Abstract
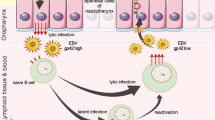
The past three decades have borne witness to many advances in the understanding of the molecular biology and treatment of nasopharyngeal carcinoma (NPC), an Epstein–Barr virus (EBV)-associated cancer endemic to southern China, southeast Asia and north Africa. In this Review, we provide a comprehensive, interdisciplinary overview of key research findings regarding NPC pathogenesis, treatment, screening and biomarker development. We describe how technological advances have led to the advent of proton therapy and other contemporary radiotherapy approaches, and emphasize the relentless efforts to identify the optimal sequencing of chemotherapy with radiotherapy through decades of clinical trials. Basic research into the pathogenic role of EBV and the genomic, epigenomic and immune landscape of NPC has laid the foundations of translational research. The latter, in turn, has led to the development of new biomarkers and therapeutic targets and of improved approaches for individualizing immunotherapy and targeted therapies for patients with NPC. We provide historical context to illustrate the effect of these advances on treatment outcomes at present. We describe current preclinical and clinical challenges and controversies in the hope of providing insights for future investigation.
Key points
-
Basic research studies elucidating the genomic, epigenomic and immune landscapes of nasopharyngeal cancer (NPC) have laid the foundation for translational research to develop new therapeutic targets and biomarkers for this malignancy.
-
Chemoradiotherapy is the treatment backbone for locoregionally advanced NPC; induction chemotherapy followed by chemoradiotherapy is the new standard-of-care therapy in this disease setting.
-
In the past decades, advances in radiotherapy, including arc-based intensity-modulated approaches, proton therapy, adaptive radiotherapy planning and incorporation of artificial intelligence, have contributed to the improvements in treatment outcomes.
-
Immunotherapy has become an important treatment modality that is being intensively investigated in different clinical settings, either as monotherapy or in combination with other agents.
-
Plasma EBV DNA has enabled risk stratification and real-time assessment of response to therapy; multiple prospective studies are evaluating its role in guiding therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Ferlay, J. et al. Data visualization tools for exploring the global cancer burden in 2020. Cancer Today https://gco.iarc.fr/today (WHO, International Agency for Research on Cancer, 2018).
Lee, A. W. et al. Changing epidemiology of nasopharyngeal carcinoma in Hong Kong over a 20-year period (1980-99): an encouraging reduction in both incidence and mortality. Int. J. Cancer 103, 680–685 (2003).
Yu, M. C. & Yuan, J. M. Epidemiology of nasopharyngeal carcinoma. Semin. Cancer Biol. 12, 421–429 (2002).
Carioli, G. et al. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int. J. Cancer 140, 2256–2264 (2017).
Wei, K. R. et al. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pacif. J. Cancer Prev. 11, 29–32 (2010).
Barnes, L., Eveson, J., Reichart, P. & Sidransky, D. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours (IARC Press, 2005).
Marks, J. E., Phillips, J. L. & Menck, H. R. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 83, 582–588 (1998).
Wang, H. Y. et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin. J. Cancer 35, 41 (2016).
Tsao, S. W. et al. Etiological factors of nasopharyngeal carcinoma. Oral. Oncol. 50, 330–338 (2014).
Liu, Z. et al. Oral hygiene and risk of nasopharyngeal carcinoma-a population-based case-control study in China. Cancer Epidemiol. Biomark. Prev. 25, 1201–1207 (2016).
Chang, E. T. et al. Active and passive smoking and risk of nasopharyngeal carcinoma: a population-based case-control study in southern china. Am. J. Epidemiol. 185, 1272–1280 (2017).
Bei, J. X., Jia, W. H. & Zeng, Y. X. Familial and large-scale case-control studies identify genes associated with nasopharyngeal carcinoma. Semin. Cancer Biol. 22, 96–106 (2012).
Lu, S. J. et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature 346, 470–471 (1990).
Xu, M. et al. Genome sequencing analysis identifies Epstein−Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 51, 1131–1136 (2019).
Young, L. S., Yap, L. F. & Murray, P. G. Epstein−Barr virus: more than 50 years old and still providing surprises. Nat. Rev. Cancer 16, 789–802 (2016).
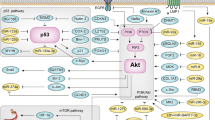
Lo, K. W., Chung, G. T. & To, K. F. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin. Cancer Biol. 22, 79–86 (2012).
Tsang, C. M., Lui, V. W. Y., Bruce, J. P., Pugh, T. J. & Lo, K. W. Translational genomics of nasopharyngeal cancer. Semin. Cancer Biol. 61, 84–100 (2020).
Tsang, C. M. et al. Cyclin D1 overexpression supports stable EBV infection in nasopharyngeal epithelial cells. Proc. Natl Acad. Sci. USA 109, E3473–E3482 (2012).
Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet 394, 64–80 (2019).
Chung, A. K. et al. Targeted sequencing of cancer-related genes in nasopharyngeal carcinoma identifies mutations in the TGF-β pathway. Cancer Med. 8, 5116–5127 (2019).
Tao, Q. & Chan, A. T. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev. Mol. Med. 9, 1–24 (2007).
Lo, K. W. et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res. 56, 2721–2725 (1996).
Lo, K. W. et al. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res. 61, 3877–3881 (2001).
