Abstract
Acute graft-versus-host disease (GVHD) is a common immune complication that can occur after allogeneic haematopoietic cell transplantation (alloHCT). Acute GVHD is a major health problem in these patients, and is associated with high morbidity and mortality. Acute GVHD is caused by the recognition and the destruction of the recipient tissues and organs by the donor immune effector cells. This condition usually occurs within the first 3 months after alloHCT, but later onset is possible. Targeted organs include the skin, the lower and upper gastrointestinal tract and the liver. Diagnosis is mainly based on clinical examination, and complementary examinations are performed to exclude differential diagnoses. Preventive treatment for acute GVHD is administered to all patients who receive alloHCT, although it is not always effective. Steroids are used for first-line treatment, and the Janus kinase 2 (JAK2) inhibitor ruxolitinib is second-line treatment. No validated treatments are available for acute GVHD that is refractory to steroids and ruxolitinib, and therefore it remains an unmet medical need.
Similar content being viewed by others
Introduction
Acute graft-versus-host disease (GVHD) was first described as a secondary disease that developed after recovery from conditioning-associated toxicity in murine models of bone marrow transplantation1. Mice developed inactivity, wasting syndrome, and skin, fur, and posture changes, and died from ‘secondary disease’. The 1966 criteria of Billingham accurately characterize standards for GVHD: the donor graft must contain immunocompetent cells, the recipient must express tissue antigens which are different from the donor’s, and the recipient must be unable to mount an adequate response to eliminate and reject the donor graft2. These criteria are applicable for both allogeneic haematopoietic cell transplantations (alloHCT)-associated GVHD and GVHD occurring after blood transfusion in individuals who are immunosuppressed.
The number of alloHCT performed annually continues to rise3, despite a transient decrease during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic4. In 2016, the last year for which data are available, 38,425 alloHCT were performed worldwide, compared with 20,333 in 2006, an increase of 89.0%5. Despite this increase, the incidence of alloHCT is higher in North America and Europe than in the rest of the world (Fig. 1). For haematological malignancies, the therapeutic efficacy of alloHCT relies on the cytotoxic effect of the conditioning regimen (Box 1) and on the immune-mediated graft-versus-tumour or graft-versus-leukaemia (GVL) effect. However, this effect is counterbalanced by the destruction of the tissue and organ by the donor immune effector cells, termed GVHD. Apart from the relapse of the underlying malignancy, GVHD remains the major complication after alloHCT and is associated with high morbidity and mortality6.
The number of alloHCT carried out per 10 million population is higher in North America and Europe than in other continents5. This difference is explained by an accessibility issue due to cost of the procedure and the low numbers of teams that perform alloHCT in Africa, the East Mediterranean region, South East Asia Pacific and West Pacific region and Latin America compared with the numbers in North America and Europe5. alloHCT, allogeneic haematopoietic cell transplantation.
GVHD can be subclassified as acute or chronic disease. Acute and chronic GVHD were previously distinguished by the timing of their onset: acute GVHD was classified as symptom presentation before 100 days after alloHCT and chronic GVHD was classified as symptom presentation >100 days after alloHCT. This classification was refined by a National Institutes of Health (NIH) consensus conference in 2005, and the distinction between acute and chronic GVHD is now based on the features of the disease7. Chronic GVHD can affect any organ in the body (acute GVHD primarily affects the skin, liver and gastrointestinal (GI) tract), with no time limit on diagnosis. Disease that fulfils the NIH definition of chronic GVHD is classified as either classic chronic GVHD or overlap chronic GVHD if acute GVHD is also present, irrespective of the time of GVHD onset7,8 (Fig. 2). GVHD with acute symptoms only is classified as classic acute GVHD (first episode within 100 days of transplantation), late onset acute GVHD (first episode more than 100 days after transplantation), recurrent acute GVHD (new episode of acute GVHD more than 100 days after transplantation in a patient with a history of classic acute GVHD), or persistent acute GVHD (classic acute GVHD symptoms that persist for more than 100 days after transplantation).
Acute graft-versus-host disease (GVHD) is classified as classic acute GVHD with onset ≤100 days after allogeneic haematopoietic cell transplantation (alloHCT), as late onset acute GVHD with first onset >100 days after alloHCT and as recurrent acute GVHD with onset >100 days after previous classic acute GVHD. Acute GVHD that persists beyond 100 days after alloHCT is classified as persistent acute GVHD. aGVHD, acute GVHD; cGVHD, chronic GVHD; D100, day 100.
Acute GVHD can also be graded based on severity as I (mild), II (moderate), III (severe) and IV (very severe), based on quantification of skin rash for skin acute GVHD, serum bilirubin level for liver acute GVHD, volume of diarrhoea for lower GI acute GVHD and persistent nausea for upper GI acute GVHD9. Grade I acute GVHD is usually not considered as clinically important given its lack of effect on patient outcome10; therefore, most studies focus on grade II–IV and severe grade III–IV acute GVHD. Several systems can be used for grading acute GVHD. The MAGIC grading system is not yet used in routine clinical practice; however, it is used in this Primer as it facilitates and helps standardize acute GVHD clinical data collection, as shown by the development and validation of the electronic eGVHD application to assist health-care professionals in the assessment of acute GVHD in clinical practice11,12.
This Primer discusses the epidemiology and pathophysiological mechanisms of acute GVHD. This Primer also discusses management, patient quality of life (QOL) and outstanding research questions including the need for more efficient prophylaxis.
Epidemiology
Incidence
In the absence of effective prophylaxis, most patients develop acute GVHD; for example, in one historical series, only 19 of 93 patients did not develop acute GVHD when no prophylaxis was administered13. Nevertheless, acute GVHD can still occur, despite the routine use of prophylaxis after alloHCT. Acute GVHD incidence varies considerably depending, predominantly, on the degree of mismatch between HLA protein and the GVHD prophylaxis administered (Supplementary Table 1). In the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1202 study14, the cumulative 100-day incidence was 62% for acute GVHD reported by centres and 49% after validation by an end point review committee14. Of note, the incidence of acute GVHD seems to be decreasing over time. In one study, the incidence of grade II–IV acute GVHD was 40% and the incidence of grade III–IV acute GVHD was 19% in patients who received a transplant during the period 1990–1995, and 28% and 11%, respectively, in those who received a transplant during the period 2011–2015 (ref. 15).
Evaluating the incidence of acute GVHD associated with specific countries or continents is not possible as it is directly dependent on the incidence of alloHCT, which is higher in Europe and North America than in other regions5 (Fig. 1). Some studies have suggested that ethnicity affects the risk of developing acute GVHD. Indeed, a higher incidence of grade III–IV acute GVHD was reported in Black patients who received alloHCT from HLA-identical sibling donors than in white patients (37% versus 21%, respectively; P = 0.047) and in those who received alloHCT from unrelated donors (61% versus 36%, respectively; P = 0.014)16. However, a higher incidence of acute GVHD in Black patients was not confirmed in more recent studies17,18. In another study, Asian patients had a significantly lower incidence of acute GVHD than white patients; the incidence of grade II–IV acute GVHD was 40.0% in Japanese patients, 42.1% in non-Japanese Asian patients and 56.5% in white patients, and the incidence of grade III–IV acute GVHD was 15.3% in Japanese patients, 15.7% in non-Japanese Asian patients and 22.6% in white patients (P < 0.001)19. Moreover, the incidence of grade III or IV acute GVHD was significantly lower in Japanese patients than in white patients irrespective of the stem cell source (n = 2,652; HR 0.74, 95% CI 0.57–0.96)20.
Survival
Despite the improvement in overall survival (OS) in patients with acute GVHD over time21,22, mortality remains high, with a 1-year OS of 70% in patients with grade II acute GVHD and 40% in patients with grade III–IV acute GVHD21. Both overall mortality and length of hospital stay were significantly increased in patients who developed acute GVHD during alloHCT admission compared with patients who did not develop acute GVHD (overall mortality 16.2% versus 5.3%; P < 0.01; length of hospital stay 42.0 versus 26.0 days; P < 0.01).
Risk factors
Numerous risk factors for acute GVHD have been identified, including degree of HLA disparity (unrelated donor or HLA-mismatched donor)23,24, stem cell source (higher risk of acute GVHD with peripheral blood and bone marrow graft versus umbilical cord blood)25, donor and recipient sex disparity (female donor to male patient)26, higher intensity of alloHCT conditioning regimen25 and type of GVHD prophylaxis. Other risk factors, such as cytomegalovirus (CMV) negativity in both donor and recipient25 and older donor age27, have also been reported as acute GVHD risk factors. In the largest study to date, unrelated donor (HR 1.61, 95% CI 1.54–1.67; P < 0.001), underlying malignancy not in complete remission at alloHCT (HR 1.25, 95% CI 1.2–1.3; P < 0.001) or untreated (HR 1.11, 95% CI 1.02–1.2; P = 0.02), bone marrow as the source of stem cells (HR 1.2, 95% CI 1.15–1.25; P < 0.001) and a female donor for a male recipient (HR 1.16, 95% CI 1.11–1.21; P < 0.001) were associated with increased risk of grade II–IV acute GVHD, whereas the use of antithymocyte globulin (ATG) or alemtuzumab prophylaxis was associated with a lower incidence (HR 0.79, 95% CI 0.74–0.84; P < 0.001)15.
Of note, some studies have suggested that the deleterious effect of HLA disparity on risk of acute GVHD can be overcome with the use of post-transplant cyclophosphamide (PTCy)28. In patients who received PTCy for GVHD prophylaxis after haploidentical alloHCT29, stem cell source or conditioning regimen had no effect on the incidences of grade II–IV acute GVHD, whereas older donor age (30 to 49 versus <29 years) was significantly associated with higher rates of grade II–IV acute GVHD (HR 1.53, 95% CI 1.11–2.12; P = 0.01)29. Although single-centre studies have identified many significant single nucleotide polymorphisms as genetic risk factors for GVHD, these findings have not been consistently reproduced in large multicentre trials30.
Mechanisms/pathophysiology
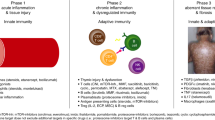
The pathophysiology of acute GVHD occurs in three phases: initiation phase, T cell activation and the effector phase (Fig. 3).
In the first (initiation) phase of acute graft-versus-host disease (GVHD), the conditioning regimen of chemotherapy and/or radiotherapy damages host tissue. This damage leads to the release of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), such as tumour necrosis factor (TNF), type I interferon (IFNγ) and lipopolysaccharide, that activate host antigen-presenting cells (APC) in the initiation phase. In addition, loss of microbiota diversity contributes to loss of epithelial and immune homeostasis. In the T cell activation phase, host APCs activate alloreactive donor CD4+ and CD8+ T cells. In the effector phase, effector T cells and pro-inflammatory cytokines damage epithelial cells of the gastrointestinal (GI) tract, skin and liver, leading to apoptosis and necroptosis, resulting in acute GVHD symptoms. CTL, cytotoxic T lymphocyte; IDO, indoleamine 2,3-dioxygenase; TH1 cell, T helper 1 cell; Treg cell, regulatory T cell.
Initiation phase
During the initiation phase, the alloHCT conditioning regimen (Box 1) damages patients’ tissues and causes release of inflammatory cytokines that lead to activation of host antigen-presenting cells (APCs).
