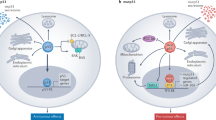
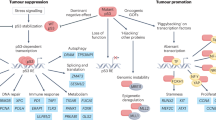
Abstract
p53, which is encoded by the most frequently mutated gene in cancer, TP53, is an attractive target for novel cancer therapies. Despite major challenges associated with this approach, several compounds that either augment the activity of wild-type p53 or restore all, or some, of the wild-type functions to p53 mutants are currently being explored. In wild-type TP53 cancer cells, p53 function is often abrogated by overexpression of the negative regulator MDM2, and agents that disrupt p53–MDM2 binding can trigger a robust p53 response, albeit potentially with induction of p53 activity in non-malignant cells. In TP53-mutant cancer cells, compounds that promote the refolding of missense mutant p53 or the translational readthrough of nonsense mutant TP53 might elicit potent cell death. Some of these compounds have been, or are being, tested in clinical trials involving patients with various types of cancer. Nonetheless, no p53-targeting drug has so far been approved for clinical use. Advances in our understanding of p53 biology provide some clues as to the underlying reasons for the variable clinical activity of p53-restoring therapies seen thus far. In this Review, we discuss the intricate interactions between p53 and its cellular and microenvironmental contexts and factors that can influence p53’s activity. We also propose several strategies for improving the clinical efficacy of these agents through the complex perspective of p53 functionality.
Key points
-
A range of cell-intrinsic and cell-extrinsic factors can affect the activity of p53, as exemplified by the regulation of p53 degradation by MDM2–MDM4 (cell-intrinsic), and the effects of cytokine-producing cancer-associated fibroblasts or a metabolite-producing microbiota on p53 levels and function (cell-extrinsic).
-
Activation of wild-type p53 in non-malignant cells can cause substantial toxicity, given that p53 levels are normally tightly controlled by MDM2–MDM4 and that unbridled p53 activity can trigger irreversible cellular demise.
-
The heterogeneity of cancer-driving TP53 mutations and the existence of tumours with wild-type TP53 that nonetheless lack functional p53 creates various forms of p53 dysfunction, which challenges the development of effective p53-reactivating therapies for large groups of patients.
-
Owing to the heterogeneous effects of different TP53 variants and p53 protein aberrations, effective therapies capable of restoring wild-type p53 function will most probably require personalization according to the specific p53 functionality of each patient.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
COSMIC. COSMIC v99, released 28-Nov-23. COSMIC https://cancer.sanger.ac.uk/cosmic (2023).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
Lang, G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
Andrysik, Z. et al. Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity. Genome Res. 27, 1645–1657 (2017).
Fischer, M. Census and evaluation of p53 target genes. Oncogene 36, 3943–3956 (2017).
Kruiswijk, F., Labuschagne, C. F. & Vousden, K. H. p53 in survival, death and metabolic health: a lifeguard with a license to kill. Nat. Rev. Mol. Cell. Biol. 16, 393–405 (2015).
Boutelle, A. M. & Attardi, L. D. p53 and tumor suppression: it takes a network. Trends Cell. Biol. 31, 298–310 (2021).
Vousden, K. H. & Prives, C. Blinded by the light: the growing complexity of p53. Cell 137, 413–431 (2009).
Levine, A. J. The many faces of p53: something for everyone. J. Mol. Cell. Biol. 11, 524–530 (2019).
Marchenko, N. D. & Moll, U. M. Mitochondrial death functions of p53. Mol. Cell. Oncol. 1, e955995 (2014).
Boettcher, S. et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 365, 599–604 (2019).
Diamond, B. et al. Tracking the evolution of therapy-related myeloid neoplasms using chemotherapy signatures. Blood 141, 2359–2371 (2023).
Sabapathy, K. & Lane, D. P. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat. Rev. Clin. Oncol. 15, 13–30 (2018).
Xiong, S. et al. Differential gain-of-function activity of three p53 hotspot mutants in vivo. Cancer Res. 82, 1926–1936 (2022).
Bernard, E. et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 26, 1549–1556 (2020).
