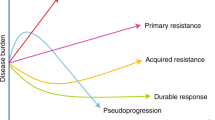
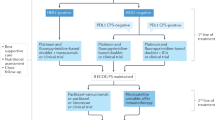
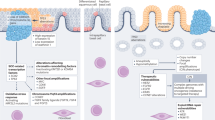
Abstract
Cancers of the upper gastrointestinal tract are a leading cause of cancer-related death world-wide and historically have a poor prognosis. The incidence and histology of these cancers have varied temporally and geographically over the last three decades, with an emerging understanding of the differences in the molecular and genetic profiles across different subgroups. Management of oesophagogastric cancers is by a multidisciplinary team with utilisation of surgery, radiotherapy and systemic treatments in combinations where appropriate. Immune checkpoint inhibition (ICI) has drastically changed the treatment landscape of multiple solid malignancies in the last 5 years. In oesophagogastric cancer, clinical trials have only recently shown activity that is often associated with the molecular characteristics of these tumours, in particular PD-L1 scores or microsatellite instability (MSI-H). This review looks to present the pivotal trials in this space, discuss the complexities between trials that may explain the disparate results and assess the benefit ICI offers in the treatment landscape at present.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–71.
Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112.
Wagner AD, Syn NLX, Moehler M, Grothe W, Yong WP, Tai B, et al. Chemotherapy for advanced gastric cancer. Cochr Database Syst Rev. 2017. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004064.pub4/pdf/full.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Bang Y-J, van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Larkin J, Chiarion-Sileni VGR. Five-year overall survival outcomes of the Check-Mate 067 phase 3 trial of nivolumab plus ipilimumab combination therapy in advanced melanoma. N Engl J Med. 2019;381:1535–46.
Herbst RS, Garon EB, Kim DW, Chul Cho B, Pérez Gracia JL, Han JY, et al. LBA4 Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol. 2018;29:mdy511–003.
Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago–gastric junction and gastric adenocarcinoma—individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol. 2009;20:885–91.
Network CGAR. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
Kato K, Sun J, Shah M, Enzinger PC, Adenis A, Doi T, et al. LBA8_PR-Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Annals of Oncology. 2020;31:S1192–3.
Chau I, Doki Y, A.Ajani J, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab plus ipilimumab or nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: first results of CheckMate 648 study. In: Nivolumab plus ipilimumab or nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: first results of CheckMate 648 study. 2021 ASCO Annual Meeting. Abstract. Vol. 4001. 2021.
Xu R, Luo H, Bai Y, Mao T, Wang J, Fan Q, et al. ESCORT-1st: a randomized, doubleblind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy vx chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC). J Clin Oncol. 2021;39:4000.
Lei M, Siemers N, Pandya D, Chang H, Sanchez T, Dorange C, et al. association of PD-L1 combined positive score and immune gene signatures with efficacy of nivolumab (NIVO) ± ipilimumab (IPI) in patients with metastatic gastroesophageal cancer (mGEC). AACR; 2019.
Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz K, et al. LBA6_PR -Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1 L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Annals of Oncology. 2020;31:S1191.
Janjigian YY, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. LBA7 Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study. Ann Oncol. 2021;32:S1329–30.
Harada K, Abdelhakeem AAF, Ajani JA. A balancing act: Dual immune-checkpoint inhibition for oesophagogastric cancer. Nat Rev Clin Oncol. 2019;16:9–10.
Boku N, Ryu M-H, Oh D, Al EA. randomised, multicenter, phase 2/3 study of nivolumab (Nivo) plus chemotherapy in patient (Pts) with previously untreated advanced or recurrent gastric (G) or gastroesophageal junction (GEJ) cancer. Annals of Oncology. 2020;31:S1142–S1215. https://doi.org/10.6084/m9.figshare.hgv.1920
Boku N, Ryu M-H, Kato K, Chung HC, Minashi K, Lee K-W, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial. Ann Oncol. 2019;30:250–8.
Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, et al. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97–106.
Bang Y-J, Kang Y-K, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828–37.
Garcia‐Pelaez J, Barbosa‐Matos R, Gullo I, Carneiro F, Oliveira C. Histological and mutational profile of diffuse gastric cancer: current knowledge and future challenges. Mol Oncol. 2021;15:2841–67.
Ohtsu A, Shah MA, van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76.
Hecht JR, Bang Y-J, Qin S, Chung H-C, Xu J-M, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. J Clin Oncol. 2013;31. https://ascopubs.org/doi/abs/10.1200/jco.2013.31.18_suppl.lba4001.
Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64:1721–31.
Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): expanded efficacy and safety data from CheckMate 649. J Clin Oncol. 2021;39:4002.
Shitara K, van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80.
Li J-Y, Chen Y-P, Li Y-Q, Liu N, Ma J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol Cancer. 2021;20:1–21.
Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu M-H, Muntean AS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39:966–77.
Chen L-T, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer. 2019;23:510–19.
Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–50.
Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens. Gastric Cancer. 2019;22:344–54.
Shitara K, Özgüroğlu M, Bang Y-J, di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Marabelle A, Le DT, Ascierto PA, di Giacomo AM, de Jesus-Acosta A, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10.
Bang Y-J, Ruiz EY, van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–60.
Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5:546–50.
Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–9.
Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48.
Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018;430:47–56.
Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–31.
Janjigian YY, Kawazoe A, Yanez PE, Luo S, Lonardi S, Kolesnik O, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. Wolters Kluwer Health; 2021.
Blum Murphy M, Xiao L, Patel VR, Maru DM, Correa AM, G. Amlashi F, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival—The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–13.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–203.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiotherapy: expanded efficacy and safety analyses from CheckMate 577. ASCO; 2021.
Oba K, Paoletti X, Alberts S, Bang Y-J, Benedetti J, Bleiberg H, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta-analysis. J Natl Cancer Inst. 2013;105:1600–7.
Ye J, Ji X, Dennis PA, Abdullah H, Mukhopadhyay P. Relationship between progression‐free survival, objective response rate, and overall survival in clinical trials of PD‐1/PD‐L1 immune checkpoint blockade: a meta‐analysis. Clin Pharmacol Ther. 2020;108:1274–88.
Shah MA, Almhanna K, Iqbal S, Thakkar P, Schneider BJ, Yantiss R, et al. Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC). Wolters Kluwer Health; 2021.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Park Y, Koh J, Na HY, Kwak Y, Lee K-W, Ahn S-H, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661.
Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26:6453–63.
Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability–high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA. 2021;7:895–902.
Author information
Authors and Affiliations
Contributions
AS: co-developed concept, analysis of data, manuscript writing, manuscript review. AR: analysis of data, manuscript writing, manuscript review. CK: analysis of data, manuscript writing, manuscript review. VB: analysis of data, manuscript writing, manuscript review. TP: co-developed concept, analysis of data, manuscript writing, manuscript review.
Corresponding authors
Ethics declarations
Competing interests
TP reports uncompensated advisory board BMS, compensated advisory board MSD. The remaining authors declare no competing interests.
Ethics approval and consent to Participate
Not Applicable
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smith, A., Roy, A., Karapetis, C.S. et al. Immunotherapy use in oesophagogastric cancers—a review of the literature. Br J Cancer 127, 21–29 (2022). https://doi.org/10.1038/s41416-022-01751-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01751-4