Intensity and type of conditioning
While in the early years of alloHCT most of the regimens in patients used high-intensity myeloablative total body irradiation (TBI) to eliminate the recipient’s haematopoiesis (blood cell production process), leukaemia and immune system, variations in the TBI dose and fractionation, and in the use of cytotoxic drugs, revealed that conditioning-induced damage itself contributed to the kinetics and severity of acute GVHD31. As an example, there were higher systemic levels of tumour necrosis factor (TNF) inducing inflammation and tissue damage itself after high-dose TBI, and overall TNF release correlated with the severity of GVHD32. The effect of the conditioning regimen on kinetics and severity of acute GVHD became more evident with the introduction of non-myeloablative conditioning. Interestingly, the severity of neutropenia caused by non-myeloablative conditioning correlates with the severity of acute GVHD; in one study, grade III–IV acute GVHD occurred in only 3% of patients without neutropenia and in 12% of patients with severe neutropenia, which was also reflected by an increase in non-relapse mortality (NRM) from 3% to 25%, respectively33. These data suggest that several parameters, including the release of inflammatory cytokines (TNF, IL-1 and IL-6), contribute to the effects of conditioning intensity on acute GVHD, not only conditioning-related damage itself, but also from the general susceptibility to inflammation. This susceptibility is influenced by several further factors, such as translocation of pathogen-associated molecular patterns (PAMPs) and the ability of the host to mount the inflammatory response (for example, by the presence of neutrophils).
Pathogen-associated molecular patterns and damage-associated molecular patterns
Molecules derived from the activation or destruction of bacteria are summarized as PAMPs, while molecules derived from the destruction of human cells (such as uric acids and others) are called damage-associated molecular patterns (DAMPs). The first PAMP associated with acute GVHD was lipopolysaccharide (LPS). The severity of acute GVHD was reduced in LPS-resistant mouse strains compared with LPS-sensitive mouse strains, and neutralization of LPS by antibodies contributed to the suppression of acute GVHD34. Other PAMPs and their receptors have also been evaluated as triggers of GVHD: TLR4 and NOD2 (also known as CARD15) were associated with acute GVHD in several studies35, although findings were not conclusive in other studies, suggesting that single pathways may not be sufficient to trigger disease. Of note, in one multicentre study, the association of acute GVHD with NOD2 was only observed in centres using specific types of GI decontamination which is widely used to reduce neutropenic infections and bacterial translocation across the damaged GI tract36.
PAMPs induce pro-inflammatory cytokines in myeloid and epithelial cells, specifically TLRs induce the myeloid differentiation factor 88 (MyD88) pathway and thus augment GVHD37. After stimulation by viral or bacterial DNA, which represents a further group of PAMPs, type I interferon (IFN) signalling via retinoic acid-inducible gene-I (RIG-I) is induced: While induction of this pathway before alloHCT protects against GVHD by stabilizing epithelial damage, it can augment GVHD if induced at later time points by activating donor CD8 cells38,39.
Tissue damage induced by pretransplant conditioning causes release of intracellular molecules, which can act as danger signals (DAMPs). Among the DAMPs, high-mobility group box 1 (HMGB1), uric acid, ATP, heat shock proteins and heparan sulfate proteoglycans have been described as triggers of acute GVHD40. Evaluation of inhibitors of these molecules such as purinergic P2X receptor 7 (P2X7R) antagonists in murine models41 or α1-antitrypsin (AAT) in patients have been tested with some success. IL-33 is a DAMP produced by endothelial and epithelial cells on inflammation and binds to its receptor suppression of tumorigenicity 2 (ST2) to induce immunoregulation; soluble ST2 is now recognized as a general biomarker of acute GVHD42.
Microbiota: more than multiple PAMPs
As previously mentioned, the main organs affected in acute GVHD are the skin, liver and GI tract. These organs represent the main epithelial surfaces that interact with commensal and pathogenic bacteria (the organ-specific microbiota). The liver is connected to the GI tract via the portal vein. Early studies demonstrated the involvement of the intestinal microbiota in acute GVHD pathophysiology, such as one study that demonstrated the absence of acute GVHD in mice grown under germ-free conditions that received bone marrow grafts43. Based on these early findings, decontamination and isolation of patients was introduced in clinical practice, and reduced the incidence of GVHD44.
In the initiation phase of acute GVHD, bacteria translocated as a consequence of epithelial damage by the conditioning regimen contribute to initiation of acute GVHD in particular through immune cell activation by PAMPs45. Experiments in murine models have demonstrated the prevention of GVHD by administration of antibiotics initiated before alloHCT and subsequent reduction of neutrophil influx in epithelial tissues. However, a study in humans found the failure of gut decontamination in ~50% of patients in whom the incidence of acute GVHD was significantly higher than in patients who achieved successful gut decontamination46. Moreover, more recent studies using 16S rRNA sequencing, have demonstrated deleterious effects of antibiotic use, namely, long-term loss of microbiota diversity, which can accelerate and aggravate acute GVHD47. Conditioning regimens and the use of broad-spectrum antibiotics during alloHCT lead to low gut microbiota diversity and intestinal domination. Indeed, one study demonstrated intestinal domination in 65% of patients, mainly related to Enterococcus faecium, which was associated with poor outcomes due to increased GVHD-related mortality48.
By contrast, some bacteria have protective effects. For example, increased numbers of bacteria belonging to the genus Blautia (order Clostridiales) is associated with reduced GVHD lethality and improved OS49. Of note, loss of Blautia is associated with the use of broad-spectrum antibiotics. These protective effects are mediated at least in part through the production of metabolites, which are essential for epithelial nutrition (such as short-chain fatty acids (SCFAs))50, intestinal stem cell support (via IL-22)51 and, most importantly, immunoregulation (as SCFAs have a major role in maintenance and support of regulatory T (Treg) cells52). In addition, these metabolites also contribute to barrier function, and their loss results in barrier damage, allowing increased translocation of pathogenic bacteria and amplifying the inflammatory cascades in GVHD53.
APC activation
PAMPs and DAMPs activate classic APCs (dendritic cells, macrophages and B cells) and non-classic APCs (MHC class II-expressing cells that can activate CD4+ T cells such as mast cells, basophils, endothelial cells and epithelial cells)54, which are then responsible for activation of donor T cells in acute GVHD. Classic APCs have a central role in acute GVHD induction, as they are sufficient to activate donor T cells. Donor T cells recognize host antigens on the surface of host APCs, leading to the initiation of CD4+ and CD8+ T cell-mediated acute GVHD (see section ‘T cell activation’)55,56. Moreover, donor-derived APCs can augment CD8+ T cell-mediated acute GVHD, presumably by acquiring and presenting host antigens by cross-priming (whereby certain professional APCs, mostly dendritic cells, take up, process and present extracellular antigens by MHC class I molecules to CD8+ T cells)57. In addition, in mixed haematopoietic chimeras, non-classic APCs, such as fibroblasts and endothelial cells, have also been shown to present antigens and induce acute GVHD after irradiation-induced damage58,59.
T cell activation
Acute GVHD occurs when donor T cells respond to HLA differences on a recipient’s tissue (usually referred to as alloreactive T cells). The central role of alloreactive T cells in acute GVHD is supported by the effectiveness of T cell depletion (TCD) for acute GVHD prophylaxis (see section ‘T cell depletion prophylaxis’).
T cell activation by minor and major HLA antigens
Donor T cells recognize HLA on the surface of host APCs, and HLA mismatch between recipient and donor is one of the most prominent risk factors for both acute and chronic GVHD, if classic immunosuppressive or non-immunosuppressive prophylaxis is used30. This holds true for HLA- A, HLA-B, HLA-DRB1 and HLA-DQB1, whereas HLA-C and HLA-DPB1 interactions are more complex, and also may contribute to activations in the context of GVL60. CD4+ T cells respond to antigens presented on MHC class II molecules (HLA DRB1, HLA-DQB1 and HLA-DPB1) while CD8+ T cells respond to antigens presented by MHC class I molecules (HLA-A, HLA-B and HLA-C)61. By contrast, minor histocompatibility antigens are the only targets of T cell alloreactivity in HLA-matched or identical alloHCT transplants with sibling donors and can lead to acute GVHD. Indeed, one study defined minor HLA antigens such as HA-1, HA-2, HA-4 and HA-5, and demonstrated a 6.4-fold increased risk of grade II–IV acute GVHD in minor mismatched transplants62.
Regulatory cells
Donor-derived host-specific natural Treg cells have a key role in suppression of acute GVHD, and are suitable for selective isolation to treat and prevent acute GVHD63,64. Besides these thymus-derived Treg cells, induced Treg cells have additional GVHD suppressive activity in peripheral target organs, especially in the GI tract65. Recent data suggest that non-lymphoid tissue Treg cells not only exert immunoregulatory effects but also are important for maintaining epithelial homeostasis66. Further subpopulations, such as CD8+ Treg cells which can be stimulated by mammalian target of rapamycin (mTOR) inhibitors67 and regulatory B cells producing IL-10, have been reported to suppress the severity of acute GVHD in experimental models68.
Innate immune cells can also exert suppressive functions in GVHD. Mesenchymal stem cells69 and myeloid-derived suppressor cells (MDSC)70 both rely on the immunosuppressive function of indoleamine 2,3-dioxygenase which shifts the balance between alloreactive effector and Treg cells; polarized type 2 macrophages have also been reported to attenuate alloreactions including GVHD71. Moreover, innate lymphoid cells 2 (ILC2) and ILC3 contribute to regulation of epithelial homeostasis72. ILC2 are depleted after conditioning and can suppress T helper 1 (TH1) and TH17 cell responses, inducing MDSCs by IL-13 (ref. 72). ILC3 release protective IL-22 and suppress T cell activation by ATP via ectoenzymes72.
Effector phase
Cellular and cytokine effectors
During the effector phase, activated alloreactive donor CD8+ T cells kill target tissues via apoptosis73,74. Apoptosis is most prominent in the stem cell niches of acute GVHD target tissues, which has been shown for keratinocytes, intestinal stem cells and associated basal Paneth cells, and neuroendocrine cells in GI crypts75. Inflammation-associated cell death, such as necroptosis76, and inhibition of inflammation-associated receptor-interacting serine–threonine kinase 1 (RIPK1) also contribute to tissue destruction, and could be targeted to develop future therapies.
Targeted cell damage by activated alloreactive CD4+ T cells is mediated by pro-inflammatory cytokines such as IFNγ and TNF; therapies targeting the latter are effective in the treatment of acute GVHD77. Of note, cytokines and other inflammatory pathways are important targets of corticosteroids and other drugs (such as JAK–signal transducer and activator of transcription (STAT) inhibitors) that are effective in the treatment of acute GVHD78.
Other targets of GVHD
An open question is the involvement of non-epithelial cells in acute GVHD. Endothelial cells are involved as endothelial cell sprouting precedes T cell infiltration in the initiation phase of GVHD, and endothelial apoptosis occurs in the effector phase79,80. Endothelial activation and damage may be the first steps not only in classic GVHD target organs but also in the less-recognized and less-characterized target tissues of acute GVHD, such as the central nervous system81 or the lung82. Of note, lymphohaematopoietic tissues are the most sensitive targets of alloreaction, which contributes to GVL and the severe immunodeficiency of GVHD.
