883–894 (2012).
Yu, D. et al. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant Huntingtin expression. Cell 150, 895–908 (2012).
Prakash, T. P. et al. Identification of metabolically stable 5′-phosphate analogs that support single-stranded siRNA activity. Nucleic Acids Res. 43, 2993–3011 (2015).
Alterman, J. F. et al. A divalent siRNA chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous system. Nat. Biotechnol. 37, 884–894 (2019). This paper describes the effect of siRNA valency on improving tissue distribution profile and potency in the CNS.
Biscans, A. et al. Docosanoic acid conjugation to siRNA enables functional and safe delivery to skeletal and cardiac muscles. Mol. Ther. 29, 1382–1394 (2021).
Brown, K. M. et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 40, 1500–1508 (2022). This paper reports on the application of lipophilic conjugates to enhance the utility of siRNA in extrahepatic tissues.
Hariharan, V. N. et al. Divalent siRNAs are bioavailable in the lung and efficiently block SARS-CoV-2 infection. Proc. Natl Acad. Sci. USA 120, e2219523120 (2023).
Elkayam, E. et al. siRNA carrying an (E)-vinylphosphonate moiety at the 5′ end of the guide strand augments gene silencing by enhanced binding to human Argonaute-2. Nucleic Acids Res. 45, 3528–3536 (2017).
Guenther, D. C. et al. Role of a “magic” methyl: 2′-Deoxy-2′-α-F-2′-β-C-methyl pyrimidine nucleotides modulate RNA interference activity through synergy with 5′-phosphate mimics and mitigation of off-target effects. J. Am. Chem. Soc. 144, 14517–14534 (2022).
Koller, E. et al. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 39, 4795–4807 (2011).
Schwarz, D. S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003).
Sano, M. et al. Effect of asymmetric terminal structures of short RNA duplexes on the RNA interference activity and strand selection. Nucleic Acids Res. 36, 5812–5821 (2008).
Kim, D.-H. et al. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 23, 222–226 (2005). This paper describes a siRNA variant that requires Dicer processing to enable RNAi activity.
Snead, N. M. et al. Molecular basis for improved gene silencing by Dicer substrate interfering RNA compared with other siRNA variants. Nucleic Acids Res. 41, 6209–6221 (2013).
Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. RNAi. Cell 101, 25–33 (2000).
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Chang, C. I. et al. Asymmetric shorter-duplex siRNA structures trigger efficient gene silencing with reduced nonspecific effects. Mol. Ther. 17, 725–732 (2009).
Jackson, A. L. et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 12, 1197–1205 (2006).
Datta, D. et al. Rational optimization of siRNA to ensure strand bias in the interaction with the RNA-induced silencing complex. Chem. Commun. 59, 6347–6350 (2023).
Chen, P. Y. et al. Strand-specific 5′-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA 14, 263–274 (2008).
Shibata, A. et al. Terminal bridging of siRNA duplex at the ribose 2′ position controls strand bias and target sequence preference. Mol. Ther. Nucleic Acids 32, 468–477 (2023).
Rossi, J. J. Bridging siRNA strands for better function. Mol. Ther. Nucleic Acids 33, 209 (2023).
Varley, A. J. & Desaulniers, J.-P. Chemical strategies for strand selection in short-interfering RNAs. RSC Adv. 11, 2415–2426 (2021).
Jackson, A. L. et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12, 1179–1187 (2006).
Anderson, E. M. et al. Experimental validation of the importance of seed complement frequency to siRNA specificity. RNA 14, 853–861 (2008).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Ferguson, C. M. et al. Silencing Apoe with divalent-siRNAs improves amyloid burden and activates immune response pathways in Alzheimer’s disease. Alzheimers Dement. https://doi.org/10.1002/alz.13703 (2024).
Janas, M. M. et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 9, 723 (2018).
Schlegel, M. K. et al. Overcoming GNA/RNA base-pairing limitations using isonucleotides improves the pharmacodynamic activity of ESC+ GalNAc–siRNAs. Nucleic Acids Res. 49, 10851–10867 (2021).
Schlegel, M. K. et al. From bench to bedside: improving the clinical safety of GalNAc–siRNA conjugates using seed-pairing destabilization. Nucleic Acids Res. 50, 6656–6670 (2022).