Hoyos, D. et al. Fundamental immune–oncogenicity trade-offs define driver mutation fitness. Nature 606, 172–179 (2022).
Klimovich, B. et al. p53 partial loss-of-function mutations sensitize to chemotherapy. Oncogene 41, 1011–1023 (2022).
Miller, L. D. et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc. Natl Acad. Sci. USA 102, 13550–13555 (2005).
Bouaoun, L. et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum. Mutat. 37, 865–876 (2016).
Kratz, C. P. et al. Analysis of the Li-Fraumeni spectrum based on an international germline TP53 variant data set: an International Agency for Research on Cancer TP53 database analysis. JAMA Oncol. 7, 1800–1805 (2021).
Pinto, E. M. et al. XAF1 as a modifier of p53 function and cancer susceptibility. Sci. Adv. 6, eaba3231 (2020).
Leung, J. C. et al. Common activities and predictive gene signature identified for genetic hypomorphs of TP53. Proc. Natl Acad. Sci. USA 120, e2212940120 (2023).
Zhou, W. et al. Full sequencing of TP53 identifies identical mutations within in situ and invasive components in breast cancer suggesting clonal evolution. Mol. Oncol. 3, 214–219 (2009).
Lomakin, A. et al. Spatial genomics maps the structure, nature and evolution of cancer clones. Nature 611, 594–602 (2022).
Galandiuk, S. et al. Field cancerization in the intestinal epithelium of patients with Crohn’s ileocolitis. Gastroenterology 142, 855–864.e8 (2012).
Griffin, R. Differential prognosis of single and multiple TP53 abnormalities in high-count MBL and untreated CLL. Blood Adv. 7, 3169–3179 (2023).
Wong, T. N. et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518, 552–555 (2015).
Lundberg, P. et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 123, 2220–2228 (2014).
Maslah, N. et al. Single-cell analysis reveals selection of TP53-mutated clones after MDM2 inhibition. Blood Adv. 6, 2813–2823 (2022).
Rasche, L. et al. The spatio-temporal evolution of multiple myeloma from baseline to relapse-refractory states. Nat. Commun. 13, 4517 (2022).
Gruber, M. et al. Growth dynamics in naturally progressing chronic lymphocytic leukaemia. Nature 570, 474–479 (2019).
Hakkarainen, M. et al. The clinical picture of ERCC6L2 disease: from bone marrow failure to acute leukemia. Blood 141, 2853–2866 (2023).
Li, C. et al. Whole exome and transcriptome sequencing reveal clonal evolution and exhibit immune-related features in metastatic colorectal tumors. Cell Death Discov. 7, 222 (2021).
Karlsson, K. et al. Deterministic evolution and stringent selection during preneoplasia. Nature 618, 383–393 (2023).
Prokocimer, M., Molchadsky, A. & Rotter, V. Dysfunctional diversity of p53 proteins in adult acute myeloid leukemia: projections on diagnostic workup and therapy. Blood 130, 699–712 (2017).
Oliner, J. D., Saiki, A. Y. & Caenepeel, S. The role of MDM2 amplification and overexpression in tumorigenesis. Cold Spring Harb. Perspect. Med. 6, a026336 (2016).
Sherr, C. J. & Weber, J. D. The ARF/p53 pathway. Curr. Op. Genet. Dev. 10, 94–99 (2000).
Trinidad, A. G. et al. Interaction of p53 with the CCT complex promotes protein folding and wild-type p53 activity. Mol. Cell. 50, 805–817 (2013).
Benor, G. et al. Transcriptional profiling reveals a subset of human breast tumors that retain wt TP53 but display mutant p53-associated features. Mol. Oncol. 14, 1640–1652 (2020).
Tuval, A. et al. Pseudo-mutant p53 is a unique phenotype of DNMT3A-mutated pre-leukemia. Haematologica 107, 2548–2561 (2022).
Haney, S. L. et al. Dnmt3a is a haploinsufficient tumor suppressor in CD8+ peripheral T cell lymphoma. PLoS Genet. 12, e1006334 (2016).