Li, L. et al. Epigenetic inactivation of the CpG demethylase TET1 as a DNA methylation feedback loop in human cancers. Sci. Rep. 6, 26591 (2016).
Jin, H. et al. Epigenetic silencing of a Ca2+-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc. Natl Acad. Sci. USA 104, 12353–12358 (2007).
Dai, W. et al. Whole-exome sequencing identifies MST1R as a genetic susceptibility gene in nasopharyngeal carcinoma. Proc. Natl Acad. Sci. USA 113, 3317–3322 (2016).
Li, Y. Y. et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat. Commun. 8, 14121 (2017).
Lin, D. C. et al. The genomic landscape of nasopharyngeal carcinoma. Nat. Genet. 46, 866–871 (2014).
Hau, P. M. et al. Targeting Epstein−Barr virus in nasopharyngeal carcinoma. Front. Oncol. 10, 600 (2020).
Lin, W. et al. Establishment and characterization of new tumor xenografts and cancer cell lines from EBV-positive nasopharyngeal carcinoma. Nat. Commun. 9, 4663 (2018).
Chen, Y. P. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 30, 1024–1042 (2020).
Jin, S. et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 30, 950–965 (2020).
Li, L. et al. Characterization of the nasopharyngeal carcinoma methylome identifies aberrant disruption of key signaling pathways and methylated tumor suppressor genes. Epigenomics 7, 155–173 (2015).
Chan, K. C. A. et al. Analysis of plasma Epstein−Barr virus DNA to screen for nasopharyngeal cancer. N. Engl. J. Med. 377, 513–522 (2017).
Zeng, Y. et al. Prospective studies on nasopharyngeal carcinoma in Epstein−Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int. J. Cancer 36, 545–547 (1985).
Zong, Y. S. et al. Immunoglobulin A against viral capsid antigen of Epstein−Barr virus and indirect mirror examination of the nasopharynx in the detection of asymptomatic nasopharyngeal carcinoma. Cancer 69, 3–7 (1992).
Ng, W. T. et al. Outcomes of nasopharyngeal carcinoma screening for high risk family members in Hong Kong. Fam. Cancer 9, 221–228 (2010).
Liu, Z. et al. Two Epstein−Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in Southern China. Am. J. Epidemiol. 177, 242–250 (2013).
Coghill, A. E. et al. Epstein−Barr virus serology as a potential screening marker for nasopharyngeal carcinoma among high-risk individuals from multiplex families in Taiwan. Cancer Epidemiol. Biomark. Prev. 23, 1213–1219 (2014).
Tay, J. K., Lim, M. Y. & Kanagalingam, J. Screening in nasopharyngeal carcinoma: current strategies and future directions. Curr. Otorhinolaryngol. Rep. 2, 1–7 (2014).
Ji, M. F. et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann. Oncol. 30, 1630–1637 (2019).
Lo, Y. M. et al. Quantitative analysis of cell-free Epstein−Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 59, 1188–1191 (1999).
Chan, K. C. et al. Early detection of nasopharyngeal carcinoma by plasma Epstein−Barr virus DNA analysis in a surveillance program. Cancer 119, 1838–1844 (2013).
Lee, A. W. et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 61, 1107–1116 (2005).
Miller, J. A., Le, Q. T., Pinsky, B. A. & Wang, H. Cost-effectiveness of nasopharyngeal carcinoma screening with Epstein−Barr virus polymerase chain reaction or serology in high-incidence populations worldwide. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa198 (2020).
Lam, W. K. J. et al. Sequencing-based counting and size profiling of plasma Epstein−Barr virus DNA enhance population screening of nasopharyngeal carcinoma. Proc. Natl Acad. Sci. USA 115, E5115–E5124 (2018).
Lam, W. K. J. et al. Methylation analysis of plasma DNA informs etiologies of Epstein−Barr virus-associated diseases. Nat. Commun. 10, 3256 (2019).
King, A. D. et al. Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann. Oncol. 30, 977–982 (2019).
King, A. D. et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 258, 531–537 (2011).
King, A. D. et al. Early detection of cancer: evaluation of MR imaging grading systems in patients with suspected nasopharyngeal carcinoma. AJNR Am. J. Neuroradiol. 41, 515–521 (2020).
Lam, W. K. J. et al. Sequencing analysis of plasma Epstein−Barr virus DNA reveals nasopharyngeal carcinoma-associated single nucleotide variant profiles. Clin. Chem. 66, 598–605 (2020).
Pan, J. J. et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 122, 546–558 (2016).
Chan, A. T. et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J. Natl Cancer Inst. 97, 536–539 (2005).
Blanchard, P. et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 16, 645–655 (2015).
Ribassin-Majed, L. et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J. Clin. Oncol. 35, 498–505 (2017).
Al-Sarraf, M. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J. Clin. Oncol. 16, 1310–1317 (1998).
Wee, J. et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J. Clin. Oncol. 23, 6730–6738 (2005).
Lee, A. W. et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother. Oncol. 98, 15–22 (2011).
Chen, Y. et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 119, 2230–2238 (2013).
Lee, A. W. et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J. Natl Cancer Inst. 102, 1188–1198 (2010).
Lee, A. W. M. et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer 123, 4147–4157 (2017).
Lee, A. W. et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J. Clin. Oncol. 23, 6966–6975 (2005).