Role of tissue homeostasis
Epithelial stem cell damage during the effector phase and the role of barrier damage in the pathophysiology of GVHD suggest that the susceptibility of the target tissue has a role in acute GVHD. Therefore, in 2017 the concept of tissue tolerance (the intrinsic, but variable, ability of tissues to tolerate or withstand damage from inflammatory immune activity during infection) was introduced in the setting of acute GVHD83. In addition to expression of inhibitors of apoptosis and epithelial protection, microbial-derived metabolites, such as SCFA metabolic pathways, have a crucial role in tissue tolerance. Moreover, mitochondrial complex II is a crucial regulator of epithelial damage in T cell-mediated diseases, including acute GVHD84. The list of metabolic changes, both in T cells and effector organs of GVHD, is continually increasing, providing further approaches for modulation of GVHD85.
Diagnosis, screening and prevention
Diagnosis
Skin acute GVHD
Skin acute GVHD is the first and most common clinical manifestation of acute GVHD in most patients14,86, and generally arises around white blood cell engraftment, which occurs 14–21 days after alloHCT87. Typically, patients with skin acute GVHD develop a maculopapular rash that initially involves sun-exposed areas such as the nape of the neck and the shoulders, or less frequently the palms of the hands and the soles of the feet (Fig. 4a,b). The rash can then spread throughout the body but does not affect the scalp. The rash is often pruritic (itchy) and can be painful. In stage 4 acute GVHD, the rash can form bullous lesions and can ulcerate. Pathological findings in the skin in acute GVHD include degeneration of the basal layer of the epidermis with apoptotic bodies, dyskeratosis (abnormal epidermal cell keratinization) with adjacent satellite lymphocytes, perivascular lymphocytic infiltration in the dermis and partial or total dermoepidermal disjunction (disjunction of the interface between the epidermal and the dermal layers of the skin)88 (Fig. 4c).
a,b, Clinical manifestation of stage 2 skin acute graft-versus-host disease (GVHD) with maculopapular rash of the skin. c, Skin biopsy sample from a patient with stage 3 skin acute GVHD, showing apoptotic bodies (blue arrow), dyskeratosis with adjacent satellite lymphocytes (black arrow), perivascular lymphocytic infiltration in the dermis (red arrow) and partial dermoepidermal disjunction (yellow arrow). d, Endoscopic finding of stage 3 lower gastrointestinal acute GVHD with diffuse erythema and scattered ulceration (black arrow). e, Rectal biopsy sample from a patient with stage 2 gastrointestinal acute GVHD, revealing with apoptotic epithelial cells (black arrow) and crypt loss (yellow arrow). Parts c and e, haematoxylin and eosin staining, original magnification ×200.
Gastrointestinal acute GVHD
The lower and upper GI tract can also be involved in acute GVHD. Diarrhoea is the prominent symptom of lower GI acute GVHD, is secretory and is usually voluminous. Abdominal pain and ileus (non-mechanical decrease or stoppage of the flow of intestinal contents) occur in severe disease. In addition, haematochezia (passage of fresh blood via the anus) occurs in severe disease owing to mucosal ulceration. Anorexia, nausea and/or vomiting are symptoms of upper GI acute GVHD89. Although upper and lower GI acute GVHD are usually associated, they can also occur in isolation.
Diarrhoea is frequent after alloHCT and can be caused by toxicity of the conditioning regimen, drug toxicity, viral infection, Clostridioides difficile colitis or neutropenic enterocolitis90. Thus, exclusion of these differential diagnoses is important to confirm lower GI acute GVHD, particularly when diarrhoea occurs in isolation. Bacteriological, virological and parasitological stool culture are usually performed, and the presence of C. difficile toxin in stool and CMV in blood is searched for to exclude differential diagnoses.
Medical imaging is not diagnostic for acute GVHD but can be performed to rule out other diagnoses. Non-specific signs of lower GI acute GVHD on CT scan include bowel wall thickening, abnormal mucosal enhancement, bowel dilatation and air or fluid levels suggestive of ileus91. Endoscopic manifestations of acute GVHD include spotted or diffuse erythema, patchy erosion, scattered ulceration and active bleeding92,93,94 (Fig. 4d). As these findings lack specificity for acute GVHD and as normal mucosa can be seen in this condition, biopsies are systematically performed for diagnosis. Histological findings include apoptotic epithelial cells, individual or multiple crypt loss and denudation (loss of the surface layer) of the epithelium95,96 (Fig. 4e).
In addition to CT and endoscopy, other imaging techniques for the diagnosis of lower GI acute GVHD show promise. Contrast-enhanced ultrasonography reveals transmural penetration of microbubbles into the bowel lumen in patients with acute GVHD, confirming bowel wall thickening and functional impairment97. However, despite a high specificity for diagnosis of GI acute GVHD in preliminary studies98,99, the use of contrast-enhanced ultrasonography is limited by the lack of prospective studies and by the fact that it can be carried out only by highly trained specialists who are not available in many hospitals. 18F-Fluorodeoxyglucose (18F-FDG) PET–CT revealed a correlation between the localization of 18F-FDG uptake in the gut and that of biopsy-proven GI acute GVHD100. Accordingly, 18F-FDG PET–CT seems to be a non-invasive, sensitive and very specific biomarker for lower GI acute GVHD diagnosis in patients with diarrhoea100; however, the lack of availability of 18F-FDG PET–CT in emergency settings strongly limits its use in these patients.
Differential diagnoses of upper GI acute GVHD include infection (oesophageal candidiasis and herpes simplex virus), conditioning regimen toxicity and peptic ulcers. Upper GI endoscopy is recommended, whenever possible, for biopsy confirmation, particularly in those with suspected isolated upper GI GVHD9. Symptom severity is also important for diagnosis of upper GI GVHD, as this condition should be considered in those with nausea for >3 days, at least two vomiting episodes per day or anorexia with weight loss.
Liver acute GVHD
The liver is the least frequently involved organ in acute GVHD and liver acute GVHD is usually associated with skin and/or GI acute GVHD101. Liver acute GVHD is characterized by an increased total serum bilirubin level (hyperbilirubinaemia), which can lead to jaundice. Liver dysfunction and hyperbilirubinaemia after alloHCT has several causes, including sinusoidal obstruction syndrome, drug toxicity and infections; therefore, biopsy confirmation is required for diagnosis of liver acute GVHD in those with no signs of acute GVHD in other organs. The most consistent histological feature of liver acute GVHD is bile duct damage102. Periportal and midzone hepatocellular necrosis and minimal lymphocytic infiltrates in the portal tract can also be observed. Liver biopsies are rarely performed early after transplantation owing to thrombocytopenia, making the diagnosis of liver acute GVHD one of exclusion.
Non-classic manifestations of acute GVHD
Other organs can be affected in acute GVHD, including lung, kidney, thymus and lymph nodes, bone marrow and the central nervous system, although acute damage is less apparent or is more difficult to distinguish from other alloHCT toxicities (drug or conditioning regimen toxicities, infectious complications). Symptoms of lung acute GVHD include fever, cough, dyspnoea (shortness of breath) and hypoxaemia (low blood oxygen levels), and are difficult to distinguish from those of other lung disease or injury103. Regarding the thymus and lymph nodes and the bone marrow, acute GVHD causes an impairment of thymic export of T cells104, a reduced haematopoiesis-supporting capacity of mesenchymal stem cells105 and a delayed B cell reconstitution and impaired antibody responses in patients106, leading to an impaired immune reconstitution and haematopoiesis. Finally, neurological deficits and abnormal MRI findings in patients developing acute GVHD have been found in several studies107.
Grading
Once a diagnosis of acute GVHD is established, disease in each involved organ (skin, GI tract and liver) is staged from 0 to 4 (whereby 4 is the most severe) (Table 1) based quantification of skin rash for skin acute GVHD, serum bilirubin level for liver acute GVHD, diarrhoea (number and/or volume of stool) for lower GI acute GVHD and persistent nausea for upper GI acute GVHD9. These stages are used to calculate the grade of acute GVHD based on the MAGIC criteria9: I (mild), II (moderate), III (severe) and IV (very severe) (Table 2). An electronic application has been developed and validated, the eGVHD App, to assist health-care professionals in the assessment of GVHD in clinical practice11,12. Other similar systems can been used for grading, including the Consensus (or modified Glucksberg) system108, the Minnesota system89,109 and the Center for International Blood and Marrow Transplant Research (CIBMTR) system110.
Biomarker screening
Researchers have aimed to identify a biomarker panel for the diagnosis of acute GVHD. A composite biomarker panel of four proteins (IL-2Rα, TNF receptor 1, IL-8, and hepatocyte growth factor) discriminates patients with and without acute GVHD, with an areas under the receiver operating characteristic curve distinguishing these two groups of 0.91 (95% CI 0.87–0.94) in the training set and 0.86 (95% CI 0.79–0.92) in the validation set111. In addition, several proteins related to GVHD organ damage have also been identified as potential biomarkers. Elafin is overexpressed in skin biopsies from patients with GVHD, and plasma levels of elafin are significantly higher at the onset of skin acute GVHD compared with those in patients without skin acute GVHD112. Moreover, regenerating islet-derived protein 3α (REG3A), hepatocyte growth factor and cytokeratin 18 (CK18) fragment levels are significantly increased in patients with lower GI acute GVHD compared with the levels in those with non-GVHD diarrhoea113,114. These three biomarkers are also elevated in patients with liver acute GVHD, but do not distinguish GVHD from other causes of hyperbilirubinaemia113.
The Ann Arbor (AA) biomarker risk uses serum concentrations of ST2 and REG3A at the onset of acute GVHD to generate a score from 1 to 3 to predict the risk of NRM and resistance to acute GVHD treatment115,116,117. The AA biomarker risk is used in large multicentre and multinational consortia (such as the MAGIC consortium) and as guidance for risk-adapted trials, but otherwise remains limited to a small number of centres. Overall, diagnostic or predictive biomarkers of acute GVHD are not used routinely in clinical practice owing to the lack of standardized commercially available assays.
Prevention
Pharmacological prophylaxis
Pharmacological prophylaxis for acute GVHD is based on the inhibition of the cytoplasmic enzyme calcineurin, which is important for T cell activation. Calcineurin inhibitors are usually combined with the anti-metabolite drug methotrexate, based on early studies which established the superiority of the calcineurin inhibitor cyclosporine combined with methotrexate over treatment with cyclosporine alone118,119. Two subsequent phase III randomized studies demonstrated a significantly lower incidence of grade II–IV acute GVHD in patients who received the calcineurin inhibitor tacrolimus combined with methotrexate compared with patients who received cyclosporine combined with methotrexate (32% versus 44% in patients with a sibling donor (P = 0.01) and 56% versus 74% in those with an unrelated donor (P = 0.0002))120,121 (Supplementary Table 1). Nevertheless, no difference in OS was found between groups. Thus, cyclosporine and tacrolimus are considered as roughly equivalent, and can be used according to centre practice. The toxic effects of cyclosporine and tacrolimus are similar and include nephrotoxicity, hypomagnesaemia, hyperkalaemia, hypertension and tremor122. Hypertrichosis (excessive hair growth) and gingival hyperplasia (overgrowth of gum tissue around the teeth) can also occur with cyclosporine treatment, and tacrolimus can be associated with alopecia. The most severe adverse effect of calcineurin inhibitors is transplant-associated thrombotic microangiopathy (TA-TMA), which is caused by direct cytotoxic damage to the endothelial cells by the calcineurin inhibitor123. The kidney is the primary site of TA-TMA, leading to proteinuria, acute kidney injury and hypertension, although multiple organs can be involved leading to intestinal thrombotic microangiopathy, pulmonary hypertension and neurotoxicity (headache, seizures, confusion and hallucinations). Therapeutic plasma exchange is usually ineffective for TA-TMA, so treatment relies on calcineurin inhibitor withdrawal and, in patients with evidence of complement activation124, complement-directed therapy (eculizumab)123. In the absence of GVHD, calcineurin inhibitors are usually tapered over 3–6 months after alloHCT.
