Abstract
The care of patients with oesophageal cancer or of individuals who have an elevated risk of oesophageal cancer has changed dramatically. The epidemiology of squamous cell and adenocarcinoma of the oesophagus has diverged over the past several decades, with a marked increase in incidence only for oesophageal adenocarcinoma. Only in the past decade, however, have molecular features that distinguish these two forms of the disease been identified. This advance has the potential to improve screening for oesophageal cancers through the development of novel minimally invasive diagnostic technologies predicated on cancer-specific genomic or epigenetic alterations. Surgical techniques have also evolved towards less invasive approaches associated with less morbidity, without compromising oncological outcomes. With improvements in multidisciplinary care, advances in radiotherapy and new tools to detect minimal residual disease, certain patients may no longer even require surgical tumour resection. However, perhaps the most anticipated advance in the treatment of patients with oesophageal cancer is the advent of immune-checkpoint inhibitors, which harness and enhance the host immune response against cancer. In this Review, we discuss all these advances in the management of oesophageal cancer, representing only the beginning of a transformation in our quest to improve patient outcomes.
Key points
-
With advances in molecular profiling technologies, the differential mechanistic underpinnings of oesophageal adenocarcinoma and oesophageal squamous cell cancer have been better defined. Improved definition and understanding of the molecular alterations will lead to the discovery of exploitable therapeutic vulnerabilities.
-
Novel technologies are being developed for the screening and surveillance of premalignant lesions, relying on the identification of the earliest molecular changes in neoplastic transformation.
-
The optimal management approach for localized oesophageal cancer continues to rely on a multidisciplinary team approach, and advances have been made in each of the subspecialties involved in the care of patients with this disease.
-
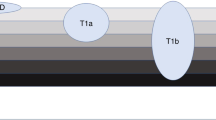
Endoscopic resection is a curative option for patients with low-risk stage T1a–b cancers; for other patients with localized oesophageal cancers, minimally invasive surgical techniques, improved perioperative care and improved radiotherapy techniques can reduce surgical morbidity without compromising oncological outcomes.
-
Immune-checkpoint inhibitors and their combinations with chemotherapy improve survival in patients with advanced-stage oesophageal cancers.
-
The development of novel targeted therapies and immunotherapies will continue to shape the treatment paradigms of oesophageal cancers for years to come.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
He, H. et al. Trends in the incidence and survival of patients with esophageal cancer: a SEER database analysis. Thorac. Cancer 11, 1121–1128 (2020).
Njei, B., McCarty, T. R. & Birk, J. W. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J. Gastroenterol. Hepatol. 31, 1141–1146 (2016).
Arnold, M., Soerjomataram, I., Henegouwen, M. B. & Soerjomataram, I. Global incidence of oesophageal cancer and gastric cancer by histology and subsite in 2018. Gut 69, 1564–1571 (2020).
Abnet, C. C., Arnold, M. & Wei, W. Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154, 360–373 (2018).
Zang, Z. et al. Dietary patterns and severity of symptom with the risk of esophageal squamous cell carcinoma and its histological precursor lesions in China: a multicenter cross-sectional latent class analysis. BMC Cancer 22, 95 (2022).
McCormack, V. A. et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int. J. Cancer 140, 259–271 (2017).
Lin, Y. et al. Esophageal cancer in high-risk areas of China: research progress and challenges. Ann. Epidemiol. 27, 215–222 (2017).
Arnal, M. J. D., Arenas, A. F. & Arbeloa, A. L. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 21, 7933–7943 (2015).
Shah, M. A. et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J. Clin. Oncol. 38, 2677–2694 (2020).
Kojima, T. et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 38, 4138–4148 (2020).
Shah, M. A. et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastateic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 5, 546–550 (2019).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastroesophageal junction, and oesophageal adenocarcioma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Shitara, K. et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 603, 942–948 (2022).
Sun, J. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398, 759–771 (2021).
Doki, Y., Ajani, J. A., Kato, K. & Xu, J. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386, 449–462 (2022).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384, 1191–1203 (2021).
Janjigian, Y. Y. et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade for HER2-positive gastric cancer. Nature 600, 727–730 (2021).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Shah, M. A. et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J. Clin. Oncol. 41, 1470–1491 (2023).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Campbell, J. D. et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 23, 194–212 (2018).
Quante, M., Wang, T. C. & Bass, A. J. Adenocarcinoma of the oesophagus: is it gastric cancer? Gut https://doi.org/10.1136/gutjnl-2022-327096 (2022).