Kodama, M. et al. Expression of mutant type-p53 products in H pylori-associated chronic gastritis. World J. Gastroenterol. 13, 1541–1546 (2007).
Wu, J., Wang, M., Li, X. & Sheng, Y. Conformation changes of p53 proteins in regulation of murine T lymphocyte proliferation. Cell Mol. Biol. Res. 39, 27–31 (1993).
Milner, J. & Watson, J. V. Addition of fresh medium induces cell cycle and conformation changes in p53, a tumour suppressor protein. Oncogene 5, 1683–1690 (1990).
Sasaki, M., Nie, L. & Maki, C. G. MDM2 binding induces a conformational change in p53 that is opposed by heat-shock protein 90 and precedes p53 proteasomal degradation. J. Biol. Chem. 282, 14626–14634 (2007).
Hainaut, P. & Milner, J. A structural role for metal ions in the “wild-type” conformation of the tumor suppressor protein p53. Cancer Res. 53, 1739–1742 (1993).
Hainaut, P. & Milner, J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 53, 4469–4473 (1993).
Furth, N. et al. Down-regulation of LATS kinases alters p53 to promote cell migration. Genes. Dev. 29, 2325–2330 (2015).
Zhang, W. & Deisseroth, A. B. Conformational change of p53 protein in growth factor-stimulated human myelogenous leukemia cells. Leuk. Lymphoma 14, 251–255 (1994).
Zhu, Y. M., Bradbury, D. & Russell, N. Expression of different conformations of p53 in the blast cells of acute myeloblastic leukaemia is related to in vitro growth characteristics. Br. J. Cancer 68, 851–855 (1993).
Rivlin, N. et al. Rescue of embryonic stem cells from cellular transformation by proteomic stabilization of mutant p53 and conversion into WT conformation. Proc. Natl Acad. Sci. USA 111, 7006–7011 (2014).
Blagosklonny, M. V., Toretsky, J. & Neckers, L. Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 11, 933–939 (1995).
Tal, P. et al. Cancer therapeutic approach based on conformational stabilization of mutant P53 protein by small peptides. Oncotarget 7, 11817–11837 (2016).
Bykov, V. J. N., Eriksson, S. E., Bianchi, J. & Wiman, K. G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 18, 89–102 (2018).
Izquierdo, E. et al. Extracellular vesicles and PD-L1 suppress macrophages, inducing therapy resistance in TP53-deficient B-cell malignancies. Blood 139, 3617–3629 (2022).
Tan, X. et al. A protumorigenic secretory pathway activated by p53 deficiency in lung adenocarcinoma. J. Clin. Invest. 131, e137186 (2021).
Maddalena, M. et al. TP53 missense mutations in PDAC are associated with enhanced fibrosis and an immunosuppressive microenvironment. Proc. Natl Acad. Sci. USA 118, e2025631118 (2021).
Arandkar, S. et al. Altered p53 functionality in cancer-associated fibroblasts contributes to their cancer-supporting features. Proc. Natl Acad. Sci. USA 115, 6410–6415 (2018).
Cheteh, E. H. et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. 6, 42 (2020).
Kadosh, E. et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586, 133–138 (2020).
Martins, C. P., Brown-Swigart, L. & Evan, G. I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323–1334 (2006).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Hsiue, E. H. et al. Targeting a neoantigen derived from a common TP53 mutation. Science 371, eabc8697 (2021).
Zhang, W. W. et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther. 29, 160–179 (2018).
Li, Y. et al. Expert consensus on the clinical application of recombinant adenovirus human p53 for head and neck cancers. Int. J. Oral. Sci. 13, 38 (2021).
Nemunaitis, J. et al. Biomarkers predict p53 gene therapy efficacy in recurrent squamous cell carcinoma of the head and neck. Clin. Cancer Res. 15, 7719–7725 (2009).
Bowman, K. E. R. et al. p53-Bad* fusion gene therapy induces apoptosis in vitro and reduces zebrafish tumor burden in hepatocellular carcinoma. Mol. Pharm. 20, 331–340 (2023).