Lin, J. C. et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J. Clin. Oncol. 21, 631–637 (2003).
Chitapanarux, I. et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur. J. Cancer 43, 1399–1406 (2007).
Dechaphunkul, T., Pruegsanusak, K., Sangthawan, D. & Sunpaweravong, P. Concurrent chemoradiotherapy with carboplatin followed by carboplatin and 5-fluorouracil in locally advanced nasopharyngeal carcinoma. Head. Neck Oncol. 3, 30 (2011).
Wu, X. et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann. Oncol. 24, 2131–2136 (2013).
Tang, L. Q. et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 19, 461–473 (2018).
Loong, H. H. et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother. Oncol. 104, 300–304 (2012).
Leung, S. F. et al. Pretherapy quantitative measurement of circulating Epstein−Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 98, 288–291 (2003).
Min, H. et al. A new staging system for nasopharyngeal carcinoma in China. Int. J. Radiat. Oncol. Biol. Phys. 30, 1037–1042 (1994).
Chen, Q. Y. et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J. Natl Cancer Inst. 103, 1761–1770 (2011).
Li, X. Y. et al. Ten-year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. Eur. J. Cancer 110, 24–31 (2019).
Xu, C. et al. Chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma: a systemic review and meta-analysis of 2138 patients. J. Cancer 8, 287–297 (2017).
Rossi, A. et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4-year multicenter randomized study. J. Clin. Oncol. 6, 1401–1410 (1988).
Kwong, D. L. et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J. Clin. Oncol. 22, 2643–2653 (2004).
Chi, K. H. et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int. J. Radiat. Oncol. Biol. Phys. 52, 1238–1244 (2002).
Kong, F. et al. Assessment of radiotherapy combined with adjuvant chemotherapy in the treatment of patients with advanced nasopharyngeal carcinoma: a prospective study. J. BUON 20, 206–211 (2015).
Chen, L. et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur. J. Cancer 75, 150–158 (2017).
Chen, Y. L., Chang, M. C. & Cheng, W. F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 400, 282–292 (2017).
Twu, C. W. et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein−Barr virus deoxyribonucleic acid. Int. J. Radiat. Oncol. Biol. Phys. 89, 21–29 (2014).
Liu, Y. C. et al. Prognostic impact of adjuvant chemotherapy in high-risk nasopharyngeal carcinoma patients. Oral. Oncol. 64, 15–21 (2017).
Chan, A. T. et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 33, 569–577 (1995).
Group, I. N. C. S. & Trial, V. I. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. Int. J. Radiat. Oncol. Biol. Phys. 35, 463–469 (1996).
Chua, D. T. et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer 83, 2270–2283 (1998).
Ma, J. et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J. Clin. Oncol. 19, 1350–1357 (2001).
Hareyama, M. et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer 94, 2217–2223 (2002).
Chua, D. T. et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J. Clin. Oncol. 23, 1118–1124 (2005).
Hui, E. P. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J. Clin. Oncol. 27, 242–249 (2009).
Fountzilas, G. et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann. Oncol. 23, 427–435 (2012).
Lee, A. W. M. et al. NPC-0501 trial on the value of changing chemoradiotherapy sequence, replacing 5-fluorouracil with capecitabine, and altering fractionation for patients with advanced nasopharyngeal carcinoma. Cancer 126, 3674–3688 (2020).
Kong, L. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer 119, 4111–4118 (2013).
Bae, W. K. et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother. Pharmacol. 65, 589–595 (2010).
Li, W. F. et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int. J. Cancer 145, 295–305 (2019).
Sun, Y. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 17, 1509–1520 (2016).
Frikha, M. et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann. Oncol. 29, 731–736 (2018).
Yang, Q. et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur. J. Cancer 119, 87–96 (2019).
Cao, S. M. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur. J. Cancer 75, 14–23 (2017).
Tan, T. et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 91, 952–960 (2015).
Hong, R. L. et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann. Oncol. 29, 1972–1979 (2018).
Zhang, L. et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 388, 1883–1892 (2016).
Zhang, Y. et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med. 381, 1124–1135 (2019).
Petit, C. et al. Network-meta-analysis of chemotherapy in nasopharyngeal carcinoma (MAC-NPC): an update on 8,221 patients. J. Clin. Oncol. 38, 6523–6523 (2020).
Tan, T. H. et al. Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: a systematic review and meta-analysis. Radiother. Oncol. 129, 10–17 (2018).
Chen, Y. P. et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin. Cancer Res. 24, 1824–1833 (2018).
Wang, B. C., Xiao, B. Y., Lin, G. H., Wang, C. & Liu, Q. The efficacy and safety of induction chemotherapy combined with concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in nasopharyngeal carcinoma patients: a systematic review and meta-analysis. BMC Cancer 20, 393 (2020).
Wang, P., Zhang, M., Ke, C. & Cai, C. The efficacy and toxicity of induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Medicine 99, e19360 (2020).
Chen, Y. P. et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J. Clin. Oncol. 39, 840–859 (2021).
Lee, A. W. et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother. Oncol. 87, 204–210 (2008).
Zhao, C. et al. locoregional control and mild late toxicity after reducing target volumes and radiation doses in patients with locoregionally advanced nasopharyngeal carcinoma treated with induction chemotherapy (ic) followed by concurrent chemoradiotherapy: 10-year results of a phase 2 study. Int. J. Radiat. Oncol. Biol. Phys. 104, 836–844 (2019).