Egli, M., Schlegel, M. K. & Manoharan, M. Acyclic (S)-glycol nucleic acid (S -GNA) modification of siRNAs improves the safety of RNAi therapeutics while maintaining potency. RNA 29, 402–414 (2023). This article introduces GNA modification at the seed region of the guide strand, which could mitigate RNAi-mediated off-targeting effects.
Yamada, K. et al. Extended nucleic acid (exNA): a novel, biologically compatible backbone that significantly enhances oligonucleotide efficacy in vivo. Preprint at https://doi.org/10.1101/2023.05.26.542506 (2023).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 (2005).
Zlatev, I. et al. Reversal of siRNA-mediated gene silencing in vivo. Nat. Biotechnol. 36, 509–511 (2018).
Ferguson et al. A combinatorial approach for achieving CNS-selective RNAi. Nucl. Acids Res. https://doi.org/10.1093/nar/gkae100 (2024).
Miller, V. M. et al. Allele-specific silencing of dominant disease genes. Proc. Natl Acad. Sci. USA 100, 7195–7200 (2003).
Schwarz, D. S. et al. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2, e140 (2006).
Conroy, F. et al. Chemical engineering of therapeutic siRNAs for allele-specific gene silencing in Huntington’s disease models. Nat. Commun. 13, 5802 (2022).
Jongejan, Y. K. et al. Small interfering RNA-mediated allele-selective silencing of von Willebrand factor in vitro and in vivo. Blood Adv. 7, 6108–6119 (2023).
Yamada, K. et al. Structurally constrained phosphonate internucleotide linkage impacts oligonucleotide–enzyme interaction, and modulates siRNA activity and allele specificity. Nucleic Acids Res. 49, 12069–12088 (2021).
Belgrad, J. et al. A programmable dual-targeting di-valent siRNA scaffold supports potent multi-gene modulation in the central nervous system. Preprint at https://doi.org/10.1101/2023.12.19.572404 (2023).
Huesken, D. et al. Design of a genome-wide siRNA library using an artificial neural network. Nat. Biotechnol. 23, 995–1001 (2005).
Shmushkovich, T. et al. Functional features defining the efficacy of cholesterol-conjugated, self-deliverable, chemically modified siRNAs. Nucleic Acids Res. 46, 10905–10916 (2018).
Khvorova, A., Reynolds, A. & Jayasena, S. D. Functional siRNAs and miRNAs exhibit strand bias. Cell 115, 209–216 (2003). This article reveals that thermodynamic properties of siRNA can induce bias in duplex unwinding and guide strand selection.
Boulias, K. & Greer, E. L. Biological roles of adenine methylation in RNA. Nat. Rev. Genet. 24, 143–160 (2023).
Das Mandal, S. & Ray, P. S. Transcriptome-wide analysis reveals spatial correlation between N6-methyladenosine and binding sites of microRNAs and RNA-binding proteins. Genomics 113, 205–216 (2021).
Kanoria, S., Rennie, W. A., Carmack, C. S., Lu, J. & Ding, Y. N6-methyladenosine enhances post-transcriptional gene regulation by microRNAs. Bioinform. Adv. 2, vbab046 (2022).
Lee, M. Machine learning for small interfering RNAs: a concise review of recent developments. Front. Genet. 14, 1226336 (2023).
Monopoli, K. R., Korkin, D. & Khvorova, A. Asymmetric trichotomous partitioning overcomes dataset limitations in building machine learning models for predicting siRNA efficacy. Mol. Ther. Nucleic Acids 33, 93–109 (2023).
Birmingham, A. et al. A protocol for designing siRNAs with high functionality and specificity. Nat. Protoc. 2, 2068–2078 (2007). This protocol outlines the fundamental principles involved in designing specific and functional siRNAs.
Birmingham, A. et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods 3, 199–204 (2006).
La Rocca, G. et al. In vivo, Argonaute-bound microRNAs exist predominantly in a reservoir of low molecular weight complexes not associated with mRNA. Proc. Natl Acad. Sci. USA 112, 767–772 (2015).
Tang, Q. et al. Multispecies-targeting siRNAs for the modulation of JAK1 in the skin. Mol. Ther. Nucleic Acids 35, 102117 (2024).
Tang, Q. et al. RNAi-based modulation of IFN-γ signaling in skin. Mol. Ther. 30, 2709–2721 (2022).