In some centres, methotrexate toxicities (mucositis and neutropenia) lead to replacement with mycophenolate mofetil (MMF). In a prospective randomized study, cyclosporine plus MMF was associated with a lower incidence of grade III–IV mucositis (21% versus 65%; P = 0.008) and a quicker neutrophil engraftment (median day 11 versus 18; P < 0.001) compared with cyclosporine plus methotrexate, with a similar incidences of grade II–IV acute GVHD in the two groups (48% versus 37%; P = 0.49)125. In a meta-analysis of retrospective studies, methotrexate was found to be associated with a lower incidence of grade III–IV acute GVHD than MMF, while the incidence of mucositis was lower with MMF, the time to engraftment was shorter with MMF, and the incidence of grade II–IV acute GVHD and OS were similar in the two groups126,127. In practice, MMF is usually given to patients who received an umbilical cord blood transplant or reduced intensity conditioning to achieve faster engraftment and avoid graft failure128,129,130,131.
Sirolimus, although not a calcineurin inhibitor, is an immunosuppressant that inhibits mTOR, thereby blocking B and T cell activation. In a phase III randomized study, no significant difference was found in the cumulative incidence of grade III–IV acute GVHD with cyclosporine and methotrexate (n = 106) compared with tacrolimus and sirolimus (13% versus 7%; P = 0.09), and no difference in OS (72% versus 71%; P = 0.71)132. However, another phase III randomized study demonstrated a significantly lower cumulative incidence of grade II–IV acute GVHD with sirolimus plus cyclosporine and MMF compared with cyclosporine and MMF (26% versus 52%; P = 0.0013) in patients who received HLA-matched unrelated donor peripheral blood stem cell (PBSC) grafts following non-myeloablative conditioning133. Moreover, a concurrent multisite phase II study of triple immunosuppression (MMF, cyclosporine and sirolimus) in recipients of HLA-mismatched PBSC grafts demonstrated a cumulative incidence of 36% for grade II–IV acute GVHD at day 100 with only 1% of patients developing grade III and none developing grade IV acute GVHD134. Similar to calcineurin inhibitors, sirolimus damages endothelial cells and seems to be associated with TA-TMA132.
The cytotoxic T cell lymphocyte 4 (CTLA4) analogue abatacept, prevents APCs from delivering the costimulatory signal to T cells135. A randomized, double-blind, placebo-controlled phase II trial found no difference in the cumulative incidence of grade III–IV acute GVHD between patients who received abatacept plus a calcineurin inhibitor and methotrexate compared with those who received placebo plus a calcineurin inhibitor and methotrexate (6.8% versus 14.8%, HR 0.45; P = 0.13)135. Severe acute GVHD-free survival was 93.2% in the abatacept group versus 82% in the placebo group (P = 0.05). These data are promising, and further investigations are expected.
T cell depletion prophylaxis
Given the important role of T cells in acute GVHD pathophysiology, TCD approaches for acute GVHD prophylaxis have been developed. We distinguish between ex vivo TCD (T cell-negative selection or CD34-positive selection) and in vivo TCD with antibodies.
Ex vivo TCD with either T cell-negative selection or CD34-positive selection is highly effective for GVHD prophylaxis; however, it is associated with a high rate of infectious complications and underlying disease relapse136,137. The most frequently used techniques to achieve TCD of the graft are based on CD34-positive selection using electromagnetic methods, which allow up to a 5-log reduction in T cells138. Contemporary studies have demonstrated reduced incidence of acute GVHD and have revealed a similar relapse risk with ex vivo TCD compared with that in patients who received an unmodified graft139,140. However, the risk of infectious complications, particularly of viral origin, remains higher with ex vivo TCD alloHCT compared with unmodified graft, owing to delays in immune recovery of CD4+ T cells.
Other ex vivo TCD approaches include αβ+ T cell receptor (TCR)/CD19 depletion (αβ+ TCR and CD19-negative selection). This approach seems to be associated with a better immune response against viral infections and a low risk of acute GVHD, by enhancing γδ T cell reconstitution and lowering αβ+ TCR counts after transplantation141,142. Of note, αβ+ TCR/CD19 depletion has been mainly investigated in children, adolescents and young adults with haploidentical donors141,142. Another TCD approach involves naive TCD of PBSC grafts. In this approach, patients receive CD34-selected PBSC grafts with a defined dose of memory T cells and depleted naive T cells. Studies investigating this protocol revealed infrequent grade III–IV acute GVHD and chronic GVHD (4% and 7%, respectively) without excess risks of relapse or NRM143. Another study evaluated co-infusion of Treg cells and conventional T cells with CD34-selected PBSC haploidentical grafts in 43 adults with high-risk acute leukaemia144, and found grade II–IV acute GVHD in 15% of patients and a cumulative incidence of relapse of 5%.
Polyclonal ATGs are the most widely used antibodies for in vivo TCD. ATGs are obtained by immunizing rabbits either with fresh human thymocytes or with the Jurkat T lymphoblastoid cell line145. Several phase III randomized studies have evaluated the addition of ATG to a calcineurin inhibitor and methotrexate or MMF in patients with an unrelated donor or a matched related donor146,147,148,149,150 (Supplementary Table 1). These studies found a lower incidence of acute and chronic GVHD and improved GVHD-free, relapse-free survival (GRFS) in patients who received ATG, despite no improvement in OS146,147,148,149,150. ATG administration can be complicated by several infusion reactions including fever, chills, erythema, oxygen desaturation, headache, hepatic cytolysis, serum sickness (5–15 days after infusion) and, exceptionally, systemic anaphylaxis151. ATG also delays immune reconstitution, and is associated with increased risk of infections, especially of viral origin145,152.
The use of PTCy for GVHD prophylaxis was developed based on pioneering work of the Baltimore group in patients with a haploidentical alloHCT donor153, and it is now a well-established GVHD prophylaxis in this setting154. PTCy acts through induction of functional impairment of alloreactive T cells without toxic effects on haematopoietic stem cells155. One trial evaluated PTCy in patients with a 9/10 mismatched unrelated donor, and found a significantly lower cumulative incidence of severe grade III–IV acute GVHD in patients who received PTCy than in those who received ATG (9% versus 19%, respectively; P = 0.04)156. Similarly, a phase II study evaluated PTCy plus sirolimus and MMF after alloHCT with a mismatched unrelated donor (n = 80)157. This trial found grade II–IV and III–IV acute GVHD rates at day 100 of 43% and 18%, respectively, in patients who received a myeloablative conditioning regimen and grade II–IV and III–IV acute GVHD rates at day 100 of 33% and 0%, respectively, in patients who received a reduced intensity conditioning regimen. Another study (a randomized phase II study) compared ATG and PTCy in patients with a matched related or unrelated donor158 and found no difference in cumulative incidence of grade II–IV acute GVHD at 6 months (36.4% for PTCy versus 24.3% for ATG; P = 0.34)158. The BMT CTN 1703 phase III study compared tacrolimus plus MMF and PTCy (n = 214) with tacrolimus plus methotrexate (n = 217) in patients with a matched related, a matched unrelated or mismatched unrelated donor159 and found a lower cumulative incidence of grade III–IV acute GVHD at day 100 in the PTCy group versus the tacrolimus plus methotrexate group (6.3% versus 14.7%; P = 0.001). Moreover, in the multivariate Cox regression model, the PTCy group had a significantly lower hazard of GRFS than the tacrolimus and methotrexate group (HR 0.641, 95% CI 0.492–0.835; P = 0.001). In another phase III study (BMT CTN 1301 (ref. 160)), PTCy monotherapy was compared with CD34-selected PBSC grafts for GVHD prophylaxis after HLA-matched alloHCT. This study found higher cumulative incidences at 100 days of grade II–IV acute GVHD (37.6% for PTCy and 16.3% for CD34 selection; P = 0.002) and grade III–IV acute GVHD (10.1% for PTCy and 2.9% for CD34 selection; P = 0.05). Nevertheless, OS was significantly higher in the PTCy group (76.2%) than in the CD34-selected group (60.1%; P = 0.019).
Similar to ATG, PTCy is associated with delayed immune reconstitution which leads to an increased incidence of viral infection161. Furthermore, particular attention must be paid to patients with a history of cardiac problems, as a higher incidence of early cardiac events (within the first 100 days after alloHCT) has been reported with PTCy treatment162. The addition of low-dose ATG to PTCy for GVHD prophylaxis in patients with a haploidentical donor shows promise, with a lower incidence of acute GVHD in those who received ATG and PTCy than in those who received PTCy alone (22% versus 12%, respectively; P = 0.029)163.
The monoclonal antibody alemtuzumab has also been evaluated for in vivo TCD. Alemtuzumab is an anti-CD52 monoclonal antibody that targets T and B cells, dendritic cells, natural killer cells, monocytes and macrophages. Although alemtuzumab was associated with a low incidence of acute GVHD in non-randomized studies164,165,166, results were not confirmed in prospective randomized studies. Furthermore, alemtuzumab can remain in the blood for up to 1–2 months after transplantation, which can substantially delay immune reconstitution, leading to a high incidence of viral infection and relapse, and no OS benefit167,168.
Finally, vedolizumab, a monoclonal antibody that selectively antagonizes α4β7 GI integrin receptors, preventing lymphocyte trafficking to the gut, has also been evaluated. A phase III randomized placebo-controlled study demonstrated improved lower GI acute GVHD-free survival at 180 days with vedolizumab prophylaxis than with placebo (85.5% versus 70.9%; P < 0.001), with no significant difference in serious adverse events between groups (69% versus 71%, respectively)169. This is the first positive phase III study for specific prevention of lower GI acute GVHD.
Management
First-line therapies
For acute GVHD treatment, consensus recommendations were published by the European Society for Blood and Marrow Transplantation131 and more specific recommendations for management of acute GVHD after umbilical cord blood alloHCT were issued by the American Society for Transplantation and Cellular Therapy170. Systemic steroids remain the standard first-line treatment for acute GVHD. However, a randomized phase III controlled trial in patients with grade I acute GVHD showed more frequent infection and no advantage regarding the development of grade III–IV acute GVHD in patients treated with 6-methylprednisolone compared with no treatment171. Accordingly, systemic treatment is recommended only in patients with acute GVHD of grade II or higher, and topical steroids alone are used in patients with grade I disease (Fig. 5). One randomized phase III study compared the use of low-dose versus standard-dose prednisone in patients with grade II acute GVHD with stage 1 GI acute GVHD, no hepatic dysfunction and stage 1 or 2 skin acute GVHD172,173. In this trial, low-dose prednisone seemed as effective as standard-dose treatment, as the low dose did not increase the risk in patients requiring secondary immunosuppressive therapy173. By contrast, in patients with grade II acute GVHD with liver or extensive skin involvement (rash ≥50% body surface area) or grade III–IV acute GVHD, the use of low-dose prednisone was associated with an increased risk of requiring secondary immunosuppressive therapy173.
Patients with acute graft-versus-host disease (GVHD) should be assessed first for skin manifestations. Isolated stage 1–2 skin acute GVHD should be treated with topical steroid alone, whereas isolated stage 3–4 skin acute GVHD should be treated with methylprednisolone. Gastrointestinal and liver acute GVHD can also be treated with methylprednisolone. If treatment fails, the dose of methylprednisolone can be increased in patients who received a low initial dose, or ruxolitinib therapy can be initiated. Third-line treatments can be used if ruxolitinib fails.
