Abstract
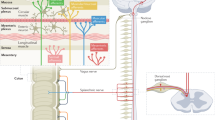
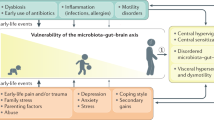
Gastrointestinal (GI) pain — a form of visceral pain — is common in some disorders, such as irritable bowel syndrome, Crohn’s disease and pancreatitis. However, identifying the cause of GI pain frequently represents a diagnostic challenge as the clinical presentation is often blurred by concomitant autonomic and somatic symptoms. In addition, GI pain can be nociceptive, neuropathic and associated with cancer, but in many cases multiple aetiologies coexist in an individual patient. Mechanisms of GI pain are complex and include both peripheral and central sensitization and the involvement of the autonomic nervous system, which has a role in generating the symptoms that frequently accompany pain. Treatment of GI pain depends on the precise type of pain and the primary disorder in the patient but can include, for example, pharmacological therapy, cognitive behavioural therapies, invasive surgical procedures, endoscopic procedures and lifestyle alterations. Owing to the major differences between organ involvement, disease mechanisms and individual factors, treatment always needs to be personalized and some data suggest that phenotyping and subsequent individual management of GI pain might be options in the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gebhart, G. F. & Bielefeldt, K. Physiology of visceral pain. Compr. Physiol. 6, 1609–1633 (2016). This review provides an overview of the basic visceral pain mechanisms.
Aziz, Q. et al. The IASP classification of chronic pain for ICD-11. Pain 160, 69–76 (2019).
Treede, R.-D. et al. A classification of chronic pain for ICD-11. Pain 156, 1003–1007 (2015).
Kosek, E. et al. Do we need a third mechanistic descriptor for chronic pain states? Pain 157, 1382–1386 (2016).
Mayer, E. A., Gupta, A., Kilpatrick, L. A. & Hong, J.-Y. Imaging brain mechanisms in chronic visceral pain. Pain 156, S50–S63 (2015).
Meerveld, B. G.-V. & Johnson, A. C. Mechanisms of stress-induced visceral pain. J. Neurogastroenterol. Motil. 24, 7–18 (2018).
Kloner, R. A. & Chaitman, B. Angina and its management. J. Cardiovasc. Pharmacol. Ther. 22, 199–209 (2017).
Picard, F., Sayah, N., Spagnoli, V., Adjedj, J. & Varenne, O. Vasospastic angina: a literature review of current evidence. Arch. Cardiovasc. Dis. 112, 44–55 (2019).
Stavropoulos, S. N., Friedel, D., Modayil, R. & Parkman, H. P. Diagnosis and management of esophageal achalasia. Br. Med. J. 354, i2785 (2016).
Kavitt, R. T., Lipowska, A. M., Anyane-Yeboa, A. & Gralnek, I. M. Diagnosis and treatment of peptic ulcer disease. Am. J. Med. 132, 447–456 (2019).
Drewes, A. M. et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 17, 720–731 (2017).
Wilkins, T., Agabin, E., Varghese, J. & Talukder, A. Gallbladder dysfunction: cholecystitis, choledocholithiasis, cholangitis, and biliary dyskinesia. Prim. Care Clin. Off. Pract. 44, 575–597 (2017).
Mayans, L. Nephrolithiasis. Prim. Care Clin. Off. Pract. 46, 203–212 (2019).
Zheng, P., Zhang, W., Leng, J. & Lang, J. Research on central sensitization of endometriosis-associated pain: a systematic review of the literature. J. Pain Res. 12, 1447–1456 (2019).
Zaidi, N., Thomas, D. & Chughtai, B. Management of chronic prostatitis (CP). Curr. Urol. Rep. 19, 88 (2018).
Enck, P. et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2, 16014 (2016). This review summarizes functional (primary) GI pain with IBS as an example.
Falcone, T. & Flyckt, R. Clinical management of endometriosis. Obstet. Gynecol. 131, 557–571 (2018).
Pape, J., Falconi, G., De Mattos Lourenco, T. R., Doumouchtsis, S. K. & Betschart, C. Variations in bladder pain syndrome/interstitial cystitis (IC) definitions, pathogenesis, diagnostics and treatment: a systematic review and evaluation of national and international guidelines. Int. Urogynecol. J. 30, 1795–1805 (2019).
Passavanti, M. B. et al. Chronic pelvic pain: assessment, evaluation, and objectivation. Pain Res. Treat. 2017, 9472925 (2017).