O’Reilly, D. et al. Di-valent siRNA-mediated silencing of MSH3 blocks somatic repeat expansion in mouse models of Huntington’s disease. Mol. Ther. 31, 1661–1674 (2023).
Schenk, S., Schoenhals, G. J., De Souza, G. & Mann, M. A high confidence, manually validated human blood plasma protein reference set. BMC Med. Genomics 1, 41 (2008).
Godinho, B. M. D. C. et al. PK-modifying anchors significantly alter clearance kinetics, tissue distribution, and efficacy of therapeutics siRNAs. Mol. Ther. Nucleic Acids 29, 116–132 (2022).
Biscans, A. et al. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 47, 1082–1096 (2019).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010). This paper presents the initial evidence of RNAi in humans after systemic administration using nanoparticles.
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Dilliard, S. A. & Siegwart, D. J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 8, 282–300 (2023).
Da Silva Sanchez, A. J. et al. Universal barcoding predicts in vivo ApoE-independent lipid nanoparticle delivery. Nano Lett. 22, 4822–4830 (2022).
Huayamares, S. G. et al. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J. Control. Rel. 357, 394–403 (2023).
Polydefkis, M. et al. Comparison of efficacy outcomes with vutrisiran vs. patisiran in hATTR amyloidosis with polyneuropathy: post-hoc analysis of the HELIOS — a study (S14.003). Neurology 100 (17 Suppl. 2), S14.003 (2023).
Debacker, A. J., Voutila, J., Catley, M., Blakey, D. & Habib, N. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol. Ther. 28, 1759–1771 (2020).
Van De Water, F. M. et al. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug. Metab. Dispos. 34, 1393–1397 (2006).
Thielmann, M. et al. Teprasiran, a small interfering RNA, for the prevention of acute kidney injury in high-risk patients undergoing cardiac surgery: a randomized clinical study. Circulation 144, 1133–1144 (2021).
Kopp, J. B. et al. Podocytopathies. Nat. Rev. Dis. Prim. 6, 68 (2020).
Anders, H.-J., Kitching, A. R., Leung, N. & Romagnani, P. Glomerulonephritis: immunopathogenesis and immunotherapy. Nat. Rev. Immunol. 23, 453–471 (2023).
Godinho, B. M. D. C. et al. Pharmacokinetic profiling of conjugated therapeutic oligonucleotides: a high-throughput method based upon serial blood microsampling coupled to peptide nucleic acid hybridization assay. Nucleic Acid. Ther. 27, 323–334 (2017).
Osborn, M. F. et al. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Res. 47, 1070–1081 (2019).
Fakih, H. H. et al. Dendritic amphiphilic siRNA: selective albumin binding, in vivo efficacy, and low toxicity. Mol. Ther. Nucleic Acids https://doi.org/10.1016/j.omtn.2023.102080 (2023).
Biscans, A., Ly, S., McHugh, N., Cooper, D. A. & Khvorova, A. Engineered ionizable lipid siRNA conjugates enhance endosomal escape but induce toxicity in vivo. J. Control. Release 349, 831–843 (2022).
Turanov, A. A. et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 36, 1164–1173 (2018).
Nanna, A. R. et al. Generation and validation of structurally defined antibody–siRNA conjugates. Nucleic Acids Res. 48, 5281–5293 (2020).
Malecova, B. et al. Targeted tissue delivery of RNA therapeutics using antibody–oligonucleotide conjugates (AOCs). Nucleic Acids Res. 51, 5901–5910 (2023). This paper describes the use of antibody–oligonucleotide conjugates as an effective strategy for delivering siRNA into muscle tissues.
Sugo, T. et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J. Control. Rel. 237, 1–13 (2016).
Klein, D. et al. Centyrin ligands for extrahepatic delivery of siRNA. Mol. Ther. 29, 2053–2066 (2021).
Ämmälä, C. et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 4, eaat3386 (2018).
McNamara, J. O. et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24, 1005–1015 (2006).
Chu, T. C. Aptamer mediated siRNA delivery. Nucleic Acids Res. 34, e73 (2006).
Zhou, J., Li, H., Li, S., Zaia, J. & Rossi, J. Novel cell type-specific aptamer-siRNA delivery system for HIV-1 therapy. Nat. Preced. https://doi.org/10.1038/npre.2007.1299.1 (2007).