Dotto, G. P. & Rustgi, A. K. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell 29, 622–637 (2016).
Yokoyama, T. et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol. Biomarkers Prev. 12, 1227–1233 (2003).
Wang, G. Q. et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut 54, 187–192 (2005).
Liu, X. et al. Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology 153, 166–177 (2017).
Liao, G. et al. Single-cell transcriptomics provides insights into the origin and microenvironment of human oesophageal high-grade intraepithelial neoplasia. Clin. Transl. Med. 12, e874 (2022).
Que, J., Garman, K. S., Souza, R. F. & Spechler, S. J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 157, 349–364.e1 (2019).
Nowicki-Osuch, K. et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science 373, 760–767 (2021).
Sawas, T. et al. Magnitude and time-trend analysis of postendoscopy esophageal adenocarcinoma: a systemic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, e31–e50 (2022).
Sawas, T. et al. Identification of prognostic phenotypes of esophageal adenocarcinoma in 2 independent cohorts. Gastroenterology 155, 1720–1728 (2018).
Stachler, M. D. et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology 155, 156–167 (2018).
Killcoyne, S. et al. Genomic copy number predicts esophageal cancer years before transformation. Nat. Med. 26, 1726–1732 (2020).
Fitzgerald, R. C. et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicenter, pragmatic, randomised controlled trial. Lancet 396, 333–344 (2020).
Wu, W. et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology 144, 956–966 (2013).
Jammula, S. et al. Identification of subtypes of Barrett’s esophagus and esophageal adenocarcinoma based on DNA methylation profiles and integration of transcriptome and genome data. Gastroenterology 158, 1682–1697 (2020).
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015).
Janjigian, Y. Y. et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 8, 49–58 (2018).
Wong, G. S. et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat. Med. 24, 968–977 (2018).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Investig. 124, 5145–5158 (2014).
Salem, M. E. et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist 23, 1319–1327 (2018).
Colom, B. et al. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 598, 510–514 (2021).
Brown, J., Stepien, A. J. & Willem, P. Landscape of copy number aberrations in esophageal squamous cell carcinoma from a high endemic region of South Africa. BMC Cancer 20, 281 (2020).
Zhou, J. et al. Pan-ERBB kinase inhibition augments CDK4/6 inhibitor efficacy in oesophageal squamous cell carcinoma. Gut 71, 665–675 (2022).
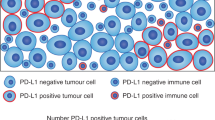
Baba, Y. et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 111, 3132–3141 (2020).
Okadome, K. et al. Prognostic and clinical impact of PD-L2 and PD-L1 expression in a cohort of 437 oesophageal cancers. Br. J. Cancer 122, 1535–1543 (2020).
Karakasheva, T. A. et al. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res. 75, 4074–4085 (2015).
Shaheen, N. J. et al. Diagnosis and management of Barrett’s esophagus: an updated ACG guideline. Am. J. Gastroenterol. 117, 559–587 (2022).
American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 140, 1084–1091 (2011).
Verbeek, R. E. et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am. J. Gastroenterol. 109, 1215–1222 (2014).
Tramontano, A. C. et al. The impact of a prior diagnosis of Barrett’s esophagus on esophageal adenocarcinoma survival. Am. J. Gastroenterol. 112, 1256–1264 (2017).
Moinova, H. R. et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 10, eaao5848 (2018).
Iyer, P. G. et al. Accurate nonendoscopic detection of Barrett’s esophagus by methylated DNA markers: a multisite case control study. Am. J. Gastroenterol. 115, 1201–1209 (2020).
Bhat, S. et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut 64, 20–25 (2015).
El-Serag, H. B. et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut 65, 1252–1260 (2016).
Corley, D. A. et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology 145, 312–319 (2013).
Abrams, J. A. et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin. Gastroenterol. Hepatol. 7, 736–742 (2009).
Runge, T. M., Abrams, J. A. & Shaheen, N. J. Epidemiology of Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol. Clin. North. Am. 44, 203–231 (2015).
Hvid-Jensen, F. et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 365, 1375–1383 (2011).
Kastelein, F. et al. Aberrant p53 protein expression is associated with increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut 62, 1676–1683 (2013).
Redston, M. et al. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology 162, 468–481 (2022).