Lei, Y. et al. A gene-expression predictor for efficacy of induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. J. Natl Cancer Inst. 113, 471−480 (2020).
Qiang, M. et al. A prognostic predictive system based on deep learning for locoregionally advanced nasopharyngeal carcinoma. J. Natl Cancer Inst. 113, 606−615 (2020).
Leung, S. F. et al. Plasma Epstein−Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J. Clin. Oncol. 24, 5414–5418 (2006).
Ai, Q. Y. et al. Extranodal extension is a criterion for poor outcome in patients with metastatic nodes from cancer of the nasopharynx. Oral. Oncol. 88, 124–130 (2019).
Zhang, B. et al. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral. Oncol. 51, 1041–1046 (2015).
Teoh, M., Clark, C. H., Wood, K., Whitaker, S. & Nisbet, A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br. J. Radiol. 84, 967–996 (2011).
Lee, F. K. et al. Dosimetric difference amongst 3 techniques: TomoTherapy, sliding-window intensity-modulated radiotherapy (IMRT), and RapidArc radiotherapy in the treatment of late-stage nasopharyngeal carcinoma (NPC). Med. Dosim. 39, 44–49 (2014).
He, L. et al. Toxicity and dosimetric analysis of nasopharyngeal carcinoma patients undergoing radiotherapy with IMRT or VMAT: a regional center’s experience. Oral. Oncol. 109, 104978 (2020).
Akbas, U. et al. Nasopharyngeal carcinoma radiotherapy with hybrid technique. Med. Dosim. 44, 251–257 (2019).
Bibault, J. E. et al. Clinical outcomes of several IMRT techniques for patients with head and neck cancer: a propensity score-weighted analysis. Int. J. Radiat. Oncol. Biol. Phys. 99, 929–937 (2017).
Moreno, A. C. et al. Intensity modulated proton therapy (IMPT) — the future of IMRT for head and neck cancer. Oral. Oncol. 88, 66–74 (2019).
Beddok, A. et al. Proton therapy for head and neck squamous cell carcinomas: a review of the physical and clinical challenges. Radiother. Oncol. 147, 30–39 (2020).
Lewis, G. D. et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head. Neck 38, E1886–E1895 (2016).
Jiří, K. et al. Proton pencil-beam scanning radiotherapy in the treatment of nasopharyngeal cancer: dosimetric parameters and 2-year results. Eur. Arch. Otorhinolaryngol. 278, 763−769 (2020).
Beddok, A. et al. Efficacy and toxicity of proton with photon radiation for locally advanced nasopharyngeal carcinoma. Acta Oncol. 58, 472–474 (2019).
Park, S. G. et al. Early clinical outcomes of helical tomotherapy/intensity-modulated proton therapy combination in nasopharynx cancer. Cancer Sci. 110, 2867–2874 (2019).
Alterio, D. et al. Mixed-beam approach in locally advanced nasopharyngeal carcinoma: IMRT followed by proton therapy boost versus IMRT-only. Evaluation of toxicity and efficacy. Acta Oncol. 59, 541–548 (2020).
Xiang, M., Chang, D. T. & Pollom, E. L. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 126, 3560–3568 (2020).
Akbaba, S. et al. Bimodal radiotherapy with active raster-scanning carbon ion radiotherapy and intensity-modulated radiotherapy in high-risk nasopharyngeal carcinoma results in excellent local control. Cancers 11, 379 (2019).
Leeman, J. E. et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 18, e254–e265 (2017).
Verma, V., Mishra, M. V. & Mehta, M. P. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 122, 1483–1501 (2016).
Lee, A., Chow, J. C. H. & Lee, N. Y. Treatment deescalation strategies for nasopharyngeal cancer: a review. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.6154 (2020).
Au, K. H. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral. Oncol. 77, 16–21 (2018).
Sommat, K. et al. Clinical and dosimetric predictors of physician and patient reported xerostomia following intensity modulated radiotherapy for nasopharyngeal cancer — a prospective cohort analysis. Radiother. Oncol. 138, 149–157 (2019).
Lan, X. et al. Saliva electrolyte analysis and xerostomia-related quality of life in nasopharyngeal carcinoma patients following intensity-modulated radiation therapy. Radiother. Oncol. 150, 97–103 (2020).
Zhang, L. L. et al. Risk assessment of secondary primary malignancies in nasopharyngeal carcinoma: a big-data intelligence platform-based analysis of 6,377 long-term survivors from an endemic area treated with intensity-modulated radiation therapy during 2003-2013. Cancer Res. Treat. 51, 982–991 (2019).
Tseng, M. et al. Emerging radiotherapy technologies and trends in nasopharyngeal cancer. Cancer Commun. 40, 395−405 (2020).
Chow, J. C. H., Au, K. H., Mang, O. W. K., Cheung, K. M. & Ngan, R. K. C. Risk, pattern and survival impact of second primary tumors in patients with nasopharyngeal carcinoma following definitive intensity-modulated radiotherapy. Asia Pac. J. Clin. Oncol. 15, 48–55 (2019).
Lee, A. W. et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother. Oncol. 126, 25–36 (2018).
Brouwer, C. L. et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG oncology and TROG consensus guidelines. Radiother. Oncol. 117, 83–90 (2015).