For GI involvement, two randomized trials compared prednisone plus beclomethasone (a non-absorbable oral steroid) and prednisone plus placebo174,175. Beclomethasone was associated with a reduced risk of GVHD treatment failure and improved survival. Based on these data, non-absorbable oral steroids (such as oral beclomethasone) are recommended in patients with acute GVHD with GI involvement131. Budesonide can be used if oral beclomethasone is not available. Similarly, in patients with skin acute GVHD who received systemic steroids, the use topical steroids in addition to systemic steroids is recommended until the skin rash disappears.
Formal assessment of acute GVHD is performed 3 and 7 days after diagnosis. Steroid-refractory GVHD is defined as disease that progresses by day 3 or disease that fails to improve by day 7 (ref. 176). The response rates to first-line treatment with prednisone are low (~50%)177; therefore, there is an important need for addition of other therapies to improve response rates.
One four-arm randomized phase II study aimed to identify the most promising agent for initial treatment of acute GVHD by evaluating methylprednisolone plus etanercept, MMF, denileukin diftitox or pentostatin178. Complete response (CR) rates at day 28 were 26% for methylprednisolone and etanercept, 60% for methylprednisolone and MMF, 53% for methylprednisolone and denileukin diftitox, and 38% for methylprednisolone and pentostatin178. The corresponding OS at 9 months was 47% for methylprednisolone and etanercept, 64% for methylprednisolone and MMF, 49% for methylprednisolone and denileukin diftitox, and 47% for methylprednisolone and pentostatin. As MMF was the most promising agent in this trial, a phase III randomized double-blind trial compared methylprednisolone plus placebo and methylprednisolone plus MMF179, and found similar CR rates at day 28 (46.6% in the methylprednisolone and MMF group versus 44.5% in the methylprednisolone and placebo group; P = 0.76), with no difference in GVHD-free survival at day 56 in patients with grade III–IV acute GVHD (54.1% in the MMF group and 51.2% in the placebo group; P = 0.8). Another randomized phase III trial found similar CR rates at day 28 for acute GVHD in patients who received infliximab plus methylprednisolone or methylprednisolone alone (62% versus 58%, respectively; P = 0.7)180.
More recently, another phase III study compared corticosteroids plus either the JAK1 inhibitor itacitinib or placebo for the initial treatment of acute GVHD (n = 439)181. This study found no difference in overall response rate (ORR) at day 28 between groups (OR 1.45, 95% CI 0.96–2.20; P = 0.078). In addition, another phase III randomized study compared sirolimus and prednisone for the initial treatment of standard risk acute GVHD (defined by the Minnesota GVHD Risk Score and Ann Arbor biomarker status)182. This study found similar CR and partial response (PR) rates at day 28 for sirolimus (64.8%, 90% CI 54.1–75.5%) versus prednisone (73%, 90% CI 63.8–82.2%), with no difference in patients’ outcomes. Moreover, sirolimus treatment was associated with reduced steroid exposure and hyperglycaemia, reduced number of grade II–III infections, improvement in immune suppression discontinuation, and patient-reported QOL, and increased risk of TA-TMA.
Second-line and third-line therapies
Until recently, no standard second-line treatments were available for steroid-resistant or steroid-dependent acute GVHD. Different treatments were used for this purpose, including ATG, AAT, anti-TNF, MMF, anti-IL-2R, alemtuzumab, sirolimus, extracorporeal photopheresis, methotrexate, mesenchymal stem cells, decidual stromal cells and faecal microbiota transplantation183,184,185. These therapies are now administered as third-line options.
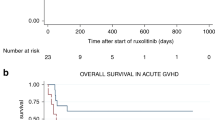
Based on promising retrospective data and a phase II clinical trial186,187, the JAK2 inhibitor ruxolitinib was evaluated in a large phase III randomized clinical trial comparing the best available treatment for steroid-refractory or steroid-dependent acute GVHD (n = 309)188. This trial found a higher overall response at day 28 in the ruxolitinib group than in the control group (62% versus 39%; OR 2.64, 95% CI 1.65–4.22; P < 0.001). The results of this study led to the approval of ruxolitinib as a second-line treatment for steroid-refractory or steroid-dependent acute GVHD by the FDA and the EMA. However, despite these positive results, in the phase III randomized clinical trial, 38% of patients who received ruxolitinib did not achieve a CR or PR by day 28, and the durable response rate at day 56 was 39.6% (indicating that 60.4% of the patients required a third-line immunosuppressive therapy or had died)188. Ruxolitinib-refractory acute GVHD can be defined as disease that progresses after 5 to 10 days of treatment with ruxolitinib, fails to improve (PR or better) after at least 14 days of treatment with ruxolitinib, or shows a loss of response at any time after initial improvement176. Management of patients with steroid-resistant and ruxolitinib-resistant acute GVHD is an unmet need, and it is recommended that such patients be included in clinical trials131. If trial enrolment is not possible, third-line treatment should be chosen according to centre practice183,184 (Fig. 5; Table 3).
Clinical trials
As treatment of acute GVHD remains associated with a high failure rate, inclusion in clinical trials of new therapies is recommended. In particular, attempts have been made to produce a risk adapted approach using the Ann Arbor (AA) biomarker risk score or the Minnesota risk score189, which predict response to steroid treatment, survival and transplant-related mortality more accurately than usual acute GVHD grading criteria89,109,110. The overall clinical response rate (CR and PR) 28 days after initiation of treatment is a validated surrogate for OS, and has been widely adopted as the primary end point in trials of treatments for acute GVHD. Secondary end points usually include the durable overall clinical response rate at day 56, OS, NRM and effect on underlying malignancy relapse (cumulative incidence of relapse), but also safety, exposure to steroids and QOL.
Treatment complications
Use of high-dose steroids and other immunosuppressive drugs increases the risk of infectious complications and other non-infectious adverse effects. Accordingly, supportive care is indispensable in patients with acute GVHD.
Close monitoring of suspected invasive fungal infections is recommended using the serum biomarker antigen galactomannan and directed CT scans190,191. In a randomized phase III study, prophylactic posaconazole was as effective as fluconazole in preventing all invasive fungal infections (incidence 5.3% and 9.0%; OR 0.56, 95% CI 0.30–1.07; P = 0.07) and was superior to fluconazole in preventing proven or probable invasive aspergillosis (2.3% versus 7.0%; OR 0.31, 95% CI 0.13–0.75; P = 0.006) in patients with severe acute GVHD192. Therefore, invasive fungal infection prophylaxis with posaconazole is recommended in patients with acute GVHD. Patients should also receive prophylaxis for Pneumocystis jirovecii pneumonia with sulfamethoxazole trimethoprim193.
Close monitoring for CMV infection is recommended in patients with acute GVHD using CMV PCR or pp65 antigen assay194. Ganciclovir or foscarnet must be initiated early in those with CMV reactivation to avoid clinical manifestations and disease. Letermovir is approved for CMV prophylaxis for up to 100 days after alloHCT195, although letermovir prophylaxis can be used after day 100 in patients at high risk of late CMV infection, including those with acute GVHD who are receiving immunosuppressive treatement196. Importantly, letermovir is not effective for prevention of varicella zoster virus infection, and patients must continue to receive valacyclovir to prevent shingles and chicken pox197,198. Close monitoring for Epstein–Barr virus infection is essential to detect early viraemia, and to permit early treatment with an anti-CD20 monoclonal antibody to prevent post-transplantation lymphoproliferative disorder199.
Patients and their family and caregivers should also receive vaccination against influenza200,201 and SARS-CoV-2 (ref. 202). Patients with influenza infection should receive neuraminidase inhibitors203. Nirmatrelvir and ritonavir are recommended in patients with COVID-19 who do not require supplemental oxygen and who have increased risk of severe disease204, and this recommendation is therefore highly relevant in patients with acute GVHD. However, ritonavir is a strong inhibitor of cytochrome P450–3A4, and interacts with several drugs, including calcineurin inhibitors, steroids and ruxolitinib; therefore, the use of nirmatrelvir plus ritonavir must be carefully assessed in patients as it is associated with withholding or reduction in the dose of some immunosuppressants205.
Although patients with humoral immune deficiency (hypogammaglobulinaemia) after alloHCT and acute GVHD treatment are more susceptible to infection with encapsulated bacteria, prophylactic use of intravenous immunoglobulin is not supported by data206. Patients should receive routine prophylaxis with penicillin or equivalent antibiotics and receive vaccinations for Streptococcus pneumoniae and Haemophilus influenzae207,208. Patients who have received steroids have a high risk of bacteraemia and septic shock; therefore, blood culture should be performed regularly in these patients even in the absence of fever. The presence of fever should prompt an infectious work-up, including blood culture, without delaying the initiation of a broad-spectrum antibiotic209.
The use of high-dose steroids is also frequently associated with diabetes mellitus, osteoporosis, aseptic osteonecrosis, amyotrophy (progressive muscle wasting) and other symptoms of iatrogenic Cushing syndrome. Close monitoring of for corticoid-induced toxicity is therefore recommended to initiate early preventive or curative treatment that can include insulin, calcium, vitamin D or bisphosphonates210,211.
Quality of life
QOL is evaluated using patient-reported outcome questionnaires. No specific QOL questionnaire is available for patients with acute GVHD; therefore, QOL in these patients is usually evaluated with a questionnaire for cancer, such as the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30)212 or for alloHCT, such as the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT)213. The FACT-BMT is based on Functional Assessment of Cancer Therapy–General (FACT-G), a 27-item cancer-specific questionnaire214, which contains supplementary questions to assess additional concerns including overall treatment effects and regret related to transplantation. The 12-item Short-Form Health Survey (SF-12) can also be used to evaluate QOL and is more practical than all other available questionnaires given its short format215.
In a cohort of 96 patients, development of grade II–IV acute GVHD was associated with a measurable decline in QOL at 6 months after alloHCT, when assessed using the FACT-BMT, compared with patients who had not developed acute GVHD216. Moreover, in patients who survived at least 1 year after alloHCT, the SF-12 Physical Component Score (PCS) and Mental Component Score (MCS) were worse in survivors of grade III–IV acute GVHD compared with those with a history of grade 0–I acute GVHD217. Furthermore, patients with a history of grade III–IV acute GVHD had significantly higher rates of late medical comorbidities than those with a history of grade 0–I acute GVHD. In particular, the cumulative incidence of major late effects (acute respiratory distress syndrome, heart failure and renal failure requiring dialysis) at 10 years after alloHCT was 42% in the grade 0–I cohort and 61% in the grade III–IV acute GVHD cohort (P < 0.001). In addition, patients who survived for >1 year after transplantation and had prior grade III–IV acute GVHD also had a worse 5-year OS (77.5% versus 83.6%; P = 0.006) and higher NRM (19.2% versus 10.6%; P < 0.001) compared with those with a history of grade 0–I acute GVHD.