Sorensen, J., Bautsita, K., Lamvu, G. & Feranec, J. Evaluation and treatment of female sexual pain: a clinical review. Cureus 10, e2379 (2018).
Sikandar, S. & Dickenson, A. H. Visceral pain: the ins and outs, the ups and downs. Curr. Opin. Support. Palliat. Care 6, 17–26 (2012).
Kennedy, P. J., Cryan, J. F., Dinan, T. G. & Clarke, G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J. Gastroenterol. 20, 14105–14125 (2014).
Giamberardino, M. A. & Vecchiet, L. Visceral pain, referred hyperalgesia and outcome: new concepts. Eur. J. Anaesthesiol. Suppl. 10, 61–66 (1995).
Sandler, R. S., Stewart, W. F., Liberman, J. N., Ricci, J. A. & Zorich, N. L. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig. Dis. Sci. 45, 1166–1171 (2000).
Russo, M. W. et al. Digestive and liver diseases statistics, 2004. Gastroenterology 126, 1448–1453 (2004).
Hotopf, M., Carr, S., Mayou, R., Wadsworth, M. & Wessely, S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. Br. Med. J. 316, 1196–1200 (1998).
Drossman, D. A. et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig. Dis. Sci. 38, 1569–1580 (1993).
Bielefeldt, K., Davis, B. & Binion, D. G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 15, 778–788 (2009).
Torres, J., Mehandru, S., Colombel, J.-F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Kleeff, J. et al. Chronic pancreatitis. Nat. Rev. Dis. Primer 3, 17060 (2017).
Olesen, S. S. et al. Towards a neurobiological understanding of pain in chronic pancreatitis: mechanisms and implications for treatment. Pain Rep. 2, e625 (2017). This review provides an overview of visceral pain mechanisms with chronic pancreatitis as an example.
Keane, M. G., Horsfall, L., Rait, G. & Pereira, S. P. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 4, e005720 (2014).
Drewes, A. M. et al. Pain in pancreatic ductal adenocarcinoma: a multidisciplinary, international guideline for optimized management. Pancreatology 18, 446–457 (2018). This paper provides a framework for multidisciplinary cancer pain management.
Rome Foundation. Rome IV online collection https://romeonline.org/product/rome-iv-online-collection-all-six-rome-iv-books-online/ (2019).
Van den Houte, K. et al. Prevalence and impact of self-reported irritable bowel symptoms in the general population. United European Gastroenterol. J. 7, 307–315 (2019).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4 (2012).
Costa, M. & Brookes, S. J. The enteric nervous system. Am. J. Gastroenterol. 89, S129–S137 (1994).
Brierley, S. M. & Blackshaw, L. A. in Chronic Abdominal and Visceral Pain, Theory and Practice (eds Pasricha, P. J., Willis, W. D. & Gebhart, G. F.) 45–66 (Taylor & Francis, 2006).
Traub, R. J. in Chronic Abdominal and Visceral Pain, Theory and Practice (eds Pasricha, P. J., Willis, W. D. & Gebhart, G. F.) 85–106 (Taylor & Francis, 2006).
Al-Chaer, E. D. &Willis, W. D. in Chronic Abdominal and Visceral Pain, Theory and Practice (eds Pasricha, P. J., Willis, W. D. & Gebhart, G. F.) 33–44 (Taylor & Francis, 2006).
Scholz, J. & Woolf, C. J. Can we conquer pain? Nat. Neurosci. 5, 1062–1067 (2002).
Drewes, A. M. et al. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut 57, 1616–1627 (2008).
Petersen, P., Gao, C., Arendt-Nielsen, L., Gregersen, H. & Drewes, A. M. Pain intensity and biomechanical responses during ramp-controlled distension of the human rectum. Dig. Dis. Sci. 48, 1310–1316 (2003).
Greenwood-Van Meerveld, B., Prusator, D. K. & Johnson, A. C. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am. J. Physiol.Gastrointest. Liver Physiol. 308, G885–G903 (2015).
Brierley, S. M. & Linden, D. R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611–627 (2014). This paper illustrates sensitization in GI diseases.
Knowles, C. H. & Aziz, Q. Basic and clinical aspects of gastrointestinal pain. Pain 141, 191–209 (2009).
Bielefeldt, K. in Chronic Abdominal and Visceral Pain, Theory and Practice (eds Pasricha, P. J., Willis, W. D. & Gebhart, G. F.) 67–84 (Taylor & Francis, 2006).