Grégoire, V. et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 110, 172–181 (2014).
Lee, A. W. et al. International guideline on dose prioritization and acceptance criteria in radiation therapy planning for nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 105, 567–580 (2019).
Mnejja, W. et al. Dosimetric impact on changes in target volumes during intensity-modulated radiotherapy for nasopharyngeal carcinoma. Rep. Pract. Oncol. Radiother. 25, 41–45 (2020).
Hu, Y. C. et al. Which nasopharyngeal cancer patients need adaptive radiotherapy? BMC Cancer 18, 1234 (2018).
Yang, H. et al. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 85, e47–e54 (2013).
Nishimura, Y. et al. A phase II study of adaptive two-step intensity-modulated radiation therapy (IMRT) with chemotherapy for loco-regionally advanced nasopharyngeal cancer (JCOG1015). Int. J. Clin. Oncol. 25, 1250–1259 (2020).
Fung, N. T. C., Hung, W. M., Sze, C. K., Lee, M. C. H. & Ng, W. T. Automatic segmentation for adaptive planning in nasopharyngeal carcinoma IMRT: time, geometrical, and dosimetric analysis. Med. Dosim. 45, 60–65 (2020).
Lim, J. Y. & Leech, M. Use of auto-segmentation in the delineation of target volumes and organs at risk in head and neck. Acta Oncol. 55, 799–806 (2016).
Tao, C. J. et al. Multi-subject atlas-based auto-segmentation reduces interobserver variation and improves dosimetric parameter consistency for organs at risk in nasopharyngeal carcinoma: a multi-institution clinical study. Radiother. Oncol. 115, 407–411 (2015).
Lin, L. et al. Deep learning for automated contouring of primary tumor volumes by MRI for nasopharyngeal carcinoma. Radiology 291, 677–686 (2019).
Lee, A. W., Ma, B. B., Ng, W. T. & Chan, A. T. Management of nasopharyngeal carcinoma: current practice and future perspective. J. Clin. Oncol. 33, 3356–3364 (2015).
Liu, Y. P. et al. Surgery for isolated regional failure in nasopharyngeal carcinoma after radiation: selective or comprehensive neck dissection. Laryngoscope 129, 387–395 (2019).
You, R. et al. Salvage endoscopic nasopharyngectomy is superior to intensity-modulated radiation therapy for local recurrence of selected T1-T3 nasopharyngeal carcinoma — a case-matched comparison. Radiother. Oncol. 115, 399–406 (2015).
Lee, A. W. M. et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat. Rev. 79, 101890 (2019).
Leong, Y. H. et al. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head. Neck 40, 622–631 (2018).
Ozyigit, G. et al. A retrospective comparison of robotic stereotactic body radiotherapy and three-dimensional conformal radiotherapy for the reirradiation of locally recurrent nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 81, e263–e268 (2011).
Lin, R. et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy–dose-volume histogram analysis. Radiology 213, 489–494 (1999).
Phan, J. et al. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int. J. Radiat. Oncol. Biol. Phys. 96, 30–41 (2016).
Romesser, P. B. et al. Proton beam reirradiation for recurrent head and neck cancer: multi-institutional report on feasibility and early outcomes. Int. J. Radiat. Oncol. Biol. Phys. 95, 386–395 (2016).
Dionisi, F. et al. Clinical results of proton therapy reirradiation for recurrent nasopharyngeal carcinoma. Acta Oncol. 58, 1238–1245 (2019).
Feehan, P. E. et al. Recurrent locally advanced nasopharyngeal carcinoma treated with heavy charged particle irradiation. Int. J. Radiat. Oncol. Biol. Phys. 23, 881–884 (1992).
Hu, J. et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer 124, 2427–2437 (2018).
Chan, O. S. & Ngan, R. K. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral. Oncol. 50, 791–797 (2014).
Zheng, W. et al. Multimodality treatment may improve the survival rate of patients with metastatic nasopharyngeal carcinoma with good performance status. PLoS ONE 11, e0146771 (2016).
Hui, E. P. et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 101, 300–306 (2004).
Pan, C. C. et al. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: A study of 1027 cases and review of the literature. Exp. Ther. Med. 4, 334–338 (2012).
Tian, Y. H. et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: a subset with better overall survival. Head. Neck 38, 1152–1157 (2016).
Zou, X. et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur. J. Cancer 77, 117–126 (2017).
Le, Q. T. et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J. Natl Cancer Inst. 111, 655–663 (2019).
Sun, X. S. et al. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein−Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer 19, 92 (2019).
Hu, J. et al. Use of radiation therapy in metastatic nasopharyngeal cancer improves survival: a seer analysis. Sci. Rep. 7, 721 (2017).
Huang, T. et al. Systemic chemotherapy and sequential locoregional radiotherapy in initially metastatic nasopharyngeal carcinoma: retrospective analysis with 821 cases. Head. Neck 42, 1970–1980 (2020).
You, R. et al. Efficacy and safety of locoregional radiotherapy with chemotherapy vs chemotherapy alone in de novo metastatic nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 6, 1345−1352 (2020).
Ma, S. X. et al. The efficacy of first-line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann. Transl. Med. 6, 201 (2018).
Chan, A. T. et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J. Clin. Oncol. 23, 3568–3576 (2005).