Importantly, acute GVHD is a risk factor for chronic GVHD23,218, which is leading cause of late NRM, morbidity219 and impaired QOL220,221. Patients also frequently experience psychosocial distress after alloHCT, with some studies finding depression in 25–35% of patients within the first year after alloHCT222,223. Overall, patients who survive for >1 year after alloHCT, and particularly those with a history of acute GVHD, are a high-risk population that must be monitored for long-term transplant complications, including chronic GVHD, multiorgan dysfunction and secondary malignancies224. Recommendations for long-term patient monitoring are provided in Table 4. Finally, available QOL questionnaires, developed for use in patients with cancer or more specifically those who undergo alloHCT, effectively evaluate QOL in patients with acute GVHD and should be used to improve patient monitoring without the need to create a specific QOL questionnaire for acute GVHD.
Outlook
Prophylaxis and treatment of acute GVHD has significantly progressed in the past few years. Indeed, development of PTCy for GVHD prophylaxis has allowed the important development of haploidentical alloHCT worldwide3 and a standard second-line treatment for steroid-refractory acute GVHD is now available188. Despite these achievements, there remain some important unmet medical needs in acute GVHD.
The most effective GVHD prophylaxis remains to be established. Further studies are needed to identify the best combinations of therapies and optimal doses to prevent severe acute GVHD without increasing the risk of infectious complications and relapse of the underlying disease. Similarly, the most effective first-line treatments for acute GVHD are still not defined, and, owing to their high failure rate and associated toxicity, steroid-sparing approaches are urgently needed. Biomarkers that are predictive of the risk of NRM and resistance to treatment in patients with acute GVHD115,116,117 have been developed and efforts must be made to implement their use in routine practice to evaluate first-line treatments for acute GVHD in patients with a high risk of resistance to steroids. This is particularly important given the irreversible tissue damage in refractory acute GVHD caused by epithelial stem suppression by steroids225 or by GVHD through reduced patient-derived IL-22 levels226,227 and increased telomere shortening228. Moreover, treatment of patients with steroid-resistant and ruxolitinib-resistant acute GVHD is an unmet medical need, and innovative approaches are urgently required. One of the most promising approaches is the development of gut microbiota manipulation, particularly faecal microbiota transplantation229,230,231.
Over the past few years, alloHCT has become more accessible across the world, partially owing to the development of haploidentical alloHCT with PTCy, which offers a cost-effective platform for low-income and middle-income countries5. However, management of severe acute GVHD will remain a challenge in these countries, particularly steroid-refractory acute GVHD, owing to the cost and lack of availability of ruxolitinib and third-line treatment. Similarly, effective prophylaxis for infectious diseases that is required during treatment of acute GVHD may be inaccessible in these countries. Accordingly, strategies developed to facilitate access to newly developed anticancer drugs in these regions232 should also include treatments for complications such as acute GVHD and anti-infection prophylaxis.
References
Van Bekkum, D., Vos, O. & Weyzen, W. W. The pathogenesis of the secondary disease after foreign bone marrow transplantation in X-irradiated mice. J. Natl Cancer Inst. 23, 75–89 (1959).
Billingham, R. E. The biology of graft-versus-host reactions. Harvey Lect. 62, 21–78 (1966).
Passweg, J. R. et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. https://doi.org/10.1038/s41409-021-01227-8 (2021).
Passweg, J. R. et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 57, 742–752 (2022).
Niederwieser, D. et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 107, 1045–1053 (2022).
Holtan, S. G. et al. Disease progression, hospital readmissions, and clinical outcomes for patients with steroid-refractory acute graft-versus-host disease: a multicenter, retrospective study. Bone Marrow Transplant. 57, 1399–1404 (2022).
Filipovich, A. H. et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 11, 945–956 (2005). This article reports the refined classification by the NIH that established that acute and chronic GVHD should be distinguished based on GVHD features and not time of onset.
Jagasia, M. H. et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 21, 389–401.e1 (2015).
Harris, A. C. et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 22, 4–10 (2016). This article refines acute GVHD diagnosis criteria to allow better standardization of data collection.
Cahn, J. Y. et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Société Française de Greffe de Moëlle et Thérapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood 106, 1495–1500 (2005).
Schoemans, H. M. et al. The eGVHD app has the potential to improve the accuracy of graft-versus-host disease assessment: a multicenter randomized controlled trial. Haematologica 103, 1698–1707 (2018).
Schoemans, H. M. et al. Accuracy and usability of the eGVHD app in assessing the severity of graft-versus-host disease at the 2017 EBMT annual congress. Bone Marrow Transplant. 53, 490–494 (2018).
Thomas, E. D. et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 49, 511–533 (1977).
Reshef, R. et al. Acute GVHD diagnosis and adjudication in a multicenter trial: a report from the BMT CTN 1202 biorepository study. J. Clin. Oncol. 39, 1878–1887 (2021).
Greinix, H. T. et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega-file study. Haematologica 107, 1054–1063 (2022). This paper is the largest and most recent analysis of acute GVHD incidence, risk factors and outcomes.
Mielcarek, M. et al. Effects of race on survival after stem cell transplantation. Biol. Blood Marrow Transplant. 11, 231–239 (2005).
Sigmund, A. M. et al. Impact of race and geographic area of residence on outcomes after allogeneic stem cell transplant. Front. Oncol. https://doi.org/10.3389/fonc.2022.801879 (2022).
Hamilton, B. K. et al. Racial differences in allogeneic hematopoietic cell transplantation outcomes among African Americans and whites. Bone Marrow Transplant. 50, 834–839 (2015).
Morishima, Y. et al. Significance of ethnicity in the risk of acute graft-versus-host disease and leukemia relapse after unrelated donor hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 19, 1197–1203 (2013).
Kanda, J. et al. Graft-versus-host disease after HLA-matched sibling bone marrow or peripheral blood stem cell transplantation: comparison of North American Caucasian and Japanese populations. Biol. Blood Marrow Transplant. 22, 744–751 (2016).
Khoury, H. J. et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 102, 958–966 (2017).
El-Jawahri, A. et al. Improved treatment-related mortality and overall survival of patients with grade IV acute GVHD in the modern years. Biol. Blood Marrow Transplant. 22, 910–918 (2016).
Flowers, M. E. et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117, 3214–3219 (2011).
Lee, S. J. et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110, 4576–4583 (2007).
Hahn, T. et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J. Clin. Oncol. 26, 5728–5734 (2008).
Gale, R. P. et al. Risk factors for acute graft-versus-host disease. Br. J. Haematol. 67, 397–406 (1987).
Kollman, C. et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98, 2043–2051 (2001).
Bacigalupo, A. Post-transplant cyclophosphamide: overcoming the HLA barrier to hematopoietic stem cell transplants. Haematologica 107, 1230–1231 (2022).
Im, A. et al. Risk factors for graft-versus-host disease in haploidentical hematopoietic cell transplantation using post-transplant cyclophosphamide. Biol. Blood Marrow Transplant. 26, 1459–1468 (2020).
Hansen, J. A., Chien, J. W., Warren, E. H., Zhao, L. P. & Martin, P. J. Defining genetic risk for graft-versus-host disease and mortality following allogeneic hematopoietic stem cell transplantation. Curr. Opin. Hematol. 17, 483–492 (2010).
Antin, J. H. & Ferrara, J. L. Cytokine dysregulation and acute graft-versus-host disease. Blood 80, 2964–2968 (1992).
Holler, E. et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood 75, 1011–1016 (1990).
Storb, R. et al. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biol. Blood Marrow Transplant. 19, 792–798 (2013).
Cooke, K. R., Olkiewicz, K., Erickson, N. & Ferrara, J. L. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J. Endotoxin Res. 8, 441–448 (2002).
Holler, E. et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood 104, 889–894 (2004).
Holler, E. et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 107, 4189–4193 (2006).
Markus, M. H. et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010).
Fischer, J. C. et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl Med. https://doi.org/10.1126/scitranslmed.aag2513 (2017).
Bader, C. S. et al. STING differentially regulates experimental GVHD mediated by CD8 versus CD4 T cell subsets. Sci. Transl Med. https://doi.org/10.1126/scitranslmed.aay5006 (2020).
Toubai, T., Mathewson, N. D., Magenau, J. & Reddy, P. Danger signals and graft-versus-host disease: current understanding and future perspectives. Front. Immunol. 7, 539 (2016).
Wilhelm, K. et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 16, 1434–1438 (2010).
Vander Lugt, M. T. et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N. Engl. J. Med. 369, 529–539 (2013).
van Bekkum, D. W., Roodenburg, J., Heidt, P. J. & van der Waaij, D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J. Natl Cancer Inst. 52, 401–404 (1974).
Storb, R. et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N. Engl. J. Med. 308, 302–307 (1983).
Hulsdunker, J. et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood 131, 1858–1869 (2018).
Vossen, J. M. et al. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS ONE 9, e105706 (2014).
Peled, J. U. et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 382, 822–834 (2020).
Stein-Thoeringer, C. K. et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 366, 1143–1149 (2019).
Jenq, R. R. et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant. 21, 1373–1383 (2015).
Riwes, M. & Reddy, P. Short chain fatty acids: postbiotics/metabolites and graft versus host disease colitis. Semin. Hematol. 57, 1–6 (2020).
Ghimire, S. et al. Low intestinal IL22 associates with increased transplant-related mortality after allogeneic stem cell transplantation. Front. Immunol. 13, 857400 (2022).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Fujiwara, H. et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 9, 3674 (2018).
Kambayashi, T. & Laufer, T. M. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat. Rev. Immunol. 14, 719–730 (2014).
Shlomchik, W. D. et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285, 412–415 (1999).
Duffner, U. A. et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 172, 7393–7398 (2004).
Matte, C. C. et al. Donor APCs are required for maximal GVHD but not for GVL. Nat. Med. 10, 987–992 (2004).
Koyama, M. & Hill, G. R. Alloantigen presentation and graft-versus-host disease: fuel for the fire. Blood 127, 2963–2970 (2016).
Koyama, M. et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat. Med. 18, 135–142 (2011).
Ruggeri, A. et al. Integrating biological HLA-DPB1 mismatch models to predict survival after unrelated hematopoietic cell transplantation. Haematologica 108, 645–652 (2023).
Neefjes, J., Jongsma, M. L., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
Goulmy, E. et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N. Engl. J. Med. 334, 281–285 (1996).
Meyer, E. H. et al. Transplantation of donor grafts with defined ratio of conventional and regulatory T cells in HLA-matched recipients. JCI Insight https://doi.org/10.1172/jci.insight.127244 (2019).
Edinger, M. et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9, 1144–1150 (2003).
Yang, J. et al. Rorc restrains the potency of ST2+ regulatory T cells in ameliorating intestinal graft-versus-host disease. JCI Insight https://doi.org/10.1172/jci.insight.122014 (2019).
Delacher, M. et al. Single-cell chromatin accessibility landscape identifies tissue repair program in human regulatory T cells. Immunity 54, 702–720.e17 (2021).
Robb, R. J. et al. Identification and expansion of highly suppressive CD8+FoxP3+ regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 119, 5898–5908 (2012).
Hu, Y. et al. Regulatory B cells promote graft-versus-host disease prevention and maintain graft-versus-leukemia activity following allogeneic bone marrow transplantation. Oncoimmunology 6, e1284721 (2017).
Le Blanc, K., Jitschin, R. & Mougiakakos, D. Myeloid-derived suppressor cells in allogeneic hematopoietic stem cell transplantation: a double-edged sword? Oncoimmunology 2, e25009 (2013).
Demosthenous, C. et al. The role of myeloid-derived suppressor cells (MDSCs) in graft-versus-host disease (GVHD). J. Clin. Med. 10, 2050 (2021).
Riquelme, P. et al. TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat. Commun. 9, 2858 (2018).