Peters, Y., van Grinsven, E. & Siersema, P. D. Systematic review with meta-analysis: the effects of family history on the risk of Barrett’s oesophagus and oesophageal adenocarcinoma. Aliment. Pharmacol. Ther. 54, 868–879 (2021).
Glamour, B. K. et al. Age of diagnosis in familial Barrett’s associated neoplasia. Fam. Cancer 21, 115–120 (2022).
Fecteau, R. E. et al. Association between germline mutation in VSIG10L and familial Barrett neoplasia. JAMA Oncol. 2, 1333–1339 (2016).
Orloff, M. et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 306, 410–419 (2011).
Banerjee, B. et al. Clinical study of ursodeoxycholic acid in Barrett’s esophagus patients. Cancer Prev. Res. 9, 528–533 (2016).
Chak, A. et al. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 13, 665–672 (2015).
Cummings, L. C. et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS ONE 12, e0184928 (2017).
Heath, E. I. et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J. Natl Canc. Inst. 99, 545–557 (2007).
Joe, A. K. et al. Phase Ib randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in patients with Barrett’s esophagus. Cancer Prev. Res. 8, 1131–1137 (2015).
Abrams, J. A. et al. Randomized controlled trial of the gastric/CCK2 receptor antagonist Netazepide in patients with Barrett’s esophagus. Cancer Prev. Res. 14, 675–682 (2021).
Jankowski, J. A. Z. et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 392, 400–408 (2018).
National Health Commission of the People’s Republic of China. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin. J. Cancer Res. 31, 223–258 (2019).
Middleton, D. R. S. et al. Minimally invasive esophageal sponge cytology sampling is feasible in a Tanzanian community setting. Int. J. Cancer 148, 1208–1218 (2021).
Roshandel, G. et al. Pilot study of cytological testing for oesophageal squamous cell dysplasia in a high-risk area in Northern Iran. Br. J. Cancer 111, 2235–2241 (2014).
Gao, Y. et al. Feasibility and accuracy of artificial intelligence-assisted sponge cytology for community-based esophageal squamous cell carcinoma screening in China. Am. J. Gastroenterol. 116, 2207–2215 (2021).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759 (2020).
Shaheen, N. J. et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 360, 2277–2288 (2009).
Canto, M. I. et al. Multifocal cryoballoon ablation for eradication of Barrett’s esophagus-related neoplasia: a prospective multicenter clinical trial. Am. J. Gastroenterol. 115, 1879–1890 (2020).
Shaheen, N. J. et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest. Endosc. 71, 680–685 (2010).
Knabe, M. et al. Hybrid APC in combination with resection for the endoscopic treatment of neoplastic Barrett’s esophagus: a prospective, multicenter study. Am. J. Gastroenterol. 117, 110–119 (2022).
Ajani, J. A. et al. Esophageal and esophagogastric junction cancers. NCCN Guidelines Version 3.2022 1-162 (2019).
Pech, O. et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 146, 652–660 (2014).
Terheggen, G. et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 66, 783–793 (2017).
Badreddine, R. J. et al. Depth of submucosal invasion does not predict lymph node metastases and survival of patients with esophageal carcinoma. Clin. Gastroenterol. Hepatol. 8, 248–253 (2010).
No Authors Listed. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 58, S3–S43 (2003).
Nieuwenhuis, E. A. et al. Analysis of metastases rates during follow-up after endoscopic resection of early “high-risk” esophageal adenocarcinoma. Gastrointest. Endosc. 5107, 237–247.e3 (2022).
Benech, N. et al. Endoscopic resection of Barrett’s adenocarcinoma: intramucosal and low-risk tumours are not associated with lymph node metastases. U. Eur. Gastroenterol. J. 9, 362–369 (2021).
Fotis, D. et al. Submucosal invasion and risk of lymph node invasion in early Barrett’s cancer: potential impact of different classification systems on patient management. U. Eur. Gastroenterol. J. 3, 505–513 (2015).
Guo, H. M., Zhang, X. Q., Chen, M., Huang, S. L. & Zou, X. P. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J. Gastroenterol. 20, 5540–5547 (2014).
Draganov, P. V., Wang, A. Y., Othman, M. O. & Fukami, N. AGA institute clinical practice update: endoscopic submucosal dissection in the United States. Clin. Gastroenterol. Hepatol. 17, 16–25 (2019).
Akutsu, Y. et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann. Surg. 264, 1009–1015 (2016).