Chua, D. T. et al. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head. Neck 30, 863–867 (2008).
Ma, B. et al. A phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma Epstein−Barr virus DNA as a biomarker of efficacy. Cancer Chemother. Pharmacol. 62, 59–64 (2008).
You, B. et al. A phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am. J. Clin. Oncol. 35, 255–260 (2012).
Elser, C. et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J. Clin. Oncol. 25, 3766–3773 (2007).
Lim, W. T. et al. A phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin. Cancer Res. 17, 5481–5489 (2011).
Hui, E. P. et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann. Oncol. 22, 1280–1287 (2011).
Li, L. et al. Apatinib, a novel VEGFR-2 tyrosine kinase inhibitor, for relapsed and refractory nasopharyngeal carcinoma: data from an open-label, single-arm, exploratory study. Invest. New Drugs 38, 1847−1853 (2020).
Ma, B. B. et al. Multicenter phase II study of the AKT inhibitor MK-2206 in recurrent or metastatic nasopharyngeal carcinoma from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group (MC1079). Invest. New Drugs 33, 985–991 (2015).
Chan, A. T. et al. Azacitidine induces demethylation of the Epstein−Barr virus genome in tumors. J. Clin. Oncol. 22, 1373–1381 (2004).
Mesia, R. et al. Phase II study of CC-486 (oral azacitidine) in previously treated patients with locally advanced or metastatic nasopharyngeal carcinoma. Eur. J. Cancer 123, 138–145 (2019).
Konteatis, Z. et al. Discovery of AG-270, a first-in-class oral MAT2A inhibitor for the treatment of tumors with homozygous MTAP deletion. J. Med. Chem. 64, 4430–4449 (2021).
Zhu, J. et al. Targeting the polycomb repressive complex-2 related proteins with novel combinational strategies for nasopharyngeal carcinoma. Am. J. Cancer Res. 10, 3267–3284 (2020).
Hui, E. P. et al. Efficacy, safety, and pharmacokinetics of axitinib in nasopharyngeal carcinoma: a preclinical and phase II correlative study. Clin. Cancer Res. 24, 1030–1037 (2018).
Chong, W. Q. et al. Integration of antiangiogenic therapy with cisplatin and gemcitabine chemotherapy in patients with nasopharyngeal carcinoma. Clin. Cancer Res. 26, 5320−5328 (2020).
Young, L. S. & Rickinson, A. B. Epstein−Barr virus: 40 years on. Nat. Rev. Cancer 4, 757–768 (2004).
Zheng, H. et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc. Natl Acad. Sci. USA 113, 11283–11288 (2016).
Chen, T. C. et al. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci. Rep. 7, 10349 (2017).
Walsh, R. J. et al. Dendritic cell therapy with CD137L-DC-EBV-VAX in locally recurrent or metastatic nasopharyngeal carcinoma (NPC). J. Clin. Oncol. 38, 6535–6535 (2020).
Xu, R.-h, Qiu, M.-Z., Zhang, Y., Wei, X.-L. & Hu, C. First-in-human dose-escalation study of anti-EGFR ADC MRG003 in patients with relapsed/refractory solid tumors [abstract]. J. Clin. Oncol. 38, 3550–3550 (2020).
Wei, J. et al. A phase I/II trial of CRISPR-Cas9-mediated PD-1 knockout Epstein−Barr virus cytotoxic lymphocytes (EBV-CTLs) for advanced stage EBV associated malignancies. J. Clin. Oncol. 36, https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS3118 (2018).
Li, Y. et al. Sequential cytokine-induced killer cell immunotherapy enhances the efficacy of the gemcitabine plus cisplatin chemotherapy regimen for metastatic nasopharyngeal carcinoma. PLoS ONE 10, e0130620 (2015).
Comoli, P. et al. Adoptive transfer of allogeneic Epstein−Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann. Oncol. 15, 113–117 (2004).
Comoli, P. et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein−Barr virus-targeted cytotoxic T lymphocytes. J. Clin. Oncol. 23, 8942–8949 (2005).
Straathof, K. C. et al. Treatment of nasopharyngeal carcinoma with Epstein−Barr virus-specific T lymphocytes. Blood 105, 1898–1904 (2005).
Louis, C. U. et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J. Immunother. 33, 983–990 (2010).
Smith, C. et al. Effective treatment of metastatic forms of Epstein−Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 72, 1116–1125 (2012).
Chia, W. K. et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol. Ther. 22, 132–139 (2014).
Lutzky, V. P. et al. Cytotoxic T cell adoptive immunotherapy as a treatment for nasopharyngeal carcinoma. Clin. Vaccin. Immunol. 21, 256–259 (2014).
Smith, C. et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: phenotype and effector function of T cells impact on clinical response. Oncoimmunology 6, e1273311 (2017).
Huang, J. et al. Epstein−Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer 123, 2642–2650 (2017).
Hui, E. P. et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein−Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 73, 1676–1688 (2013).
Sinha, D., Smith, C. & Khanna, R. Joining forces: improving clinical response to cellular immunotherapies with small-molecule inhibitors. Trends Mol. Med. 27, 75−90 (2020).
Hsu, C. et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J. Clin. Oncol. 35, 4050–4056 (2017).
Ma, B. B. Y. et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 36, 1412–1418 (2018).