Mayer, E. A. et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol. Motil. 21, 579–596 (2009).
Willis, W. D. & Westlund, K. N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 14, 2–31 (1997).
Drewes, A. M. et al. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp. Brain Res. 174, 443–452 (2006).
Bonaz, B., Sinniger, V. & Pellissier, S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol. Motil. 28, 455–462 (2016). This review provides an overview of vagal function in GI disorders.
Sengupta, J. N. & Gebhart, G. F. in Physiology of the Gastrointestinal Tract (eds Johnson, L. R., Alpers, D. H., Christensen, J., Jacobson, E. D. & Walsh, J. H.) 483–519 (Raven Press, 1994).
Ren, K., Randich, A. & Gebhart, G. F. Effects of electrical stimulation of vagal afferents on spinothalamic tract cells in the rat. Pain 44, 311–319 (1991).
Heinricher, M. M., Tavares, I., Leith, J. L. & Lumb, B. M. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 60, 214–225 (2009).
Vanegas, H. & Schaible, H.-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res. Brain Res. Rev. 46, 295–309 (2004).
Arendt-Nielsen, L. et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 22, 216–241 (2018). This paper explains central sensitization in chronic (including GI) pain.
Wilder-Smith, C. H., Schindler, D., Lovblad, K., Redmond, S. M. & Nirkko, A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53, 1595–1601 (2004).
Olesen, S. S. et al. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 8, 724–730 (2010).
Kuhlmann, L. et al. Patient and disease characteristics associate with sensory testing results in chronic pancreatitis. Clin. J. Pain 35, 786–793 (2019).
Dimcevski, G. et al. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology 132, 1546–1556 (2007).
Olesen, S. S., Frøkjær, J. B., Lelic, D., Valeriani, M. & Drewes, A. M. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology 10, 742–751 (2010).
Lelic, D., Olesen, S. S., Hansen, T. M., Valeriani, M. & Drewes, A. M. Functional reorganization of brain networks in patients with painful chronic pancreatitis. Eur. J. Pain 18, 968–977 (2014).
Frokjaer, J. B. et al. Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut 60, 1554–1562 (2011).
Frøkjær, J. B. et al. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 10, 434–438.e1 (2012).
Wells, C. I., O’Grady, G. & Bissett, I. P. Colonic electromechanical abnormalities underlying post-operative ileus: a systematic and critical review. J. Neurogastroenterol. Motil. 25, 36–47 (2019).
Wang, X., Gong, Z., Wu, K., Wang, B. & Yuang, Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 18, 57–62 (2003).
Morales-Soto, W. & Gulbransen, B. D. Enteric glia: a new player in abdominal pain. Cell. Mol. Gastroenterol. Hepatol. 7, 433–445 (2019).
Pokusaeva, K. et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29, e12904 (2017).
Schemann, M., Frieling, T. & Enck, P. To learn, to remember, to forget—how smart is the gut? Acta Physiol. 7, e13296 (2019).
Andrews, P. L. R. & Sanger, G. J. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr. Opin. Pharmacol. 2, 650–656 (2002).
Dinan, T. G. & Cryan, J. F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89 (2017).
Mayer, E. A., Tillisch, K. & Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938 (2015). This paper reviews the function of microbiota in the brain and pain processing.
Luczynski, P. et al. Microbiota regulates visceral pain in the mouse. eLife 6, e25887 (2017).
Kannampalli, P. et al. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 26, 1694–1704 (2014).
Heiss, C. N. & Olofsson, L. E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 31, e12684 (2019).
Cervero, F., Connell, L. A. & Lawson, S. N. Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J. Comp. Neurol. 228, 422–431 (1984).
Jänig, W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol. Psychol. 42, 29–51 (1996).
Sugiura, Y., Terui, N. & Hosoya, Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J. Neurophysiol. 62, 834–840 (1989).
Arendt-Nielsen, L., Laursen, R. J. & Drewes, A. M. Referred pain as an indicator for neural plasticity. Prog. Brain Res. 129, 343–356 (2000).
Qin, C., Malykhina, A. P., Akbarali, H. I. & Foreman, R. D. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 129, 1967–1978 (2005).
Grundy, L. & Brierley, S. M. Cross-organ sensitization between the colon and bladder: to pee or not to pee? Am. J. Physiol. Gastrointest. Liver Physiol. 314, G301–G308 (2018). This article describes the complex interactions in cross-organ sensitization.