Hassin, O. & Oren, M. Drugging p53 in cancer: one protein, many targets. Nat. Rev. Drug. Discov. 22, 127–144 (2023).
Nishikawa, S. & Iwakuma, T. Drugs targeting p53 mutations with FDA approval and in clinical trials. Cancers 15, 429 (2023).
Daver, N. G. et al. TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discov. 12, 2516–2529 (2022).
Zhu, H. et al. Targeting p53-MDM2 interaction by small-molecule inhibitors: learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 15, 91 (2022).
Kussie, P. H. et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274, 948–953 (1996).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell. 21, 307–315 (2006).
Klein, A. M., de Queiroz, R. M., Venkatesh, D. & Prives, C. The roles and regulation of MDM2 and MDMX: it is not just about p53. Genes. Dev. 35, 575–601 (2021).
Feeley, K. P., Adams, C. M., Mitra, R. & Eischen, C. M. Mdm2 is required for survival and growth of p53-deficient cancer cells. Cancer Res. 77, 3823–3833 (2017).
Adams, C. M. et al. Targeted MDM2 degradation reveals a new vulnerability for p53-inactivated triple-negative breast cancer. Cancer Discov. 13, 1210–1229 (2023).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Yee, K. et al. Murine double minute 2 inhibition alone or with cytarabine in acute myeloid leukemia: results from an idasanutlin phase 1/1b study. Leuk. Res. 100, 106489 (2021).
Konopleva, M. Y. et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 6, 4147–4156 (2022).
Daver, N. G. et al. Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood 141, 1265–1276 (2023).
Napolitano, R. et al. Kevetrin induces apoptosis in TP53 wild-type and mutant acute myeloid leukemia cells. Oncol. Rep. 44, 1561–1573 (2020).
Carvajal, L. A. et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 10, eaao3003 (2018).
Saleh, M. N. et al. Phase 1 trial of ALRN-6924, a dual inhibitor of MDMX and MDM2, in patients with solid tumors and lymphomas bearing wild-type TP53. Clin. Cancer Res. 27, 5236–5247 (2021).
Ueda, K. et al. MDMX acts as a pervasive preleukemic-to-acute myeloid leukemia transition mechanism. Cancer Cell 39, 529–547.e7 (2021).
Peng, X. et al. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis. 4, e881 (2013).
Tessoulin, B. et al. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood 124, 1626–1636 (2014).
Haffo, L. et al. Inhibition of the glutaredoxin and thioredoxin systems and ribonucleotide reductase by mutant p53-targeting compound APR-246. Sci. Rep. 8, 12671 (2018).
Liu, D. S. et al. Inhibiting the system xC−/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 8, 14844 (2017).
Rabik, C. A. & Dolan, M. E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 33, 9–23 (2007).
Mohell, N. et al. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 6, e1794 (2015).
Maslah, N. et al. Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 105, 1539–1551 (2020).
Bally, C. et al. Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk. Res. 38, 751–755 (2014).
Bewersdorf, J. P. et al. Clinical outcomes and characteristics of patients with TP53-mutated acute myeloid leukemia or myelodysplastic syndromes: a single center experience. Leuk. Lymphoma 61, 2180–2190 (2020).
Sallman, D. A. et al. Clonal suppression of TP53 mutant MDS and oligoblastic AML with hypomethylating agent therapy improves overall survival. Blood 132, 1817 (2018).
Montalban-Bravo, G. et al. Genomic context and TP53 allele frequency define clinical outcomes in TP53-mutated myelodysplastic syndromes. Blood Adv. 4, 482–495 (2020).
Cluzeau, T. et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe Francophone des Myélodysplasies (GFM). J. Clin. Oncol. 39, 1575–1583 (2021).
Sallman, D. et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J. Clin. Oncol. 39, 1584–1594 (2021).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT03745716?tab=results (2022).
Mishra, A. et al. Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J. Clin. Oncol. 40, 3985–3993 (2022).
Ghosh, A. et al. Increased p53 expression induced by APR-246 reprograms tumor-associated macrophages to augment immune checkpoint blockade. J. Clin. Invest. 132, e148141 (2022).