Dassie, J. P. et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotechnol. 27, 839–846 (2009).
Neff, C. P. et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci. Transl. Med. 3, 66ra6 (2011).
Wheeler, L. A. et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J. Clin. Invest. 121, 2401–2412 (2011).
Wheeler, L. A. et al. Durable knockdown and protection from HIV transmission in humanized mice treated with gel-formulated CD4 aptamer-siRNA chimeras. Mol. Ther. 21, 1378–1389 (2013).
Zhou, J. & Rossi, J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 16, 181–202 (2017). This review discusses aptamers as targeted therapeutics and siRNA delivery agents.
Zhang, Y. et al. Immunotherapy for breast cancer using EpCAM aptamer tumor-targeted gene knockdown. Proc. Natl Acad. Sci. USA 118, e2022830118 (2021).
Xie, S. et al. Aptamer-based targeted delivery of functional nucleic acids. J. Am. Chem. Soc. 145, 7677–7691 (2023).
Lee, H. et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 7, 389–393 (2012).
Fakhoury, J. J., McLaughlin, C. K., Edwardson, T. W., Conway, J. W. & Sleiman, H. F. Development and characterization of gene silencing DNA cages. Biomacromolecules 15, 276–282 (2014).
Chen, Y.-J., Groves, B., Muscat, R. A. & Seelig, G. DNA nanotechnology from the test tube to the cell. Nat. Nanotechnol. 10, 748–760 (2015).
Bujold, K. E., Fakih, H. H. & Sleiman, H. F. in RNA Interference and Cancer Therapy (ed. Dinesh Kumar, L.) 69–81. Methods in Molecular Biology vol. 1974 (Springer, 2019).
Zhang, H. et al. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl Acad. Sci. USA 116, 7543–7548 (2019).
Zhang, H. et al. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 15, 3064–3087 (2020).
No authors listed.Lipophilic siRNA conjugates yield durable silencing in extrahepatic tissues. Nat. Biotechnol. 40, 1439–1440 (2022).
Cheng, S.-Y. et al. Single intravitreal administration of a tetravalent siRNA exhibits robust and efficient gene silencing in rodent and swine photoreceptors. Mol. Ther. Nucleic Acids 35, 102088 (2023).
Ng, B. et al. Intratracheal administration of siRNA triggers mRNA silencing in the lung to modulate T cell immune response and lung inflammation. Mol. Ther. Nucleic Acids 16, 194–205 (2019).
Ferguson, C. M. et al. Comparative route of administration studies using therapeutic siRNAs show widespread gene modulation in Dorset sheep. JCI Insight 6, e152203 (2021).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 3, 17071 (2017).
Masters, C. L. et al. Alzheimer’s disease. Nat. Rev. Dis. Prim. 1, 15056 (2015).
Hsu, T. & Mitragotri, S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc. Natl Acad. Sci. USA 108, 15816–15821 (2011).
Zheng, D. et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl Acad. Sci. USA 109, 11975–11980 (2012).
Liang, X. et al. Skin delivery of siRNA using sponge spicules in combination with cationic flexible liposomes. Mol. Ther. Nucleic Acids 20, 639–648 (2020).
Dharamdasani, V. et al. Topical delivery of siRNA into skin using ionic liquids. J. Control. Rel. 323, 475–482 (2020).
Lee, K. et al. Study and evaluation of the potential of lipid nanocarriers for transdermal delivery of siRNA. Biotechnol. J. 15, 2000079 (2020).
Mandal, A. et al. Treatment of psoriasis with NFKBIZ siRNA using topical ionic liquid formulations. Sci. Adv. 6, eabb6049 (2020).
Aldawsari, M., Chougule, M. B. & Babu, R. J. Progress in topical siRNA delivery approaches for skin disorders. Curr. Pharm. Des. 21, 4594–4605 (2015).
Tang, Q. et al. Rational design of a JAK1-selective siRNA inhibitor for the modulation of autoimmunity in the skin. Nat. Commun. 14, 7099 (2023).
Cui, J. et al. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat. Commun. 8, 191 (2017).
Brüggenwirth, I. M. A. & Martins, P. N. RNA interference therapeutics in organ transplantation: the dawn of a new era. Am. J. Transplant. 20, 931–941 (2020).