Garrison, D. W., Chandler, M. J. & Foreman, R. D. Viscerosomatic convergence onto feline spinal neurons from esophagus, heart and somatic fields: effects of inflammation. Pain 49, 373–382 (1992).
Brock, C. et al. Central pain mechanisms following combined acid and capsaicin perfusion of the human oesophagus. Eur. J. Pain 14, 273–281 (2010).
Sami, S. A. K. et al. Cortical changes to experimental sensitization of the human esophagus. Neuroscience 140, 269–279 (2006).
Giamberardino, M. A. et al. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain 151, 307–322 (2010).
Olivar, T. & Laird, J. M. Differential effects of N-methyl-D-aspartate receptor blockade on nociceptive somatic and visceral reflexes. Pain 79, 67–73 (1999).
Colloca, L. et al. Neuropathic pain. Nat. Rev. Dis. Primer 3, 17002 (2017).
Drewes, A. in Pain from Unrelated Treatment (ed. Jarrell, J.) 55–69 (Wolters Kluwer, 2018).
Søfteland, E. et al. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J. Diabetes Complic. 28, 370–377 (2014).
Frøkjaerl, J. B. et al. Gastrointestinal symptoms in type-1 diabetes: is it all about brain plasticity? Eur. J. Pain 15, 249–257 (2011).
Treede, R.-D. et al. Chronic pain as a symptom or a disease. Pain 160, 19–27 (2019).
Mearin, F. et al. Bowel disorders. Gastroenterology 150, 1393–1407.e5 (2016).
Chang, L. et al. Functional bowel disorders: a roadmap to guide the next generation of research. Gastroenterology 154, 723–735 (2018).
Paice, J. A. et al. AAPT diagnostic criteria for chronic cancer pain conditions. J. Pain 18, 233–246 (2017).
Ceyhan, G. O. et al. Pancreatic neuropathy and neuropathic pain — a comprehensive pathomorphological study of 546 cases. Gastroenterology 136, 177–186.e1 (2009).
Sharma, M., Simpson, K., Bennett, M. & Gupta, S. (eds) Practical Management of Complex Cancer Pain (Oxford Univ. Press, 2014).
Brown, M. & Farquhar-Smith, P. Pain in cancer survivors; filling in the gaps. Br. J. Anaesth. 119, 723–736 (2017).
Drewes, A. M. et al. Experimental pain in the stomach: a model based on electrical stimulation guided by gastroscopy. Gut 41, 753–757 (1997).
Graven-Nielsen, T. & Arendt-Nielsen, L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat. Rev. Rheumatol. 6, 599–606 (2010).
Mertz, H., Fullerton, S., Naliboff, B. & Mayer, E. A. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut 42, 814–822 (1998).
Stawowy, M., Funch-Jensen, P., Arendt-Nielsen, L. & Drewes, A. M. Somatosensory changes in the referred pain area in patients with cholecystolithiasis. Eur. J. Gastroenterol. Hepatol. 17, 865–870 (2005).
Bielefeldt, K., Christianson, J. A. & Davis, B. M. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterol. Motil. 17, 488–499 (2005).
Talley, N. J., Zinsmeister, A. R. & Melton, L. J. Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am. J. Epidemiol. 142, 76–83 (1995).
Vardeh, D., Mannion, R. J. & Woolf, C. J. Toward a mechanism-based approach to pain diagnosis. J. Pain 17, T50–T69 (2016).
Mujagic, Z. et al. Systematic review: instruments to assess abdominal pain in irritable bowel syndrome. Aliment. Pharmacol. Ther. 42, 1064–1081 (2015).
Olesen, A. E., Farmer, A. D., Olesen, S. S., Aziz, Q. & Drewes, A. M. Management of chronic visceral pain. Pain Manag. 6, 469–486 (2016).
Farrar, J. T. Jr, Y., J. P., LaMoreaux, L., Werth, J. L. & Poole, R. M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158 (2001).
Akbar, A. et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 59, 767–774 (2010).
Olesen, S. S., Bouwense, S. A. W., Wilder-Smith, O. H. G., van Goor, H. & Drewes, A. M. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology 141, 536–543 (2011).
Teo, K., Johnson, M. H., Drewes, A. M. & Windsor, J. A. A comprehensive pain assessment tool (COMPAT) for chronic pancreatitis: development, face validation and pilot evaluation. Pancreatology 17, 706–719 (2017).