Park, H. et al. Phase Ib study of eprenetapopt (APR-246) in combination with pembrolizumab in patients with advanced or metastatic solid tumors. ESMO Open. 7, 100573 (2022).
Garcia-Manero, G. et al. Eprenetapopt combined with venetoclax and azacitidine in TP53-mutated acute myeloid leukaemia: a phase 1, dose-finding and expansion study. Lancet Haematol. 10, e272–e283 (2023).
DiNardo, C. D. et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 135, 791–803 (2020).
DiNardo, C. D. et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383, 617–629 (2020).
Bao, W. et al. PRIMA-1Met/APR-246 induces wild-type p53-dependent suppression of malignant melanoma tumor growth in 3D culture and in vivo. Cell Cycle 10, 301–307 (2011).
Zhang, Q. et al. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 9, 439 (2018).
Khairul, I., Wang, Q. Q., Jiang, Y. H., Wang, C. & Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 8, 23905–23926 (2017).
Lo-Coco, F. et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369, 111–121 (2013).
Chen, S. et al. Arsenic trioxide rescues structural p53 mutations through a cryptic allosteric site. Cancer Cell 39, 225–239.e8 (2021).
Dumbrava, E. E. et al. First-in-human study of PC14586, a small molecule structural corrector of Y220C mutant p53, in patients with advanced solid tumors harboring a TP53 Y220C mutation [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 3003 (2022).
Linde, L. & Kerem, B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 24, 552–563 (2008).
Howard, M., Frizzell, R. A. & Bedwell, D. M. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat. Med. 2, 467–469 (1996).
Burke, J. F. & Mogg, A. E. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 13, 6265–6272 (1985).
Floquet, C., Deforges, J., Rousset, J. P. & Bidou, L. Rescue of non-sense mutated p53 tumor suppressor gene by aminoglycosides. Nucleic Acids Res. 39, 3350–3362 (2011).
Floquet, C., Hatin, I., Rousset, J. P. & Bidou, L. Statistical analysis of readthrough levels for nonsense mutations in mammalian cells reveals a major determinant of response to gentamicin. PLoS Genet. 8, e1002608 (2012).
Zhang, M. et al. Synergistic rescue of nonsense mutant tumor suppressor p53 by combination treatment with aminoglycosides and Mdm2 inhibitors. Front. Oncol. 7, 323 (2018).
Friesen, W. J. et al. The nucleoside analog clitocine is a potent and efficacious readthrough agent. RNA 23, 567–577 (2017).
Crawford, D. K., Alroy, I., Sharpe, N., Goddeeris, M. M. & Williams, G. ELX-02 generates protein via premature stop codon read-through without inducing native stop codon read-through proteins. J. Pharmacol. Exp. Ther. 374, 264–272 (2020).
Trzaska, C. et al. 2,6-Diaminopurine as a highly potent corrector of UGA nonsense mutations. Nat. Commun. 11, 1509 (2020).
Baradaran-Heravi, A. et al. Effect of small molecule eRF3 degraders on premature termination codon readthrough. Nucleic Acids Res. 49, 3692–3708 (2021).
Palomar-Siles, M. et al. Translational readthrough of nonsense mutant TP53 by mRNA incorporation of 5-fluorouridine. Cell Death Dis. 13, 997 (2022).
Ivanov, A. et al. PABP enhances release factor recruitment and stop codon recognition during translation termination. Nucleic Acids Res. 44, 7766–7776 (2016).
Kariv, R. et al. Resorting the function of the colorectal cancer gatekeeper adenomatous polyposis coli. Int. J. Cancer 146, 1064–1074 (2020).
Wang, Z. et al. The anti-cancer agent APR-246 can activate several programmed cell death processes to kill malignant cells. Cell Death Differ. 30, 1033–1046 (2023).
Birsen, R. et al. APR-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 107, 403–416 (2022).
Fujihara, K. M. et al. Eprenetapopt triggers ferroptosis, inhibits NFS1 cysteine desulfurase, and synergizes with serine and glycine dietary restriction. Sci. Adv. 8, eabm9427 (2022).