Wang, F. et al. Recombinant humanized anti-PD-1 monoclonal antibody (JS001) in patients with refractory/metastatic nasopharyngeal carcinoma: interim results of an open-label phase II clinical study. J. Clin. Oncol. 37, 6017–6017 (2019).
Fang, W. et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 19, 1338–1350 (2018).
Wang, S. et al. Preliminary results with tislelizumab, an investigational anti-PD-1 antibody, in Chinese patients with nasopharyngeal cancer (NPC). J. Clin. Oncol. 37, 2556–2556 (2019).
Shen, L. et al. Atezolizumab monotherapy in Chinese patients with locally advanced or metastatic solid tumors. Eur. Soc. Med. Oncol. Asia. Singap. Ann. Oncol. 29, 20 (2018).
Wang, F. H. et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J. Clin. Oncol. 39, 704–712 (2021).
Wang, B. C. et al. The efficacy and safety of PD-1/PD-L1 inhibitors in patients with recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral. Oncol. 104, 104640 (2020).
Lim, D. et al. Phase II study of spartalizumab (PDR001) vs chemotherapy (CT) in patients with recurrent/metastatic nasopharyngeal cancer (NPC). Cancer Res. 79, CT150 (2019).
Tang, J. et al. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 17, 854–855 (2018).
Kao, H. et al. Combination ipilimumab and nivolumab in recurrent/metastatic nasopharyngeal carcinoma (R/M NPC) — updated efficacy and safety analysis of NCT03097939. Ann. Oncol. 31, S1347–S1354 (2020).
Lam, W. K. J., Chan, K. C. A. & Lo, Y. M. D. Plasma Epstein−Barr virus DNA as an archetypal circulating tumour DNA marker. J. Pathol. 247, 641–649 (2019).
Ma, B. B. Y. et al. Recent advances in the development of biomarkers and chemoradiotherapeutic approaches for nasopharyngeal carcinoma. Am. Soc. Clin. Oncol. Educ. Book 40, 1–11 (2020).
Xie, X., Ren, Y., Wang, K. & Yi, B. Molecular prognostic value of circulating epstein-Barr viral DNA in nasopharyngeal carcinoma: a meta-analysis of 27,235 cases in the endemic area of southeast Asia. Genet. Test. Mol. Biomark. 23, 448–459 (2019).
Hui, E. P. et al. Integrating postradiotherapy plasma Epstein−Barr virus DNA and TNM stage for risk stratification of nasopharyngeal carcinoma to adjuvant therapy. Ann. Oncol. 31, 769−779 (2020).
Guo, R. et al. Proposed modifications and incorporation of plasma Epstein−Barr virus DNA improve the TNM staging system for Epstein−Barr virus-related nasopharyngeal carcinoma. Cancer 125, 79–89 (2019).
Lee, V. H. et al. The addition of pretreatment plasma Epstein−Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int. J. Cancer 144, 1713–1722 (2019).
Chan, A. T. C. et al. Analysis of plasma Epstein−Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.2018.77.7847 (2018).
Lv, J. et al. Liquid biopsy tracking during sequential chemo-radiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat. Commun. 10, 3941 (2019).
Fang, W. et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget 5, 12189–12202 (2014).
Lee, V. H. et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS ONE 11, e0157969 (2016).
Zhu, Q. et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology 6, e1312240 (2017).
Zhang, J. et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med. Oncol. 32, 86 (2015).
Zhou, Y. et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci. Rep. 7, 43627 (2017).
Cao, Y. et al. Expression and clinical significance of PD-L1 and BRAF expression in nasopharyngeal carcinoma. BMC Cancer 19, 1022 (2019).
Huang, Z. L. et al. The prognostic significance of PD-L1 and PD-1 expression in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Cancer Cell Int. 19, 141 (2019).
Goodman, A. M. et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 4, 1237–1244 (2018).
Liu, N. et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 13, 633–641 (2012).
Jiang, W. et al. Genome-wide identification of a methylation gene panel as a prognostic biomarker in nasopharyngeal carcinoma. Mol. Cancer Ther. 14, 2864–2873 (2015).
Tang, X. R. et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 19, 382–393 (2018).
Wang, Y. Q. et al. Development and validation of an immune checkpoint-based signature to predict prognosis in nasopharyngeal carcinoma using computational pathology analysis. J. Immunother. Cancer 7, 298 (2019).
Yang, M. & Huang, W. Circular RNAs in nasopharyngeal carcinoma. Clin. Chim. Acta 508, 240–248 (2020).
Bruce, J. P. et al. Identification of a microRNA signature associated with risk of distant metastasis in nasopharyngeal carcinoma. Oncotarget 6, 4537–4550 (2015).
Li, Q. et al. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Medicine 96, e8084 (2017).
Ma, B. et al. Prospective evaluation of plasma Epstein−Barr virus DNA clearance and fluorodeoxyglucose positron emission scan in assessing early response to chemotherapy in patients with advanced or recurrent nasopharyngeal carcinoma. Br. J. Cancer 118, 1051–1055 (2018).
Zhang, Y. et al. Prognostic value of the primary lesion apparent diffusion coefficient (ADC) in nasopharyngeal carcinoma: a retrospective study of 541 cases. Sci. Rep. 5, 12242 (2015).
Hong, J. et al. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 149, 707–713 (2013).