Spiegel, B. et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment. Pharmacol. Ther. 30, 1159–1170 (2009).
Spiegel, B. M. R. et al. Characterizing abdominal pain in IBS: guidance for study inclusion criteria, outcome measurement and clinical practice. Aliment. Pharmacol. Ther. 32, 1192–1202 (2010).
Olesen, S. S. et al. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLOS ONE 8, e57963 (2013).
Olesen, S. S., van Goor, H., Bouwense, S. A. W., Wilder-Smith, O. H. G. & Drewes, A. M. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg. Anesth. Pain Med. 37, 530–536 (2012).
Bouin, M. et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 122, 1771–1777 (2002).
Bouwense, S. A. et al. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLOS ONE 8, e55460 (2013).
Kuhlmann, L., Olesen, S. S., Olesen, A. E., Arendt-Nielsen, L. & Drewes, A. M. Mechanism-based pain management in chronic pancreatitis – is it time for a paradigm shift? Expert Rev. Clin. Pharmacol. 12, 249–258 (2019).
Botha, C. et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 64, 611–617 (2015).
Fukudo, S. et al. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage 47, 946–951 (2009).
Tanaka, Y. et al. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLOS ONE 11, e0157347 (2016).
Lee, I.-S., Wang, H., Chae, Y., Preissl, H. & Enck, P. Functional neuroimaging studies in functional dyspepsia patients: a systematic review. Neurogastroenterol. Motil. 28, 793–805 (2016).
Lelic, D. et al. Brain networks encoding rectal sensation in type 1 diabetes. Neuroscience 237, 96–105 (2013).
Farmer, A. D. & Aziz, Q. Mechanisms of visceral pain in health and functional gastrointestinal disorders. Scand. J. Pain 5, 51–60 (2014).
Srinath, A., Young, E. & Szigethy, E. Pain management in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 20, 2433–2449 (2014).
Beckers, A. B. et al. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: a review for the gastroenterologist. Neurogastroenterol. Motil. 29, e13013 (2017).
Sharpstone, D. & Colin-Jones, D. G. Chronic, non-visceral abdominal pain. Gut 35, 833–836 (1994).
[No authors listed] Abdominal wall tenderness test: could Carnett cut costs? Lancet 337, 1134 (1991).
Drenth, J. P. & van der Meer, J. W. Hereditary periodic fever. N. Engl. J. Med. 345, 1748–1757 (2001).
Kim, E. N. et al. Median arcuate ligament syndrome — review of this rare disease. JAMA Surg. 151, 471–477 (2016).
Sato, Y. & Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 8, 255–263 (2015).
Longhurst, H. & Cicardi, M. Hereditary angio-oedema. Lancet 379, 474–481 (2012).
Bissell, D. M., Anderson, K. E. & Bonkovsky, H. L. Porphyria. N. Engl. J. Med. 377, 862–872 (2017).
McQuay, H. J., Derry, S., Eccleston, C., Wiffen, P. J. & Moore, A. R. Evidence for analgesic effect in acute pain—50 years on. Pain 153, 1364–1367 (2012).
Hermanns, T. et al. Is there a role for tamsulosin in the treatment of distal ureteral stones of 7 mm or less? Results of a randomised, double-blind, placebo-controlled trial. Eur. Urol. 56, 407–412 (2009).
Ford, A. C. et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. Br. Med. J. 337, a2313 (2008).
Schug, S. A. & Goddard, C. Recent advances in the pharmacological management of acute and chronic pain. Ann. Palliat. Med. 3, 263–275 (2014).
O’Brien, T. et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur. J. Pain 21, 3–19 (2017).
Szigethy, E., Knisely, M. & Drossman, D. Opioid misuse in gastroenterology and non-opioid management of abdominal pain. Nat. Rev. Gastroenterol. Hepatol. 15, 168–180 (2017).
Hacker, K. E., Reynolds, R. K. & Uppal, S. Ongoing strategies and updates on pain management in gynecologic oncology patients. Gynecol. Oncol. 149, 410–419 (2018).
Portenoy, R. K. Treatment of cancer pain. Lancet 377, 2236–2247 (2011).
Enck, P., Klosterhalfen, S. & Weimer, K. Unsolved, forgotten, and ignored features of the placebo response in medicine. Clin. Ther. 39, 458–468 (2017).