Milne, J. V. et al. Transketolase regulates sensitivity to APR-246 in p53-null cells independently of oxidative stress modulation. Sci. Rep. 11, 4480 (2021).
Guiley, K. Z. & Shokat, K. M. A small molecule reacts with the p53 somatic mutant Y220C to rescue wild-type thermal stability. Cancer Discov. 13, 56–69 (2023).
Tang, Y. et al. Repurposing antiparasitic antimonials to noncovalently rescue temperature-sensitive p53 mutations. Cell Rep. 39, 110622 (2022).
Friedman, D. N. et al. Clonal hematopoiesis in survivors of childhood cancer. Blood Adv. 7, 4102–4106 (2023).
Calapre, L. et al. Identification of TP53 mutations in circulating tumour DNA in high grade serous ovarian carcinoma using next generation sequencing technologies. Sci. Rep. 13, 278 (2023).
Synnott, N. C. et al. The mutant p53-targeting compound APR-246 induces ROS-modulating genes in breast cancer cells. Transl. Oncol. 11, 1343–1349 (2018).
Ali, D. et al. Anti-leukaemic effects induced by APR-246 are dependent on induction of oxidative stress and the NFE2L2/HMOX1 axis that can be targeted by PI3K and mTOR inhibitors in acute myeloid leukaemia cells. Br. J. Haematol. 174, 117–126 (2016).
Maiti, A. & Daver, N. G. Eprenetapopt in the post-transplant setting: mechanisms and future directions. J. Clin. Oncol. 40, 3994–3997 (2022).
Ceder, S. et al. A thiol-bound drug reservoir enhances APR-246-induced mutant p53 tumor cell death. EMBO Mol. Med. 13, e10852 (2021).
Ho, J. N. H. G. et al. Targeting MDM2 enhances antileukemia immunity after allogeneic transplantation via MHC-II and TRAIL-R1/2 upregulation. Blood 140, 1167–1181 (2022).
Zhou, J. et al. The ubiquitin ligase MDM2 sustains STAT5 stability to control T cell-mediated antitumor immunity. Nat. Immunol. 22, 460–470 (2021).
Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 29, 49–58 (2023).
Kotler, E. et al. A systematic p53 mutation library links differential functional impact to cancer mutation pattern and evolutionary conservation. Mol. Cell. 71, 178–190.e8 (2018).
Lipkova, J. et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 40, 1095–1110 (2022).
Joerger, A. C. & Fersht, A. R. The tumor suppressor p53: from structures to drug discovery. Cold. Spring Harb. Perspect. Biol. 2, a000919 (2010).
Zhang, Q. et al. Evolutionary history of the p53 family DNA-binding domain: insights from an Alvinella pompejana homolog. Cell Death Dis. 13, 214 (2022).
National Cancer Institute. The TP53 database. The TP53 Database tp53.isb-cgc.org/ (2023).
Soussi, T. & Wiman, K. G. TP53: an oncogene in disguise. Cell Death Differ. 22, 1239–1249 (2015).
Sabapathy, K. & Lane, D. P. Understanding p53 functions through p53 antibodies. J. Mol. Cell Biol. 11, 317–329 (2019).
Light, N. et al. Germline TP53 mutations undergo copy number gain years prior to tumor diagnosis. Nat. Commun. 14, 77 (2023).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Martincorena, I. et al. Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917 (2018).
Fabre, M. A. et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606, 335–342 (2022).
Colom, B. et al. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 598, 510–514 (2021).
Sperling, A. S. et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood 140, 1753–1763 (2022).
Lodé, L. et al. Emergence and evolution of TP53 mutations are key features of disease progression in myelodysplastic patients with lower-risk del(5q) treated with lenalidomide. Haematologica 103, e143–e146 (2018).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52, 1219–1226 (2020).
Marcellino, B. K. et al. Transient expansion of TP53 mutated clones in polycythemia vera patients treated with idasanutlin. Blood Adv. 4, 5735–5744 (2020).
Raver-Shapira, N. et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell. 26, 731–743 (2007).
Vennin, C. et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 10, 3637 (2019).