Qamar, S. et al. Pre-treatment intravoxel incoherent motion diffusion-weighted imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur. J. Radiol. 129, 109127 (2020).
Qin, Y. et al. Prognostic value of the pretreatment primary lesion quantitative dynamic contrast-enhanced magnetic resonance imaging for nasopharyngeal carcinoma. Acad. Radiol. 26, 1473–1482 (2019).
Qamar, S. et al. Pre-treatment amide proton transfer imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur. Radiol. 30, 6339–6347 (2020).
Ai, Q. Y. et al. Prediction of distant metastases from nasopharyngeal carcinoma: Improved diagnostic performance of MRI using nodal volume in N1 and N2 stage disease. Oral. Oncol. 69, 74–79 (2017).
Bossi, P. et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32, 452−465 (2020).
Luo, X. et al. DNMT1 mediates metabolic reprogramming induced by Epstein−Barr virus latent membrane protein 1 and reversed by grifolin in nasopharyngeal carcinoma. Cell Death Dis. 9, 619 (2018).
Tsai, C. L. et al. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH(2)-terminal kinase signaling. Cancer Res. 66, 11668–11676 (2006).
Shi, F. et al. Wild-type IDH2 contributes to Epstein−Barr virus-dependent metabolic alterations and tumorigenesis. Mol. Metab. 36, 100966 (2020).
Alajez, N. M. et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis. 1, e85 (2010).
Tong, Z. T. et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 31, 583–594 (2012).
Shu, X. S. et al. FEZF2, a novel 3p14 tumor suppressor gene, represses oncogene EZH2 and MDM2 expression and is frequently methylated in nasopharyngeal carcinoma. Carcinogenesis 34, 1984–1993 (2013).
Song, L. B. et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J. Clin. Invest. 119, 3626–3636 (2009).
Yip, Y. L. et al. Expression of Epstein−Barr virus-encoded LMP1 and hTERT extends the life span and immortalizes primary cultures of nasopharyngeal epithelial cells. J. Med. Virol. 82, 1711–1723 (2010).
Liu, H. et al. Promoter methylation inhibits BRD7 expression in human nasopharyngeal carcinoma cells. BMC Cancer 8, 253 (2008).
Shu, X. S. et al. The epigenetic modifier PRDM5 functions as a tumor suppressor through modulating WNT/β-catenin signaling and is frequently silenced in multiple tumors. PLoS ONE 6, e27346 (2011).
He, X. et al. Chromatin remodeling factor LSH drives cancer progression by suppressing the activity of fumarate hydratase. Cancer Res. 76, 5743–5755 (2016).
Acknowledgements
This work is dedicated to the memory of S.-F. Leung for his contribution to research in nasopharyngeal cancer. The work of K.W.L. and K.F.T. is supported by the Hong Kong Research Grant Council (Collaborative Research Fund no. C4001-18GF and Area of Excellence scheme no. AoE/M-401/20). The work of L.L. and Q.T. is supported by the Hong Kong Research Grant Council Fund no. 14115920. We thank K. Fung (Hong Kong Cancer Institute) for providing clerical support. This work is supported in part by the Kingboard Foundation. We also thank medical physicist M.L.M. Cheung (Chinese University of Hong Kong) for his advice on the section on radiotherapy.
Author information
Authors and Affiliations
Contributions
K.C.W.W., B.B.Y.M. and A.T.C.C. were responsible for the overall design and preparation of this manuscript. All authors contributed to all other aspects of manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
E.P.H. has received speaker’s honoraria from Merck Serono and Merck Sharpe and Dohme (MSD), and is a consultant and advisory board member for MSD. W.K.J.L. holds equity in Grail, and holds patents and patent applications related to molecular diagnostics. K.C.A.C. holds equity in Dra Company Limited (DRA), Grail and Take2, is a consultant to Grail, a director of DRA and Take2, holds patents and patent applications related to molecular diagnostics, and receives royalties from DRA, Grail, Illumina, Take2 and Xcelom. B.B.Y.M. is an advisory board member and receives speaker’s honoraria from Bristol Myers Squibb, Merck Serono, MSD and Novartis, and is a consultant for Y-Biologics. A.T.C.C. receives research funding from Merck Serono, MSD and Pfizer, and is an advisory board member for MSD and Tessa Therapeutics. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks B.C. Goh and C.M. Lim for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov database: https://clinicaltrials.gov
Supplementary Information
Rights and permissions
About this article
Cite this article
Wong, K.C.W., Hui, E.P., Lo, KW. et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol 18, 679–695 (2021). https://doi.org/10.1038/s41571-021-00524-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00524-x
This article is cited by
-
EBV DNA methylation profiles and its application in distinguishing nasopharyngeal carcinoma and nasal NK/T-cell lymphoma
Clinical Epigenetics (2024)
-
High expression of PPP1CC promotes NHEJ-mediated DNA repair leading to radioresistance and poor prognosis in nasopharyngeal carcinoma
Cell Death & Differentiation (2024)
-
CYLD induces high oxidative stress and DNA damage through class I HDACs to promote radiosensitivity in nasopharyngeal carcinoma
Cell Death & Disease (2024)
-
Epstein–Barr virus at 60
Nature (2024)
-
TSPAN1 inhibits metastasis of nasopharyngeal carcinoma via suppressing NF-kB signaling
Cancer Gene Therapy (2024)