Vase, L. & Wartolowska, K. Pain, placebo, and test of treatment efficacy: a narrative review. Br. J. Anaesth. 123, e254–e262 (2019).
Drewes, A. M. et al. Controversies on the endoscopic and surgical management of pain in patients with chronic pancreatitis: pros and cons! Gut 68, 1343–1351 (2019).
Johnson, A. C. & Greenwood-Van Meerveld, B. The pharmacology of visceral pain. Adv. Pharmacol. 75, 273–301 (2016).
Drossman, D. A. et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome Foundation Working Team report. Gastroenterology 154, 1140–1171.e1 (2018). This report reviews the pharmacological mechanisms when treating functional visceral pain.
Colombel, J.-F., Shin, A. & Gibson, P. R. AGA clinical practice update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Clin. Gastroenterol. Hepatol. 17, 380–390.e1 (2019).
Edwards, R. R. et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 157, 1851–1871 (2016).
Camilleri, M., Lembo, A. & Katzka, D. A. Opioids in gastroenterology: treating adverse effects and creating therapeutic benefits. Clin. Gastroenterol. Hepatol. 15, 1338–1349 (2017).
Kilgallon, E. et al. Chronic continuous abdominal pain: evaluation of diagnostic features, iatrogenesis and drug treatments in a cohort of 103 patients. Aliment. Pharmacol. Ther. 49, 1282–1292 (2019).
Iskandar, H. N. et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J. Clin. Gastroenterol. 48, 423–429 (2014).
Saito, Y. A. et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome. Aliment. Pharmacol. Ther. 49, 389–397 (2019).
Muthuraman, A., Singh, N., Jaggi, A. S. & Ramesh, M. Drug therapy of neuropathic pain: current developments and future perspectives. Curr. Drug Targets 15, 210–253 (2014).
Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 18, 2483 (2017).
Tack, J. et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin. Gastroenterol. Hepatol. 14, 385–392.e4 (2016).
Camilleri, M. & Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66, 966–974 (2017).
Han, S. et al. Discovery of APD371: identification of a highly potent and selective CB2 agonist for the treatment of chronic pain. ACS Med. Chem. Lett. 8, 1309–1313 (2017).
Ahmed, W. & Katz, S. Therapeutic use of cannabis in inflammatory bowel disease. Gastroenterol. Hepatol. 12, 668–679 (2016).
Camilleri, M. Cannabinoids and gastrointestinal motility: pharmacology, clinical effects, and potential therapeutics in humans. Neurogastroenterol. Motil. 30, e13370 (2018).
Olesen, A. E. et al. The absorption profile of pregabalin in chronic pancreatitis. Basic Clin. Pharmacol. Toxicol. 111, 385–390 (2012).
Aghazadeh-Habashi, A. & Jamali, F. Pharmacokinetics of meloxicam administered as regular and fast dissolving formulations to the rat: influence of gastrointestinal dysfunction on the relative bioavailability of two formulations. Eur. J. Pharm. Biopharm. 70, 889–894 (2008).
Li, J. et al. Prediction of drug disposition in diabetic patients by means of a physiologically based pharmacokinetic model. Clin. Pharmacokinet. 54, 179–193 (2015).
Smith, B. S., Yogaratnam, D., Levasseur-Franklin, K. E., Forni, A. & Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 141, 1327–1336 (2012).
Olesen, A. E., Brokjaer, A., Fisher, I. W. & Larsen, I. M. Pharmacological challenges in chronic pancreatitis. World J. Gastroenterol. 19, 7302–7307 (2013).
Olausson, E. A. et al. Postprandial plasma glucose response and gastrointestinal symptom severity in patients with diabetic gastroparesis. J. Diabetes Sci. Technol. 8, 881–888 (2014).
Farmer, A. D. et al. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United European Gastroenterol. J. 7, 7–20 (2019).
Hatton, G. B., Madla, C. M., Rabbie, S. C. & Basit, A. W. All disease begins in the gut: influence of gastrointestinal disorders and surgery on oral drug performance. Int. J. Pharm. 548, 408–422 (2018).
Tennant, F. Why oral opioids may not be effective in a subset of chronic pain patients. Postgrad. Med. 128, 18–22 (2016).
Leppert, W., Malec-Milewska, M., Zajaczkowska, R. & Wordliczek, J. Transdermal and topical drug administration in the treatment of pain. Molecules 23, 1–16 (2018).