Li, B. et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 146, 1198–1203 (2013).
Kato, K. et al. Parallel-group controlled trial of surgery versus chemoradiotherapy in patients with stage I esophageal squamous cell carcinoma. Gastroenterology 161, 1878–1886 (2021).
Dimick, J. B., Wainess, R. M., Upchurch, G. R., Iannettoni, M. D. & Orringer, M. B. National trends in outcomes for esophageal resection. Ann. Thorac. Surg. 79, 212–216 (2005).
Mitzman, B., Schipper, P. H., Edwards, M. A., Kim, S. & Ferguson, M. K. Complications after esophagectomy are associated with extremes of body mass index. Ann. Thorac. Surg. 106, 973–980 (2018).
Kuppusamy, M. K. & Low, D. E., International Esodata Study Group (IESG). Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of >6000 patients using ECCG definitions and the online Esodata database. Ann. Surg. 275, 515–525 (2022).
Voeten, D. M. et al. Outcomes of esophagogastric cancer surgery during eight years of surgical auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 274, 866–873 (2021).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366, 2074–2084 (2012).
Haverkamp, L., Seesing, M. F. J., Ruurda, J. P., Boone, J. & Hillegersberg, R. V. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis. Esophagus 30, 1–7 (2017).
Schlottman, F., Strassle, P. D. & Patti, M. G. Transhiatal vs transthoracic esophagectomy: a NSQIP analysis of postoperative outcomes and risk factors for morbidity. J. Gastrointest. Surg. 21, 1757–1763 (2017).
Hulscher, J. B. et al. Extending transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N. Engl. J. Med. 347, 1662–1669 (2002).
van Workum, F., Fransen, L., Dp Luyer, M. & Rosman, C. Learning curves in minimally invasive esophagectomy. World J. Gastroenterol. 24, 4974–4978 (2018).
Blears, E., Fernando, H. C., Shahoud, J. & Weksler, B. Factors associated with access and approach to esophagectomy for cancer: a National Cancer Database study. Surg. Endosc. 36, 7016–7024 (2022).
Straatman, J. et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann. Surg. 266, 232–236 (2017).
Biere, S. S. et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 379, 1887–1892 (2012).
Nuytens, F. et al. Five-year survival outcomes of hybrid minimally invasive esophagectomy in esophageal cancer: results of the MIRO randomized clinical trial. JAMA Surg. 156, 323–332 (2021).
Yang, Y. et al. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE Trial. Ann. Surg. 275, 646–653 (2022).
Groth, S. S. et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J. Thorac. Cardiovasc. Surg. 139, 612–620 (2010).
Peyre, C. G. et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann. Surg. 248, 549–556 (2008).
Rizk, N. P. et al. Optimum lymphadenectomy for esophageal cancer. Ann. Surg. 251, 46–50 (2010).
Altorki, N. et al. Ten-year survival and recurrence patterns after three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005627 (2022).
Eyck, B. M. et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J. Clin. Oncol. 39, 1995–2004 (2021).
Kamel, M. K. et al. Extended lymphadenectomy improves survival after induction chemoradiation for esophageal cancer: a propensity matched analysis of the National Cancer Database. Ann. Surg. 277, e772–e776 (2023).
Visser, E., van Rossum, P. S. N., Ruurda, J. P. & van Hillegersberg, R. Impact of lymph node yield on overall survival in patients treated with neoadjuvant chemoradiotherapy followed by esophagectomy for cancer: a population-based cohort study in the Netherlands. Ann. Surg. 266, 863–869 (2017).
Isono, K., Sato, H. & Nakayama, K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 48, 411–420 (1991).
Li, B. et al. Esophagectomy with three-field vs two-field lymphadenectomy for middle and lower thoracic esophageal cancer: long-term outcomes of a randomized clinical trial. J. Thorac. Oncol. 16, 310–317 (2021).
Lerut, T. et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann. Surg. 240, 962–972 (2004).
Low, D. E. et al. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS) society recommendations. World J. Surg. 434, 299–330 (2019).
Minnella, E. M. et al. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 153, 1081–1089 (2018).
Allen, S. K. et al. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: effect on cardiopulmonary exercise test performance, muscle mass and quality of life — a pilot randomized trial. Ann. Surg. Oncol. 29, 1839–1850 (2022).