Quatrini, L. et al. Helper innate lymphoid cells in allogenic hematopoietic stem cell transplantation and graft versus host disease. Front. Immunol. 11, 582098 (2020).
Luft, T. et al. Serum cytokeratin-18 fragments as quantitative markers of epithelial apoptosis in liver and intestinal graft-versus-host disease. Blood 110, 4535–4542 (2007).
Glucksberg, H. et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18, 295–304 (1974).
Fu, Y. Y. et al. T cell recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity 51, 90–103.e3 (2019).
Matsuzawa-Ishimoto, Y. et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J. Exp. Med. 214, 3687–3705 (2017).
Hill, G. R., Krenger, W. & Ferrara, J. L. The role of cytokines in acute graft-versus-host disease. Cytokines Cell. Mol. Ther. 3, 257–266 (1997).
Spoerl, S. et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123, 3832–3842 (2014).
Dietrich, S. et al. Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol. Blood Marrow Transplant. 19, 22–27 (2013).
Biedermann, B. C. et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet 359, 2078–2083 (2002).
Mathew, N. R. et al. Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia. J. Clin. Invest. 130, 1315–1329 (2020).
Hildebrandt, G. C. et al. Donor-derived TNF-α regulates pulmonary chemokine expression and the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 104, 586–593 (2004).
Wu, S. R. & Reddy, P. Tissue tolerance: a distinct concept to control acute GVHD severity. Blood 129, 1747–1752 (2017).
Fujiwara, H. et al. Mitochondrial complex II in intestinal epithelial cells regulates T cell-mediated immunopathology. Nat. Immunol. 22, 1440–1451 (2021).
Mohamed, F. A. et al. Recent metabolic advances for preventing and treating acute and chronic graft versus host disease. Front. Immunol. 12, 757836 (2021).
Holtan, S. G. et al. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review. Bone Marrow Transplant. 57, 1581–1585 (2022).
Champlin, R. E. et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood 95, 3702–3709 (2000).
Lerner, K. G. et al. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant. Proc. 6, 367–371 (1974).
Weisdorf, D. J. et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood 76, 624–629 (1990).
Cox, G. J. et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology 107, 1398–1407 (1994).
Kalantari, B. N. et al. CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. AJR Am. J. Roentgenol. 181, 1621–1625 (2003).
Thompson, B., Salzman, D., Steinhauer, J., Lazenby, A. J. & Wilcox, C. M. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 38, 371–376 (2006).
Tarantino, G. et al. Gastrointestinal complications after allogeneic hematopoietic stem cell transplant: a multidisciplinary approach with early endoscopic evaluation. Clin. Hematol. Int. 3, 161–168 (2021).
Shabbir, E., Farooq, U., Yanes, B. & Magalhaes-Silverman, M. Repeat endoscopy affects patient management in gastrointestinal graft-versus-host disease. Clin. Hematol. Int. 2, 69–73 (2020).
Sale, G. E., Shulman, H. M., McDonald, G. B. & Thomas, E. D. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am. J. Surg. Pathol. 3, 291–299 (1979).
Epstein, R. J., McDonald, G. B., Sale, G. E., Shulman, H. M. & Thomas, E. D. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology 78, 764–771 (1980).
Schreyer, A. G. et al. Transmural penetration of intravenously applied microbubbles during contrast-enhanced ultrasound as a new diagnostic feature in patients with GVHD of the bowel. Bone Marrow Transplant. 46, 1006–1011 (2011).
Schreyer, A. G. et al. Contrast-enhanced ultrasound for differential diagnosis of suspected GvHD in patients after allogeneic transplantation. Clin. Hemorheol. Microcirc. 49, 129–136 (2011).
Benedetti, E. et al. Prospective qualitative and quantitative non-invasive evaluation of intestinal acute GVHD by contrast-enhanced ultrasound sonography. Bone Marrow Transplant. 48, 1421–1428 (2013).
Stelljes, M. et al. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood 111, 2909–2918 (2008).
Gooley, T. A. et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 363, 2091–2101 (2010).
Snover, D. C., Weisdorf, S. A., Ramsay, N. K., McGlave, P. & Kersey, J. H. Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology 4, 123–130 (1984).
Cooke, K. R. & Yanik, G. Acute lung injury after allogeneic stem cell transplantation: is the lung a target of acute graft-versus-host disease. Bone Marrow Transplant. 34, 753–765 (2004).
Krenger, W., Blazar, B. R. & Holländer, G. A. Thymic T-cell development in allogeneic stem cell transplantation. Blood 117, 6768–6776 (2011).
Ding, L. et al. Tumor necrosis factor α in aGVHD patients contributed to the impairment of recipient bone marrow MSC stemness and deficiency of their hematopoiesis-promotion capacity. Stem Cell Res. Ther. 11, 119 (2020).
Mensen, A. et al. Bone marrow T-cell infiltration during acute GVHD is associated with delayed B-cell recovery and function after HSCT. Blood 124, 963–972 (2014).
Shortt, J., Hutton, E., Faragher, M. & Spencer, A. Central nervous system graft-versus-host disease post allogeneic stem cell transplant. Br. J. Haematol. 132, 245–247 (2006).
Przepiorka, D. et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 15, 825–828 (1995).
MacMillan, M. L. et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol. Blood Marrow Transplant. 8, 387–394 (2002).
Rowlings, P. A. et al. IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br. J. Haematol. 97, 855–864 (1997).
Paczesny, S. et al. A biomarker panel for acute graft-versus-host disease. Blood 113, 273–278 (2009).
Paczesny, S. et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci. Transl Med. 2, 13ra12 (2010).
Harris, A. C. et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood 119, 2960–2963 (2012).
Ferrara, J. L. et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 118, 6702–6708 (2011).
Hartwell, M. J. et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI insight 2, e89798 (2017).
Srinagesh, H. K. et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Advan. 3, 4034–4042 (2019).
Zewde, M. G. et al. Evaluation of elafin as a prognostic biomarker in acute graft-versus-host disease. Transplant. Cell Ther. 27, 988.e1–988.e7 (2021).
Storb, R. et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 314, 729–735 (1986). To our knowledge, the first randomized phase III study that established the combination of methotrexate and a calcineurin as the standard GVHD prophylaxis after alloHCT.
Storb, R. et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 73, 1729–1734 (1989).
Ratanatharathorn, V. et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 92, 2303–2314 (1998).
Nash, R. A. et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 96, 2062–2068 (2000).
Farouk, S. S. & Rein, J. L. The many faces of calcineurin inhibitor toxicity – what the FK? Adv. Chronic Kidney Dis. 27, 56–66 (2020).
Young, J. A., Pallas, C. R. & Knovich, M. A. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 56, 1805–1817 (2021).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Bolwell, B. et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 34, 621–625 (2004).
Kharfan-Dabaja, M. et al. Mycophenolate mofetil versus methotrexate for prevention of graft-versus-host disease in people receiving allogeneic hematopoietic stem cell transplantation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010280.pub2 (2014).
Ram, R., Yeshurun, M., Vidal, L., Shpilberg, O. & Gafter-Gvili, A. Mycophenolate mofetil vs. methotrexate for the prevention of graft-versus-host-disease-systematic review and meta-analysis. Leuk. Res 38, 352–360 (2014).
Mohty, M. et al. Mycophenolate mofetil and cyclosporine for graft-versus-host disease prophylaxis following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 34, 527–530 (2004).
Brunstein, C. G. et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 110, 3064–3070 (2007).
Brissot, E. et al. Prophylaxis with mycophenolate mofetil and CsA can decrease the incidence of severe acute GVHD after antithymocyte globulin-based reduced-intensity preparative regimen and allo-SCT from HLA-matched unrelated donors. Bone Marrow Transplant. 45, 786–788 (2010).
Penack, O. et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 7, e157–e167 (2020).
Törlén, J. et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica 101, 1417–1425 (2016).
Sandmaier, B. M. et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial. Lancet Haematol. 6, e409–e418 (2019).
Kornblit, B. et al. Sirolimus with CSP and MMF as GVHD prophylaxis for allogeneic transplantation with HLA antigen-mismatched donors. Blood 136, 1499–1506 (2020).
Watkins, B. et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J. Clin. Oncol. 39, 1865–1877 (2021).
Urbano-Ispizua, A. et al. Rapid engraftment without significant graft-versus-host disease after allogeneic transplantation of CD34+ selected cells from peripheral blood. Blood 89, 3967–3973 (1997).
Wagner, J. E., Thompson, J. S., Carter, S. L. & Kernan, N. A. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet 366, 733–741 (2005).
Keever-Taylor, C. A. et al. Characteristics of CliniMACS® System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia – Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol. Blood Marrow Transplant. 18, 690–697 (2012).
Hobbs, G. S. et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 50, 493–498 (2015).
Barba, P. et al. CD34+ cell selection versus reduced-intensity conditioning and unmodified grafts for allogeneic hematopoietic cell transplantation in patients age >50 years with acute myelogenous leukemia and myelodysplastic syndrome. Biol. Blood Marrow Transplant. 24, 964–972 (2018).
Bertaina, A. et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 124, 822–826 (2014).
Salzmann-Manrique, E. et al. Joint modeling of immune reconstitution post haploidentical stem cell transplantation in pediatric patients with acute leukemia comparing CD34+-selected to CD3/CD19-depleted grafts in a retrospective multicenter study. Front. Immunol. 9, 1841 (2018).
Bleakley, M. et al. Naive T-cell depletion to prevent chronic graft-versus-host disease. J. Clin. Oncol. 40, 1174–1185 (2022).
Martelli, M. F. et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124, 638–644 (2014).
Mohty, M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21, 1387–1394 (2007).
Bacigalupo, A. et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 98, 2942–2947 (2001). To our knowledge, the first randomized study showing that in vivo T cell depletion of antithymocyte globulin added to standard pharmacological GVHD prophylaxis decreases the incidence of acute GVHD.
Finke, J. et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 10, 855–864 (2009).
Kröger, N. et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl. J. Med. 374, 43–53 (2016).
Walker, I. et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 17, 164–173 (2016).
Soiffer, R. J. et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J. Clin. Oncol. 35, 4003–4011 (2017).
Bonifazi, F. in The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies (eds Carreras, E., Dufour, C., Mohty, M. & Kröger, N.) 183–187 (Springer, 2019).
Soiffer, R. J. et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 117, 6963–6970 (2011).
Luznik, L. et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 14, 641–650 (2008). To our knowledge, the first study reporting the use of post-transplant cyclophosphamide for acute GVHD in patients with a haploidentical donor.
Bashey, A. et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J. Clin. Oncol. 31, 1310–1316 (2013).
Wachsmuth, L. P. et al. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J. Clin. Investig. 129, 2357–2373 (2019).
Battipaglia, G. et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood 134, 892–899 (2019).
Shaw, B. E. et al. A national marrow donor program sponsored multi-center, phase II trial of HLA- mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J. Clin. Oncol. 39, 1971–1982 (2021).
Brissot, E. et al. Post-transplantation cyclophosphamide vs. antithymocyte globulin after RIC regimen allo-HCT: first analysis of a prospective randomized multicenter trial in recipients of 10/10 matched donors [abstract O001]. Bone Marrow Transplant. 56 (Suppl. 1), 12 (2021).
Holtan, S. G. et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for graft-versus-host disease (GVHD) prophylaxis in reduced intensity conditioning: results from phase III BMT CTN 1703 [abstract]. Blood 140 (Suppl. 2), LBA-4 (2022).