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion — from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
No authors listed. Buying time for transplants. Nat. Biotechnol. 35, 801 (2017).
Senior, M. Beating the organ clock. Nat. Biotechnol. 36, 488–492 (2018).
Theruvath, J. et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 28, 333–344 (2022).
Gulhati, P. et al. Targeting T cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results in antitumor immunity and durable response in pancreatic cancer. Nat. Cancer 4, 62–80 (2022).
Richardson, P. G. et al. Mezigdomide plus dexamethasone in relapsed and refractory multiple myeloma. N. Engl. J. Med. 389, 1009–1022 (2023).
Trajanoska, K. et al. From target discovery to clinical drug development with human genetics. Nature 620, 737–745 (2023).
Wu, T., Cooper, S. A. & Shah, V. H. Omics and AI advance biomarker discovery for liver disease. Nat. Med. 28, 1131–1132 (2022).
Liu, S. et al. Multi-organ landscape of therapy-resistant melanoma. Nat. Med. 29, 1123–1134 (2023).
Theodoris, C. V. et al. Transfer learning enables predictions in network biology. Nature 618, 616–624 (2023).
Larsson, E., Sander, C. & Marks, D. mRNA turnover rate limits siRNA and microRNA efficacy. Mol. Syst. Biol. 6, 433 (2010).
Judge, A. D. et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Invest. 119, 661–673 (2009).
Gonzalez-Gonzalez, E. et al. siRNA silencing of keratinocyte-specific GFP expression in a transgenic mouse skin model. Gene Ther. 16, 963–972 (2009).
Omi, K., Tokunaga, K. & Hohjoh, H. Long‐lasting RNAi activity in mammalian neurons. FEBS Lett. 558, 89–95 (2004).
McDonagh, M., Peterson, K., Holzhammer, B. & Fazio, S. A systematic review of PCSK9 inhibitors alirocumab and evolocumab. Manag. Care Spec. Pharm. 22, 641–653q (2016).
Flower, M. et al. MSH3 modifies somatic instability and disease severity in Huntington’s and myotonic dystrophy type 1. Brain 142, 1876–1886 (2019).
Takahashi, M., Koi, M., Balaguer, F., Boland, C. R. & Goel, A. MSH3 mediates sensitization of colorectal cancer cells to cisplatin, oxaliplatin, and a poly(ADP-ribose) polymerase inhibitor. J. Biol. Chem. 286, 12157–12165 (2011).
Adam, R. et al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am. J. Hum. Genet. 99, 337–351 (2016).
Iyama, T. & Wilson, D. M. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 12, 620–636 (2013).
Bogdanovich, S. et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420, 418–421 (2002).
Kota, J. et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci. Transl. Med. 1, 6ra15 (2009).
Lee, S.-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Invest. 131, e148372 (2021).
Van Der Worp, H. B. et al. Can animal models of disease reliably inform human studies? PLoS Med. 7, e1000245 (2010).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Perlman, R. L. Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health 2016, 170–176 (2016).
Gusella, J. F. et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306, 234–238 (1983).
The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72, 971–983 (1993).
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Sutherland, J. E. et al. Nonclinical safety profile of revusiran, a 1st-generation GalNAc-siRNA conjugate for treatment of hereditary transthyretin-mediated amyloidosis. Nucleic Acid Ther. 30, 33–49 (2020).
Gouda, M. A., Buschhorn, L., Schneeweiss, A., Wahida, A. & Subbiah, V. N-of-1 trials in cancer drug development. Cancer Discov. 13, 1301–1309 (2023).
Tran, H. et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat. Med. 28, 117–124 (2022).
Matlin, A. J., Clark, F. & Smith, C. W. J. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6, 386–398 (2005).
Nilsen, T. W. & Graveley, B. R. Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463 (2010).
Prinos, P. et al. Alternative splicing of SYK regulates mitosis and cell survival. Nat. Struct. Mol. Biol. 18, 673–679 (2011).
Schwerk, J. et al. RNA-binding protein isoforms ZAP-S and ZAP-L have distinct antiviral and immune resolution functions. Nat. Immunol. 20, 1610–1620 (2019).