Steffens, D. et al. Prehabilitation with preoperative exercise and education for patients undergoing major abdominal surgery: protocol for a multicenter randomised controlled trial (PRIORITY TRIAL). BMC Cancer 22, 443 (2022).
Mariette, C. et al. Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase III controlled trial: the MIRO trial. Ann. Surg. 271, 1023–1029 (2020).
Noordman, B. J. et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for esophageal cancer: a stepped-wedge cluster randomized trial. BMC Cancer https://doi.org/10.1186/s12885-018-4034-1 (2018).
Derogar, M. & Lagergren, P. Health-relted quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J. Clin. Oncol. 30, 413–418 (2012).
Schreurs, L. M. et al. Impact of 18-fluorodeoxyglucose positron emission tomography on computed tomoography defined target volumes in radiation treatment planning of esophageal cancer: reduction in geographic misses with equal interobserver variability: PET/CT improves esophageal target definition. Dis. Esophagus 23, 493–501 (2010).
Vosmik, M. et al. Technological advances in radiotherapy for esophageal cancer. World J. Gastroenterol. 16, 5555–5564 (2010).
Wang, J. et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int. J. Rad. Oncol. Biol. Phys. 86, 885–891 (2013).
Lin, S. H. et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J. Clin. Oncol. 38, 1569–1579 (2020).
Minsky, B. et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J. Clin. Oncol. 20, 1167–1174 (2002).
Hulshof, M. C. C. et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO Study). J. Clin. Oncol. 39, 2816–2824 (2021).
Wang, X. et al. High versus standard radiation dose of definitive concurrent chemoradiotherapy for esophageal cancer: a systemic review and meta-analysis of randomized clinical trials. Radiother. Oncol. 180, 109463 (2023).
Penniment, M. G. et al. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in adavced oesophageal cancer: a multicenter randomised controlled trial (TROG 03.01). Lancet Gastroenterol. Hepatol. 3, 114–124 (2017).
Bass, G. A., Furlong, H., O’Sullivan, K. E., Hennessy, T. P. & Walsh, T. N. Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur. J. Cancer 50, 1065–1075 (2014).
Boonstra, J. J. et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer 11, 181 (2011).
Lv, J. et al. Long-term efficacy of periopeartive chemoradiotherapy on esophageal squamous cell carcinoma. World J. Gastroenterol. 16, 1649–1654 (2010).
Tepper, J. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092 (2008).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20 (2006).
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I. & Langley, R. E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 27, 5062–5067 (2009).
Zhao, Y. et al. Periopertive versus preoperative chemotherapy with surgery in patients with resectable squamous cell carcinoma of esophagus: a phase III randomized trial. J. Thorac. Oncol. 10, 1349–1356 (2015).
Ando, N. et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 19, 68–74 (2012).
Ychou, M. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 29, 1715–1721 (2011).
Obermannova, R. et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 992–1004 (2022).
Klevebro, F. et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 27, 660–667 (2016).
Stahl, M. et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinoma of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur. J. Cancer 81, 183–190 (2017).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Bedenne, L. et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 25, 1160–1168 (2007).
Stahl, M. et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 23, 2310–2317 (2005).
Swisher, S. G. et al. Final results of NRG Oncology RTOG 0246: an organ-preserving selective resection strategy in esophageal cancer patients treated with definitive chemoradiation. J. Thorac. Oncol. 12, 368–374 (2017).
van der Wilk, B. J. et al. Active surveillance versus immediate surgery in clinical complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity matched study. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003636 (2019).
Bolger, J. C., Donohoe, C. L., Lowery, M. & Reynolds, J. V. Advances in the curative management of oesophageal cancer. Br. J. Cancer 126, 706–717 (2022).
Cheedella, N. K. et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann. Oncol. 24, 1262–1266 (2013).
Noordman, B. J. et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicenter, diagnostic cohort study. Lancet Oncol. 19, 965–974 (2018).
Azad, T. D. et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology 158, 494–505 (2020).
Wieder, H. A. et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J. Clin. Oncol. 22, 900–908 (2004).
Ott, K. et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J. Clin. Oncol. 24, 4692–4698 (2006).
Lordick, F. et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 8, 797–805 (2007).
Goodman, K. A. et al. Randomized phase II study of PET response-adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (Alliance) trial. J. Clin. Oncol. 39, 2803–2815 (2021).
Cunningham, D. et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council STO3): primary analysis results of a multicenter open-label, randomised phase 2-3 trial. Lancet Oncol. 18, 357–370 (2017).