Luznik, L. et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J. Clin. Oncol. 40, 356–368 (2022).
Atilla, E., Atilla, P. A., Bozdag, S. C. & Demirer, T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 45, 403–411 (2017).
Duléry, R. et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol. 3, 250–259 (2021).
El-Cheikh, J. et al. Impact of adding antithymocyte globulin to posttransplantation cyclophosphamide in haploidentical stem-cell transplantation. Clin. Lymphoma Myeloma Leuk. 20, 617–623 (2020).
Pérez-Simón, J. A. et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood 100, 3121–3127 (2002).
Kottaridis, P. D. et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 96, 2419–2425 (2000).
Chakraverty, R. et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood 99, 1071–1078 (2002).
Malladi, R. K. et al. Alemtuzumab markedly reduces chronic GVHD without affecting overall survival in reduced-intensity conditioning sibling allo-SCT for adults with AML. Bone Marrow Transplant. 43, 709–715 (2009).
van Besien, K. et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol. Blood Marrow Transplant. 15, 610–617 (2009).
Chen, Y.-B. et al. Vedolizumab for prophylaxis of lower gastrointestinal acute graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation from unrelated donors: a phase 3, randomized, double-blind, placebo-controlled, multicenter study. Presented at the 49th EBMT Annual Meeting (2023).
Ponce, D. M. et al. Guidelines for the prevention and management of graft-versus-host disease after cord blood transplantation. Transplant. Cell Ther. 27, 540–544 (2021).
Bacigalupo, A. et al. Steroid treatment of acute graft-versus-host disease grade I: a randomized trial. Haematologica 102, 2125–2133 (2017).
Mielcarek, M. et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood 113, 2888–2894 (2009).
Mielcarek, M. et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica 100, 842–848 (2015).
Hockenbery, D. M. et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood 109, 4557–4563 (2007).
McDonald, G. B. et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology 115, 28–35 (1998).
Mohty, M. et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood 136, 1903–1906 (2020).
MacMillan, M. L., DeFor, T. E. & Weisdorf, D. J. The best endpoint for acute GVHD treatment trials. Blood 115, 5412–5417 (2010).
Alousi, A. M. et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 114, 511–517 (2009).
Bolanos-Meade, J. et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood 124, 3221–3227 (2014).
Couriel, D. R. et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol. Blood Marrow Transplant. 15, 1555–1562 (2009).
Zeiser, R. et al. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): a randomised, multicentre, double-blind, phase 3 trial. Lancet Haematol. 9, e14–e25 (2022).
Pidala, J. et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood 135, 97–107 (2020).
Martin, P. J. et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 18, 1150–1163 (2012).
Martin, P. J. How I treat steroid-refractory acute graft-versus-host disease. Blood 135, 1630–1638 (2020).
Malard, F., Huang, X. J. & Sim, J. P. Y. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia 34, 1229–1240 (2020).
Zeiser, R. et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 29, 2062–2068 (2015).
Jagasia, M. et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label, phase 2 trial. Blood https://doi.org/10.1182/blood.2020004823 (2020).
Zeiser, R. et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 382, 1800–1810 (2020). To our knowledge, the first randomized study showing the superiority of a second-line treatment, ruxolitinib, for steroid-refractory acute GVHD.
MacMillan, M. L. et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol. Blood Marrow Transplant. 21, 761–767 (2015).
Marchetti, O. et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 47, 846–854 (2012).
Yuan, Y. et al. High incidence of resistant breakthrough invasive fungal infections (IFD) in patients treated for acute gastrointestinal graft-versus-host disease (GI GVHD) following allogeneic haematopoietic cell transplantation. Bone Marrow Transplant. 57, 1712–1715 (2022).
Ullmann, A. J. et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356, 335–347 (2007).
Maertens, J. et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 71, 2397–2404 (2016).
Ljungman, P. et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e260–e272 (2019).
Wolfe, D. et al. Letermovir prophylaxis and cytomegalovirus reactivation in adult hematopoietic cell transplant recipients with and without acute graft versus host disease. Cancers https://doi.org/10.3390/cancers13215572 (2021).
Bansal, R. et al. Extended letermovir administration, beyond day 100, is effective for CMV prophylaxis in patients with graft versus host disease. Transplant. Infect. Dis. 23, e13487 (2021).
Boeckh, M., Kim, H. W., Flowers, M. E., Meyers, J. D. & Bowden, R. A. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation – a randomized double-blind placebo-controlled study. Blood 107, 1800–1805 (2006).
Klein, A., Miller, K. B., Sprague, K., DesJardin, J. A. & Snydman, D. R. A randomized, double-blind, placebo-controlled trial of valacyclovir prophylaxis to prevent zoster recurrence from months 4 to 24 after BMT. Bone Marrow Transplant. 46, 294–299 (2011).
Stocker, N. et al. Pre-emptive rituximab treatment for Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes. Bone Marrow Transplant. 55, 586–594 (2020).
Engelhard, D., Mohty, B., de la Camara, R., Cordonnier, C. & Ljungman, P. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transplant. Infect. Dis. 15, 219–232 (2013).
Piñana, J. L. et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional, prospective, observational study. Clin. Infect. Dis. 68, 1894–1903 (2019).
Cesaro, S. et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 36, 1467–1480 (2022).
Machado, C. M. et al. Use of oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 34, 111–114 (2004).
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408 (2022).
Lemaitre, F. et al. Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19. Ther. Drug Monit. 45, 191–199 (2023).
Raanani, P. et al. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J. Clin. Oncol. 27, 770–781 (2009).
Ljungman, P. et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 35, 737–746 (2005).
Cordonnier, C. et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e200–e212 (2019).
Averbuch, D. et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 98, 1826–1835 (2013).
Seibel, M. J., Cooper, M. S. & Zhou, H. Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 1, 59–70 (2013).
Li, J.-X. & Cummins, C. L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 18, 540–557 (2022).
Aaronson, N. K. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
McQuellon, R. P. et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 19, 357–368 (1997).
Lent, L., Hahn, E., Eremenco, S., Webster, K. & Cella, D. Using cross-cultural input to adapt the Functional Assessment of Chronic Illness Therapy (FACIT) scales. Acta Oncol. 38, 695–702 (1999).
Ware, J. Jr., Kosinski, M. & Keller, S. D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care 34, 220–233 (1996).
Lee, S. J. et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 38, 305–310 (2006).
Rashid, N. et al. Late effects of severe acute graft-versus-host disease on quality of life, medical comorbidities, and survival. Transplant. Cell Ther. https://doi.org/10.1016/j.jtct.2022.08.027 (2022).
Grube, M. et al. Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation – results from a single-center observational study. Biol. Blood Marrow Transplant. 22, 1781–1791 (2016).
Boyiadzis, M. et al. Impact of chronic graft-versus-host disease on late relapse and survival on 7,489 patients after myeloablative allogeneic hematopoietic cell transplantation for leukemia. Clin. Cancer Res. 21, 2020–2028 (2015).
Pidala, J., Anasetti, C. & Jim, H. Quality of life after allogeneic hematopoietic cell transplantation. Blood 114, 7–19 (2009).
Brice, L. et al. Predictors of quality of life in allogeneic hematopoietic stem cell transplantation survivors. J. Psychosoc. Oncol. 39, 534–552 (2021).
Norkin, M., Hsu, J. W. & Wingard, J. R. Quality of life, social challenges, and psychosocial support for long-term survivors after allogeneic hematopoietic stem-cell transplantation. Semin. Hematol. 49, 104–109 (2012).
Loberiza, F. R. Jr. et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J. Clin. Oncol. 20, 2118–2126 (2002).
Clark, C. A., Savani, M., Mohty, M. & Savani, B. N. What do we need to know about allogeneic hematopoietic stem cell transplant survivors. Bone Marrow Transplant. 51, 1025–1031 (2016).
Arnhold, V. et al. Corticosteroid treatment impairs epithelial regeneration, limiting intestinal recovery in experimental graft vs host disease [abstract]. Blood 138 (Suppl. 1), 88 (2021).
Hanash, A. M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Lindemans, C. A. et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564 (2015).
Hummel, S. et al. Telomere shortening in enterocytes of patients with uncontrolled acute intestinal graft-versus-host disease. Blood 126, 2518–2521 (2015).
Malard, F., Gaugler, B. & Mohty, M. Faecal microbiota transplantation in patients with haematological malignancies undergoing cellular therapies: from translational research to routine clinical practice. Lancet Haematol. 9, e776–e785 (2022).
Shouval, R., Geva, M., Nagler, A. & Youngster, I. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin. Hematol. Int. 1, 28–35 (2019).
Qiao, X. et al. Safety and efficacy of fecal microbiota transplantation in the treatment of graft-versus-host disease. Bone Marrow Transplant. https://doi.org/10.1038/s41409-022-01824-1 (2022).
Sirohi, B. & Mathew, A. Access to and affordability of cancer medicines: time to focus on the last mile. Lancet Oncol. 22, 1342–1343 (2021).
Bleakley, M. & Riddell, S. R. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat. Rev. Cancer 4, 371–380 (2004).
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813–1826 (2006).
Bacigalupo, A. et al. Defining the intensity of conditioning regimens: working definitions. Biol. Blood Marrow Transplant. 15, 1628–1633 (2009).
Gragert, L. et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US registry. N. Engl. J. Med. 371, 339–348 (2014).
Kanakry, C. G., Fuchs, E. J. & Luznik, L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat. Rev. Clin. Oncol. 13, 10–24 (2016).
Acknowledgements
The authors are grateful to J. V. Melo (University of Adelaide, Australia) for her critical review of this manuscript.
Author information
Authors and Affiliations
Contributions
Introduction (F.M., M.M.); Epidemiology (F.M. and M.M.); Mechanisms/pathophysiology (F.M., E.H. and M.M.); Diagnosis, screening and prevention (F.M., B.M.S. and M.M.); Management (F.M. and M.M.); Quality of life (F.M., H.H. and M.M.); Outlook (F.M. and M.M.); Overview of the Primer (F.M. and M.M.).
Corresponding authors
Ethics declarations
Competing interests
F.M. reports honoraria from Therakos/Mallinckrodt, Janssen, Biocodex, Sanofi, Jazz Pharmaceuticals, Gilead, Novartis and Astellas, all outside the scope of this work. E.H. reports grants and honoraria from MaaT Pharma, Pharmabiome, Medac and Novartis, all outside the scope of this work. M.M. reports grants, lecture honoraria and research support from Adaptive Biotechnologies, Amgen, Astellas, BMS-Celgene, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, Takeda and Sanofi, all outside the scope of this work. The other authors do not declare any competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks J. Wagner, R. Champlin, Y. Atsuta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 4.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malard, F., Holler, E., Sandmaier, B.M. et al. Acute graft-versus-host disease. Nat Rev Dis Primers 9, 27 (2023). https://doi.org/10.1038/s41572-023-00438-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-023-00438-1
This article is cited by
-
Altered microbial bile acid metabolism exacerbates T cell-driven inflammation during graft-versus-host disease
Nature Microbiology (2024)
-
Post-transplant cyclophosphamide, calcineurin inhibitor, and mycophenolate mofetil compared to anti-thymocyte globulin, calcineurin inhibitor, and methotrexate combinations as graft-versus-host disease prophylaxis post allogeneic stem cell transplantation from sibling and unrelated donors in patients with acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2024)