Ray, T. A. et al. Comprehensive identification of mRNA isoforms reveals the diversity of neural cell-surface molecules with roles in retinal development and disease. Nat. Commun. 11, 3328 (2020).
Nikom, D. & Zheng, S. Alternative splicing in neurodegenerative disease and the promise of RNA therapies. Nat. Rev. Neurosci. 24, 457–473 (2023).
Bradley, R. K. & Anczuków, O. RNA splicing dysregulation and the hallmarks of cancer. Nat. Rev. Cancer 23, 135–155 (2023).
Marx, V. Method of the year: long-read sequencing. Nat. Methods 20, 6–11 (2023).
Shaffer, C. Mist begins to clear for lung delivery of RNA. Nat. Biotechnol. 38, 1110–1112 (2020).
Dusheiko, G., Agarwal, K. & Maini, M. K. New approaches to chronic hepatitis B. N. Engl. J. Med. 388, 55–69 (2023).
Ambike, S. et al. Targeting genomic SARS-CoV-2 RNA with siRNAs allows efficient inhibition of viral replication and spread. Nucleic Acids Res. 50, 333–349 (2022).
Estes, J. D., Wong, S. W. & Brenchley, J. M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 18, 390–404 (2018).
Dennis, C. The brave new world of RNA. Nature 418, 122–124 (2002).
Matsui, M. & Corey, D. R. Non-coding RNAs as drug targets. Nat. Rev. Drug. Discov. 16, 167–179 (2017).
Slack, F. J. & Chinnaiyan, A. M. The role of non-coding RNAs in oncology. Cell 179, 1033–1055 (2019).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 20, 629–651 (2021).
Statello, L., Guo, C.-J., Chen, L.-L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023).
Balusu, S. et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science 381, 1176–1182 (2023).
Gaya, A. et al. Results of a phase 1/2 study of cemdisiran in healthy subjects and patients with paroxysmal nocturnal hemoglobinuria. eJHaem 4, 612–624 (2023).
Devalaraja-Narashimha, K. et al. Pharmacokinetics and pharmacodynamics of pozelimab alone or in combination with cemdisiran in non-human primates. PLoS ONE 17, e0269749 (2022).
Griffin, M. Pozelimab/cemdisiran. Complement protein C5 inhibitor, treatment of complement-mediated diseases. Drugs Fut. 48, 93 (2023).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998). This paper discovers that double-stranded RNA can induce catalytic cleavage of target mRNAs in C. elegans.
Hannon, G. J. RNA interference. Nature 418, 244–251 (2002).
Dorsett, Y. & Tuschl, T. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 3, 318–329 (2004).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426–433 (2009).
Hannon, G. J. & Rossi, J. J. Unlocking the potential of the human genome with RNA interference. Nature 431, 371–378 (2004).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Caplen, N. J., Parrish, S., Imani, F., Fire, A. & Morgan, R. A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl Acad. Sci. USA 98, 9742–9747 (2001). References 213 and 214 were among the pioneering studies that demonstrated RNAi in mammalian cells.
McCaffrey, A. P. et al. RNA interference in adult mice. Nature 418, 38–39 (2002).
Hamar, P. et al. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 101, 14883–14888 (2004).
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004).
Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134, 577–586 (2008).
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 23, 709–717 (2005).
Palliser, D. et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439, 89–94 (2006).
Li, B. et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 11, 944–951 (2005).
Woodrow, K. A. et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 8, 526–533 (2009).
Song, E. et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9, 347–351 (2003).
Dykxhoorn, D. M. & Lieberman, J. Knocking down disease with siRNAs. Cell 126, 231–235 (2006).
De Fougerolles, A., Vornlocher, H.-P., Maraganore, J. & Lieberman, J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 6, 443–453 (2007).
Sledz, C. A., Holko, M., De Veer, M. J., Silverman, R. H. & Williams, B. R. G. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–839 (2003).
Frantz, S. Studies reveal potential pitfalls of RNAi. Nat. Rev. Drug Discov. 2, 763–764 (2003).
Marques, J. T. & Williams, B. R. G. Activation of the mammalian immune system by siRNAs. Nat. Biotechnol. 23, 1399–1405 (2005).
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23, 457–462 (2005).
Hornung, V. et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11, 263–270 (2005).