Brock, C. et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 72, 1847–1865 (2012).
Poulsen, J. L., Brock, C., Olesen, A. E., Nilsson, M. & Drewes, A. M. Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Ther. Adv. Gastroenterol. 8, 360–372 (2015).
Farmer, A. D. et al. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 3, 203–212 (2018).
Dumonceau, J.-M. et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut 56, 545–552 (2007).
Chandra, A. & Quinones-Baldrich, W. J. Chronic mesenteric ischemia: how to select patients for invasive treatment. Semin. Vasc. Surg. 23, 21–28 (2010).
Uden, S. et al. Antioxidant therapy for recurrent pancreatitis: biochemical profiles in a placebo-controlled trial. Aliment. Pharmacol. Ther. 6, 229–240 (1992).
Rustagi, T. & Njei, B. Antioxidant therapy for pain reduction in patients with chronic pancreatitis: a systematic review and meta-analysis. Pancreas 44, 812–818 (2015).
Mitchell, H., Porter, J., Gibson, P. R., Barrett, J. & Garg, M. Review article: implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 49, 124–139 (2019).
Mekhail, N. A. et al. Clinical applications of neurostimulation: forty years later. Pain Pract. 10, 103–112 (2010).
Roy, H., Offiah, I. & Dua, A. Neuromodulation for pelvic and urogenital pain. Brain Sci. 8, 180 (2018).
Rombouts, S. J. E. et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br. J. Surg. 102, 182–193 (2015).
Apkarian, A. V., Baliki, M. N. & Geha, P. Y. Towards a theory of chronic pain. Prog. Neurobiol. 87, 81–97 (2009).
Gallagher, R. M. Chronification to maldynia: biopsychosocial failure of pain homeostasis. Pain Med. 12, 993–995 (2011).
Keefer, L. et al. Centrally mediated disorders of gastrointestinal pain. Gastroenterology 150, 1408–1419 (2016).
Burns, J. W., Johnson, B. J., Mahoney, N., Devine, J. & Pawl, R. Cognitive and physical capacity process variables predict long-term outcome after treatment of chronic pain. J. Consult. Clin. Psychol. 66, 434–439 (1998).
Peters, M. L. Emotional and cognitive influences on pain experience. Mod. Trends Pharmacopsychiatry 30, 138–152 (2015).
Walk, D. & Poliak-Tunis, M. Chronic pain management: an overview of taxonomy, conditions commonly encountered, and assessment. Med. Clin. North Am. 100, 1–16 (2016).
Palsson, O. S. & Whitehead, W. E. Psychological treatments in functional gastrointestinal disorders: a primer for the gastroenterologist. Clin. Gastroenterol. Hepatol. 11, 208–216 (2013).
Berrill, J. W., Sadlier, M., Hood, K. & Green, J. T. Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. J. Crohns Colitis 8, 945–955 (2014).
Ballou, S. & Keefer, L. Psychological interventions for irritable bowel syndrome and inflammatory bowel diseases. Clin. Transl. Gastroenterol. 8, e214 (2017).
Regueiro, M., Greer, J. B. & Szigethy, E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 152, 430–439.e4 (2017).
Kinsinger, S. W. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol. Res. Behav. Manag. 10, 231–237 (2017).
Lee, H. H., Choi, Y. Y. & Choi, M.-G. The efficacy of hypnotherapy in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J. Neurogastroenterol. Motil. 20, 152–162 (2014).
Palsson, O. S. Hypnosis treatment of gastrointestinal disorders: a comprehensive review of the empirical evidence. Am. J. Clin. Hypn. 58, 134–158 (2015).
Flik, C. E. et al. Efficacy of individual and group hypnotherapy in irritable bowel syndrome (IMAGINE): a multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 4, 20–31 (2019).
Aucoin, M., Lalonde-Parsi, M.-J. & Cooley, K. Mindfulness-based therapies in the treatment of functional gastrointestinal disorders: a meta-analysis. Evid. Based Complement. Altern. Med. 2014, 140724 (2014).
Juel, J., Abrahamsen, R., Olesen, S. S. & Drewes, A. M. A pilot-study of hypnotherapy as complementary treatment for pain in chronic pancreatitis. J. Complement. Integr. Med. 15, 20170084 (2018).
Dworkin, R. H. et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 9, 105–121 (2008).
Olesen, S. S. et al. Pain severity reduces life quality in chronic pancreatitis: implications for design of future outcome trials. Pancreatology 14, 497–502 (2014).