Barak, Y., Juven, T., Haffner, R. & Oren, M. Mdm2 expression is induced by wild type-p53 activity. EMBO J. 12, 461–468 (1993).
Gu, J. et al. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J. Biol. Chem. 277, 19251–19254 (2002).
Wade, M., Li, Y. C. & Wahl, G. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 13, 83–96 (2013).
Lambert, J. M. R. et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 15, 376–388 (2009).
Degtjarik, O. et al. Structural basis of reactivation of oncogenic p53 mutants by a small molecule: methylene quinuclidinone (MQ). Nat. Commun. 12, 7057 (2021).
Liu, X. et al. Small molecule induced reactivation of mutant p53 in cancer cells. Nucleic Acids Res. 41, 6034–6044 (2013).
NCCN Guidelines Version 2.2024 Breast, Ovarian, and/or Pancreatic Cancer Genetic Assessment, https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed 13 December 2023).
Li, F. P. et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 48, 5358–5362 (1988).
Chompret, A. et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J. Med. Genet. 38, 43–47 (2001).
Bougeard, G. et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J. Clin. Oncol. 33, 2345–2352 (2015).
Kato, S. et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl Acad. Sci. USA 100, 8424–8429 (2003).
Giacomelli, A. O. et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat. Genet. 50, 1381–1387 (2018).
Ben-Cohen, G. et al. TP53_PROF: a machine learning model to predict impact of missense mutations in TP53. Brief. Bioinform. 23, bbab524 (2022).
The TP53 Web Site. UMD TP53 mutation database.The TP53 Web Site p53.fr/the-database (2017).
Béroud, C. & Soussi, T. The UMD-p53 database: new mutations and analysis tools. Hum. Mutat. 21, 176–181 (2003).
Carbonnier, V., Leroy, B., Rosenberg, S. & Soussi, T. Comprehensive assessment of TP53 loss of function using multiple combinatorial mutagenesis libraries. Sci. Rep. 10, 20368 (2020).
Donehower, L. A. Integrated analysis of TP53 gene and pathway alterations in The Cancer Genome Atlas. Cell Rep. 28, 1370–1384.e5 (2019).
Levine, A. J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 20, 471–480 (2020).
Sermeus, A. & Michiels, C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2, e164 (2011).
Wang, Y., Pakunlu, R. I., Tsao, W., Pozharov, V. & Minko, T. Bimodal effect of hypoxia in cancer: role of hypoxia inducible factor in apoptosis. Mol. Pharm. 1, 156–165 (2004).
Tashakori, M. et al. TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia. Blood 140, 58–72 (2022).
Saft, L. et al. p53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q). Haematologica 99, 1041–1049 (2014).
McGraw, K. L. et al. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse clinical outcome in myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica 101, e320–e323 (2016).
Acknowledgements
This work was supported by grants from the European Research Council (advanced ERC grant no. 694825-TRANSREAD), the Swedish Research Council (VR 2017-01509; 2021-02064), the Swedish Cancer Fund (Cancerfonden 18 0774; 21 1473 Pj 01 H), the Swedish Childhood Cancer Fund (Barncancerfonden PR2020-0042) and Radiumhemmets Forskingsfonder (201383). We apologize to all authors whose work could not be referenced owing to limited space.
Author information
Authors and Affiliations
Contributions
All authors researched data for this manuscript. A.T. and K.G.W. made a substantial contribution to discussions of content. All authors wrote the manuscript and edited and/or reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
K.G.W. has received research funding from, is a cofounder and shareholder of, and has previously received a salary from Aprea Therapeutics, a company that develops p53-based cancer therapies including eprenetapopt. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks M. Oren, T. Iwakuma and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuval, A., Strandgren, C., Heldin, A. et al. Pharmacological reactivation of p53 in the era of precision anticancer medicine. Nat Rev Clin Oncol 21, 106–120 (2024). https://doi.org/10.1038/s41571-023-00842-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00842-2
This article is cited by
-
p53 biology and reactivation for improved therapy in MDS and AML
Biomarker Research (2024)