Okines, A. F. C. et al. Biomarker analysis in oesophageal cancer: results from the REAL3 and TransMAGIC trials. Eur. J. Cancer 49, 2116–2125 (2013).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Ruhstaller, T. et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann. Oncol. 29, 1386–1393 (2018).
Crosby, T. et al. SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br. J. Cancer 116, 709–716 (2017).
Suntharalingam, M. et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer. The NRG Oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol. 3, 1520–1528 (2017).
Safran, H. P. et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicenter, randomised, phase 3 trial. Lancet Oncol. 23, 259–269 (2022).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Lynce, F. & Swain, S. M. Pertuzumab for the treatment of breast cancer. Cancer Invest. 32, 430–438 (2014).
Hofheinz, R. D. et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2-positive resectable esophagogastric adenocarcinoma: a randomized phase II trial of the AIO EGA study group. J. Clin. Oncol. 40, 3750–3761 (2022).
Shah, M. A. Update on metastatic gastric and esophageal cancers. J. Clin. Oncol. 33, 1760–1769 (2015).
Al-Batran, S. E. et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 26, 1435–1442 (2008).
Cunningham, D. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358, 36–46 (2008).
Kang, Y. K. et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann. Oncol. 20, 666–673 (2009).
Van Cutsem, E. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24, 4991–4997 (2006).
Shah, M. A. et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J. Clin. Oncol. 33, 3874–3879 (2015).
Lorenzen, S. et al. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann. Oncol. 18, 1673–1679 (2007).
Al-Batran, S. E. et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann. Oncol. 19, 1882–1887 (2008).
Lu, B. et al. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol. Res. 50, 269–275 (2011).
Ohigashi, Y. et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 11, 2947–2953 (2005).
Shah, M. A. et al. T cell-inflamed gene expression profile and PD-L1 expression and pembrolizumab efficacy in advanced esophageal cancer. Future Oncol. https://doi.org/10.2217/fon-2021-1134 (2022).
Kato, K. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (Attraction-3): a multicenter, randomised, open label phase 3 trial. Lancet Oncol. 20, 1506–1517 (2019).
Huang, J. et al. Camrelizumab versus investigator’s choice of chemotherapy as 2nd line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicenter, randomised, open-label, phase 3 study. Lancet Oncol. 21, 832–842 (2020).
Shen, L. et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (Rationale-302): a randomized phase III study. J. Clin. Oncol. 40, 3065–3076 (2022).
Bang, Y. J. et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-esophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann. Oncol. 29, 2052–2060 (2018).
Luo, H. et al. Effect of camrelizumab vs. placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st Randomized Clinical Trial. JAMA 326, 916–925 (2021).
Lu, Z. et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinioma (ORIENT-15): multicenter, randomised, double blind, phase 3 trial. BMJ 377, e068714 (2022).
Wang, Z. X. et al. Toripalimab plus chemotherapy in treatment-naive advanced esophageal squamous cell carcinoma (Jupiter-06): a multi-center phase 3 trial. Cancer Cell 40, 277–288 (2022).
Chaganty, B. K. R. et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNy secretion. Cancer Lett. 430, 47–56 (2018).
Varadan, V. et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin. Cancer Res. 22, 3249–3259 (2016).
Song, Y. et al. First-line serplulimab or placebo plus chemotherapy in PD-L1 positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat. Med. 29, 473–482 (2023).
Author information
Authors and Affiliations
Contributions
M.A.S., N.A., P.P., A.B. and J.A.A. researched data for the article and reviewed and/or edited the manuscript before submission. All authors contributed to the discussion of content and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.A.S. has received research funding from Bristol Myers Squibb, Merck and Oncolys Biopharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks J. Ajani, K. Kato and F. Lordick for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/ct2/homeInternational Clinical Trials Registry Platform Search Portal: https://trialsearch.who.int/Default.aspx
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, M.A., Altorki, N., Patel, P. et al. Improving outcomes in patients with oesophageal cancer. Nat Rev Clin Oncol 20, 390–407 (2023). https://doi.org/10.1038/s41571-023-00757-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00757-y
This article is cited by
-
CD276 promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma through the TGF-β/SMAD signaling
Clinical & Experimental Metastasis (2024)
-
Aberrant epithelial cell interaction promotes esophageal squamous-cell carcinoma development and progression
Signal Transduction and Targeted Therapy (2023)