Jackson, A. L. et al. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 21, 635–637 (2003). This article demonstrates that siRNAs can induce off-targeting effects with limited target sequence similarity.
Karikó, K., Bhuyan, P., Capodici, J. & Weissman, D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J. Immunol. 172, 6545–6549 (2004). This paper demonstrates the role of Toll-like receptor 3 in mediating siRNA-induced immune activation.
Ledford, H. Drug giants turn their backs on RNA interference. Nature 468, 487–487 (2010).
Schmidt, C. RNAi momentum fizzles as pharma shifts priorities. Nat. Biotechnol. 29, 93–94 (2011).
Check Hayden, E. RNA interference rebooted. Nature 508, 443–443 (2014).
Amrite, A., Fuentes, E., Marbury, T. C. & Zhang, S. Safety, pharmacokinetics, and exposure–response modeling of nedosiran in participants with severe chronic kidney disease. Clin. Pharmacol. Drug Dev. 12, 1164–1177 (2023).
Desai, A. S. et al. Zilebesiran, an RNA interference therapeutic agent for hypertension. N. Engl. J. Med. 389, 228–238 (2023). This article reports on the clinical efficacy of siRNA in treating the prevalent disease hypertension.
Fernández-Ruiz, I. Promising novel siRNA for the treatment of hypertension. Nat. Rev. Cardiol. 20, 647–647 (2023).
Raal, F. J. et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med. 382, 1520–1530 (2020). This article reports on the clinical efficacy of siRNA in treating the prevalent disease hypercholesterolaemia.
Lamb, Y. N. Inclisiran: first approval. Drugs 81, 389–395 (2021).
Long, D. et al. Potent effect of target structure on microRNA function. Nat. Struct. Mol. Biol. 14, 287–294 (2007).
Małecka, E. M. & Woodson, S. A. Ribosomes clear the way for siRNA targeting. Nat. Struct. Mol. Biol. 27, 775–777 (2020). References 241 and 242 describe how mRNA secondary structure is a factor impacting siRNA/miRNA efficacy.
Vert, J.-P., Foveau, N., Lajaunie, C. & Vandenbrouck, Y. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics 7, 520 (2006).
Shabalina, S. A., Spiridonov, A. N. & Ogurtsov, A. Y. Computational models with thermodynamic and composition features improve siRNA design. BMC Bioinformatics 7, 65 (2006).
Matveeva, O. et al. Comparison of approaches for rational siRNA design leading to a new efficient and transparent method. Nucleic Acids Res. 35, e63 (2007).
Ichihara, M. et al. Thermodynamic instability of siRNA duplex is a prerequisite for dependable prediction of siRNA activities. Nucleic Acids Res. 35, e123 (2007).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Brown, K. M., Chu, C. & Rana, T. M. Target accessibility dictates the potency of human RISC. Nat. Struct. Mol. Biol. 12, 469–470 (2005). This article reports on the effect of increased target accessibility in improving RISC potency.
Heale, B. S. E. siRNA target site secondary structure predictions using local stable substructures. Nucleic Acids Res. 33, e30 (2005).
Kedde, M. et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007).
Kim, S. et al. The regulatory impact of RNA-binding proteins on microRNA targeting. Nat. Commun. 12, 5057 (2021).
Davis, S. M. Guidelines for designing therapeutic siRNAs. PhD Thesis, UMass Chan Medical School (2023).
Bahar Halpern, K. et al. Nuclear retention of mRNA in mammalian tissues. Cell Rep. 13, 2653–2662 (2015).
Wegener, M. & Müller-McNicoll, M. Nuclear retention of mRNAs — quality control, gene regulation and human disease. Semin. Cell Dev. Biol. 79, 131–142 (2018).
Didiot, M.-C. et al. Nuclear localization of Huntingtin mRNA is specific to cells of neuronal origin. Cell Rep. 24, 2553–2560.e5 (2018).
Ferguson, C. M., Echeverria, D., Hassler, M., Ly, S. & Khvorova, A. Cell type impacts accessibility of mRNA to silencing by RNA interference. Mol. Ther. Nucleic Acids 21, 384–393 (2020). This paper describes how the subcellular localization of mRNA transcripts in different cell types contributes to affecting siRNA efficacy.
Dawson, M. A. et al. Nuclear JAK2. Blood 118, 6987–6988 (2011).