McHorney, C. A., Ware, J. E. & Raczek, A. E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 31, 247–263 (1993).
Fitzsimmons, D. et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur. J. Cancer 35, 939–941 (1999).
Wassef, W. et al. Pancreatitis quality of life instrument: a psychometric evaluation. Am. J. Gastroenterol. 111, 1177–1186 (2016).
Eypasch, E. et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br. J. Surg. 82, 216–222 (1995).
Stevens, T. et al. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 10, 186–191.e1 (2012).
Baghdadi, S., Abbas, M. H., Albouz, F. & Ammori, B. J. Systematic review of the role of thoracoscopic splanchnicectomy in palliating the pain of patients with chronic pancreatitis. Surg. Endosc. 22, 580–588 (2008).
Gewandter, J. S. et al. Research design characteristics of published pharmacologic randomized clinical trials for irritable bowel syndrome and chronic pelvic pain conditions: an ACTTION systematic review. J. Pain 19, 717–726 (2018).
Giannetti, E. et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Gastroenterol. 51, e5–e10 (2017).
Guy, W. ECDEU Assessment Manual for Psychopharmacology. (U.S. Department of Health, Education, and Welfare, 1976).
Boom, M. et al. Fentanyl utility function: a risk-benefit composite of pain relief and breathing responses. Anesthesiology 119, 663–674 (2013).
Roozekrans, M. et al. Benefit versus severe side effects of opioid analgesia. Anesthesiology 128, 932–942 (2018).
Olesen, A. E. et al. A pragmatic utility function to describe the risk-benefit composite of opioid and nonopioid analgesic medication. J. Pharmacol. Exp. Ther. 371, 1–6 (2018).
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Tack, J. et al. The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 66, 1403–1413 (2017).
Wouters, M. M. et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150, 875–887.e9 (2016).
Kovacic, K. et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol. Hepatol. 2, 727–737 (2017).
Aroniadis, O. C., Drossman, D. A. & Simrén, M. A perspective on brain–gut communication: the American Gastroenterology Association and American Psychosomatic Society Joint Symposium on Brain–Gut Interactions and the Intestinal Microenvironment. Psychosom. Med. 79, 847–856 (2017).
Pusceddu, M. M. & Gareau, M. G. Visceral pain: gut microbiota, a new hope? J. Biomed. Sci. 25, 73 (2018).
Oʼ Mahony, S. M., Dinan, T. G. & Cryan, J. F. The gut microbiota as a key regulator of visceral pain. Pain 158, S19–S28 (2017).
Crouzet, L. et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 25, e272–e282 (2013).
Ringel, Y. The gut microbiome in irritable bowel syndrome and other functional bowel disorders. Gastroenterol. Clin. North Am. 46, 91–101 (2017).
Hungin, A. P. S. et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice—an evidence-based international guide. Aliment. Pharmacol. Ther. 38, 864–886 (2013).
Author information
Authors and Affiliations
Contributions
Introduction (A.M.D. and E.S.); Epidemiology (S.S.O. and A.D.F.); Mechanisms/pathophysiology (A.M.D. and V.R.); Diagnosis, screening and prevention (A.M.D., S.S.O. and A.D.F.); Management (A.E.O., E.S., V.R., S.S.O. and A.M.D.); Quality of life (E.S. and S.S.O.); Outlook (S.S.O., A.M.D. and A.E.O.); Overview of Primer (A.M.D.).
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks S. Fukudo, J. Jarrell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drewes, A.M., Olesen, A.E., Farmer, A.D. et al. Gastrointestinal pain. Nat Rev Dis Primers 6, 1 (2020). https://doi.org/10.1038/s41572-019-0135-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-019-0135-7
This article is cited by
-
Spanish transcultural adaptation of the 4AT score for the evaluation of delirium in the emergency department: a prospective diagnostic test accuracy study
BMC Nursing (2024)
-
Susceptibility to acute cognitive dysfunction in aged mice is underpinned by reduced white matter integrity and microgliosis
Communications Biology (2024)
-
mRNA nuclear retention reduces AMPAR expression and promotes autistic behavior in UBE3A-overexpressing mice
EMBO Reports (2024)
-
The structure of psychiatric comorbidity without selection and assortative mating
Translational Psychiatry (2024)
-
Structural models of genome-wide covariance identify multiple common dimensions in autism
Nature Communications (2024)