Narykov, O., Johnson, N. T. & Korkin, D. Predicting protein interaction network perturbation by alternative splicing with semi-supervised learning. Cell Rep. 37, 110045 (2021).
Dua, K. et al. The potential of siRNA based drug delivery in respiratory disorders: recent advances and progress. Drug. Dev. Res. 80, 714–730 (2019).
Donoso, L. A. et al. Autosomal dominant stargardt-like macular dystrophy. Surv. Ophthalmol. 46, 149–163 (2001).
Ayuso, C. & Millan, J. M. Retinitis pigmentosa and allied conditions today: a paradigm of translational research. Genome Med. 2, 34 (2010).
Meng, D., Ragi, S. D. & Tsang, S. H. Therapy in rhodopsin-mediated autosomal dominant retinitis pigmentosa. Mol. Ther. 28, 2139–2149 (2020).
Samson, N. & Ablasser, A. The cGAS–STING pathway and cancer. Nat. Cancer 3, 1452–1463 (2022).
Kaya, T. et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 25, 1446–1457 (2022).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023).
Fitzgerald, K., Frank-Kamenetsky, M. & Charisse, K. Dual targeting siRNA. US patent 9,187,746B2 (2015).
Lee, S. H., Mok, H., Jo, S., Hong, C. A. & Park, T. G. Dual gene targeted multimeric siRNA for combinatorial gene silencing. Biomaterials 32, 2359–2368 (2011).
Brown, J. M. et al. Ligand conjugated multimeric siRNAs enable enhanced uptake and multiplexed gene silencing. Nucleic Acid Ther. 29, 231–244 (2019).
Chareddy, Y. et al. P2.11-03 Synergistic co-targeting of MYC and KRAS in lung cancer by novel ligand-directed inverted chimeric RNAi molecules. J. Thorac. Oncol. 18, S360–S361 (2023).
No authors listed. Undruggable KRAS — time to rebrand? Lancet Oncol. 22, 289 (2021).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Stojic, L. et al. Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions. Nat. Commun. 7, 10406 (2016).
Acknowledgements
The authors acknowledge the nucleic acid–medicine community who contributed to the technology innovations, and whose excellent work could not be included here owing to lack of space. This work was supported by the NIH (grant nos. R35 GM131839 and R01 NS104022 to A.K.; and K99 AR082987 to Q.T.). The authors thank M. B. Dziewietin for her administrative assistance and D. Conte at RNA Therapeutics Institute for help in revising and proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A.K. is a founder of Atalanta Therapeutics and Comanche Biopharma; serves on the Scientific Advisory Board of Aldena Therapeutics, Prime Medicine, Alltrna, Advirna and Evox Therapeutics. A.K. and Q.T. are listed as inventors of RNAi technology patents and patent applications.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Nedosiran: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215842s000lbl.pdf
Revusiran: https://www.alnylam.de/wp-content/uploads/2017/08/Revusiran-RNAi-Roundtable_FINAL2_08092017.pdf
A phase I/II study: https://www.aviditybiosciences.com/wp-content/uploads/2023/04/230330-AAN-2023-MARINA-Prelim-Data-Oral-Presentation_v6.0_FINAL.pdf
ABX1100: https://www.arobiotx.com/aro-receives-fda-orphan-drug-designation-for-abx1100
FDA Takes New Steps Aimed at Advancing Development of Individualized Medicines to Treat Genetic Diseases: https://www.fda.gov/news-events/press-announcements/fda-brief-fda-takes-new-steps-aimed-advancing-development-individualized-medicines-treat-genetic
FDA Takes Steps to Provide Clarity on Developing New Drug Products in the Age of Individualized Medicine: https://www.fda.gov/news-events/press-announcements/fda-takes-steps-provide-clarity-developing-new-drug-products-age-individualized-medicine
Regeneron and Alnylam announce a broad collaboration: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-alnylam-announce-broad-collaboration-discover
The Alnylam GEMINI platform: https://capella.alnylam.com/wp-content/uploads/2022/05/Theile_TIDES-2022.pdf
The Human Protein Atlas database: https://www.proteinatlas.org
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Q., Khvorova, A. RNAi-based drug design: considerations and future directions. Nat Rev Drug Discov (2024). https://doi.org/10.1038/s41573-024-00912-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-024-00912-9